Abstract
Supine hypertension is difficult to manage in patients with pure autonomic failure because treatment can worsen orthostatic hypotension. Supine hypertension in pure autonomic failure has been associated with left ventricular hypertrophy, but end-organ damage in the kidney has not been assessed. We reviewed hemodynamic and laboratory data of 64 male patients with pure autonomic failure, 69±11 (mean±SD) years old. Systolic blood pressure fell by 67±40 mmHg within 10 minutes of standing, with an inappropriately low 13±11 beats per minute increase in heart rate. Plasma norepinephrine levels were below normal (0.62±0.32 nmol/L supine and 1.28±1.25 nmol/L standing). A control dataset of 75 males (67±12 years) was obtained from a de-identified version of Vanderbilt University Medical Center's electronic medical record database. Compared with controls, pure autonomic failure patients had lower hemoglobin (8.3±0.9 mmol/L vs. 9.3±0.8 mmol/L, P<0.001), packed cell volume (0.40±0.04 vs. 0.45±0.04, P<0.001) and red blood cell count (4.4±0.5 × 1012 cells/L vs. 5.0±0.5 × 1012 cells/L, P<0.001). Serum creatinine and blood urea nitrogen were elevated in patients. Forty-eight percent of patients with pure autonomic failure had supine hypertension (supine systolic blood pressure≥150 mmHg). Serum creatinine was higher in patients with supine hypertension (133±44 μmol/L vs. 106±27 μmol/L, P=0.021), and estimated glomerular filtration rate was lower (57±22 mL/min/1.73m2 vs. 70±20 mL/min/1.73m2, P=0.022), compared with patients who did not have supine hypertension. These findings may indicate that renal function is diminished in pure autonomic failure in association with supine hypertension.
Keywords: Pure Autonomic Failure, Orthostatic Hypotension, Supine Hypertension, Renal Insufficiency, Anemia
Introduction
Pure autonomic failure (PAF) is an uncommon primary autonomic disorder characterized by orthostatic hypotension, defined as a drop in systolic blood pressure (BP) of ≥20 mm Hg or diastolic BP of ≥10 mm Hg1. A decrease in systolic BP of 50 mm Hg or greater is not unusual in patients with PAF. Approximately 50% of patients with severe autonomic failure have supine hypertension (sHTN), which can induce a nighttime pressure natriuresis that worsens morning orthostatic symptoms. Managing sHTN in patients with orthostatic hypotension is challenging. However, its treatment becomes more crucial with evidence of left ventricular hypertrophy in autonomic failure patients with sHTN 2.
Renal failure is not considered to be a feature of PAF, though varying degrees of diminished renal function are encountered in other forms of profound orthostatic hypotension. For example, patients with Familial Dysautonomia (FD), an autosomal recessive disorder distinguished by sensory and autonomic dysfunction, are much more likely to develop chronic kidney disease than the general US population3. Patients with the greatest postural change in BP experience the most severe renal damage3. Our laboratory has accumulated evidence of elevated BUN and serum creatinine in patients who lack the enzyme required to synthesize norepinephrine (NE) as a result of dopamine-beta-hydroxylase (DBH) deficiency4-6. The reason for the abnormal renal function parameters in DBH deficiency is unknown, but these patients lack noradrenergic sympathetic activity and experience such extreme orthostatic hypotension that they are often unable to stand longer than 30 seconds without losing consciousness.
Indirect evidence suggests that renal function may also be altered in PAF7-9. By reviewing our data on BUN and serum creatinine, this study tested the hypothesis that renal function is impaired in PAF. We additionally evaluated the influence of sHTN on renal function by comparing BUN and creatinine in patients with and without sHTN. The current study also assessed hemoglobin (Hgb), packed cell volume (PCV) and red blood cell count (RBC) to confirm previous findings of anemia in patients with PAF.
Methods
The electronic database of the Autonomic Dysfunction Clinic (ADC) at Vanderbilt University contains data on patients who have come to the Center for evaluation and treatment of their autonomic disorders. Findings from a complete history and physical exam, autonomic function testing, and catecholamine analyses are included. A diagnosis of PAF is triggered by signs and symptoms indicating failure of the sympathetic and parasympathetic nervous systems, recurring orthostatic hypotension, reduced catecholamine levels, and lack of cerebellar, striatal, pyramidal, or extrapyramidal dysfunction. A review of the electronic database yielded 64 white, male patients with a diagnosis of PAF, whose records were complete for admission laboratory tests, posture studies and tests of autonomic function. Patients whose medical history included known causes of anemia or renal impairment were excluded from evaluation. Only patients with a supine plasma NE less than 1.18 nmol/L (200 pg/mL) were included. Since 95% of our PAF population was white and non-Hispanic, and to avoid gender-related differences in biochemical and hematological values10-12, we restricted our study to white males. All 64 patients included in this study were evaluated between 1995 and 2008. All protocols and procedures were approved by the institutional review board at Vanderbilt University and written informed consent was obtained.
Blood samples for routine chemistry and hematology studies were collected before any testing was carried out. The analyses were performed in the clinical pathology laboratory of Vanderbilt University Medical Center, and the reference ranges are those used by these laboratories. Glomerular filtration rate was estimated (eGFR) from serum creatinine using the equation developed from The Modification of Diet in Renal Disease study13.
Following admission, patients received a diet containing 150 mEq of sodium and 60-80 mEq of potassium per day. All medications were withheld during their autonomic evaluation. A battery of autonomic function tests was used to assess sympathetic and parasympathetic control of heart rate and BP. These included an orthostatic stress test, Valsalva maneuver, sinus arrhythmia, cold pressor test, and isometric handgrip, as described previously14, 15. Heart rate was measured by continuous ECG, and BP was measured by photoplethysmography (Finapres Medical Systems, the Netherlands). Supine and standing oscillometric brachial BPs (Dinamap, GE Medical Systems Information Technologies; one measurement at each time point) were also measured following an overnight supine rest and fast (posture study). Although there are limitations associated with the use of an automatic cuff rather than manual measurement with Korotkoff sounds or an arterial line, the oscillometric BP has good predictive value16. Blood samples for fractionated catecholamines, renin activity and aldosterone analyses17 were collected from an antecubital vein during the posture study. Patients were asked to stand for as long as they were able, up to 30 minutes, and upright blood samples were collected at the end of this period. Normal values for autonomic function testing were determined from a set of 70 healthy controls (36 ± 10 yr, 44 females, 62 Caucasians) in the ADC database.
The control dataset for the laboratory data was obtained from Vanderbilt University Medical Center's Synthetic Derivative (SD). The SD is a database containing clinical information derived from Vanderbilt's electronic medical record, labeled with a unique research ID, and stripped of personal identifiers. The SD search interface allows the user to input basic clinical and demographic information, such as ICD-9 codes, medications, lab values, age and gender and returns de-identified data to the user for review and selection. Use of the SD was approved by the institutional review board at Vanderbilt. Search criteria included white males ages 45 to 94 years with an ICD-9 code for a routine medical examination (V70.0). The dataset was limited to “healthy” individuals by excluding those with a number of neurologic and cardiovascular disorders, orthostatic hypotension, diabetes, hypertension and kidney disease. Records from an initial dataset of 318 individuals were reviewed and the list was culled to 148, based on ICD-9 codes or clinical notes that indicated cancer or another active disease, lack of laboratory data, or vital signs that included repeated systolic BPs above 140 mmHg. Finally, 75 individuals with an age distribution similar to that in our patient group were selected. Orthostatic vital signs are not generally collected during routine clinic visits in normal subjects, but the seated pressures for our control group averaged 124±13/74±9 mmHg.
Demographic, clinical and biochemical data are expressed as mean±S.D or mean (95% confidence intervals). The relationship between sHTN and renal function was assessed by stratifying the patient group according to supine systolic BP (sHTN defined as supine systolic BP≥150 mmHg) and comparing groups with and without sHTN. Differences between patients and controls and between patients with and without sHTN were assessed by the Mann-Whitney U test. The chi-square test was used for analysis of categorical variables. Statistical analyses were carried out using the statistical software SPSS for Windows, Version 15.0 (SPSS Inc., Chicago, IL). All tests were two-sided and differences with P<0.05 were considered statistically significant.
Results
During a 10-minute orthostatic challenge, patients with PAF stood for 268 ± 175 sec (range 30-600 sec). Systolic BP fell dramatically (-67 ± 40 mmHg), accompanied by an inappropriately low increase in heart rate (13 ± 11 beats per minute; Table 1). Results of autonomic function testing reflected autonomic failure and included: attenuated cardiovagal responses to sinus arrhythmia and the Valsalva maneuver; lack of systolic BP recovery during phase II of the Valsalva maneuver and absent phase IV systolic BP overshoot; blunted sympathetic vasopressor responses with the cold pressor and handgrip tests; and deficient sympathetically mediated reflex vasoconstriction during hyperventilation. Plasma NE levels were below normal (0.62 ± 0.32 nmol/L supine and 1.28 ± 1.25 nmol/L standing). Plasma renin activity was low and failed to appropriately increase with standing.
Table 1.
Results of autonomic function tests
| Parameters | PAF | Normal values* |
|---|---|---|
| Orthostatic vital signs | ||
| ΔSBP (mmHg) | -67 ± 40† | -1 ± 18 |
| ΔHR (bpm) | 13 ± 11 | 15 ± 13 |
| Autonomic function tests | ||
| Sinus arrhythmia ratio | 1.1 ± 0.2 | 1.4 ± 0.2 |
| Valsalva phase IIL ΔSBP (mmHg) | -58 ± 30 | -17 ± 17 |
| Valsalva phase IV ΔSBP (mmHg) | -25 ± 23 | 24 ± 15 |
| Valsalva ratio | 1.1 ± 0.1 | 1.7 ± 0.4 |
| Hyperventilation ΔSBP (mmHg) | -28 ± 26 | -6 ± 12 |
| Cold pressor ΔSBP (mmHg) | 11 ± 18 | 20 ± 15 |
| Handgrip ΔSBP (mmHg) | 9 ± 12 | 17 ± 13 |
| Plasma norepinephrine (nmol/L) | ||
| Supine | 0.62 ± 0.32 | 1.21 ± 0.82 |
| Upright | 1.28 ± 1.25 | 3.06 ± 1.32 |
| Plasma renin activity (μg/L/h) | ||
| Supine | 0.4 ± 0.5 | 0.7 ± 0.5 |
| Upright | 0.5 ± 1.0 | 2.2 ± 2.0 |
Normal values are from the Vanderbilt Autonomic Dysfunction Center database.
Means ± SD. SBP: systolic blood pressure; HR: heart rate
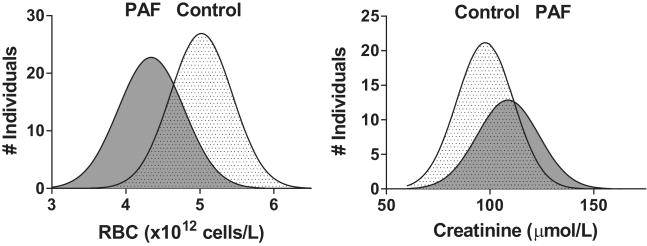
Compared with a healthy population (67 ± 12 yr), admission laboratory values for patients with PAF (69 ± 11 yr, P=0.160) included a number of divergences (Table 2, Figure 1). Hemoglobin, PCV, and RBC for PAF patients were each significantly below the control values (P<0.001 for each comparison). Furthermore, Hgb, PCV and RBC measures were all below the reference range in 63% of patients with PAF compared with 12% of our control group (P<0.001). Mean serum creatinine (115 ± 35 vs. 97 ± 18 μmol/L, P<0.001) and BUN (8.2 ± 2.5 vs. 5.7 ± 1.4 mmol/L, P<0.001) were elevated in patients. Serum creatinine was above the reference range (> 133 μmol/L) in 20% of PAF patients and BUN was above the reference range (> 8.9 mmol/L) in 30% of patients. The mean eGFR of the control group was 73 ± 14 mL/min/1.73m2 (range 41 to 104 mL/min/1.73m2). In our patients with PAF, eGFR was 63 ± 22 mL/min/1.73m2 (range 23 to 119 mL/min/1.73m2; P <0.001). Defining a normal GFR to be ≥ 60 mL/min/1.73m2, 44% of PAF patients had a decreased eGFR. In comparison, GFR was below 60 mL/min/1.73m2 in 13% of our control population. Routine urinalysis results were available for 56 patients and 39 members of our control dataset. Of these, 25% of patients had at least trace levels of albumin, compared with 8% of the controls (results not shown; P=0.030). Body weights for the two groups were similar.
Table 2.
Clinical laboratory results for patients with pure autonomic failure (PAF) and controls
| Lab | Reference Range | Controls | PAF | P |
|---|---|---|---|---|
| Age (years) | 67 ± 12 | 69 ± 11 | 0.160 | |
| Weight (kg) | 85.1 ± 12.2 | 83.4 ± 13.8 | 0.788 | |
| Hgb (mmol/L) | 8.7 - 11.2 | 9.3 ± 0.8 | 8.3 ± 0.9 | <0.001 |
| PCV | 0.42 - 0.50 | 0.45 ± 0.04 | 0.40 ± 0.04 | <0.001 |
| RBC (× 1012 cells/L) | 4.7 - 6.1 | 5.0 ± 0.5 | 4.4 ± 0.5 | <0.001 |
| BUN (mmol/L) | 1.8 - 8.9 | 5.7 ± 1.4 | 8.2 ± 2.5 | <0.001 |
| Creatinine (μmol/L) | 62 - 133 | 97 ± 18 | 115 ± 35 | <0.001 |
| eGFR (mL/min/1.73m2) | > 60 | 73 ± 14 | 63 ± 22 | <0.001 |
Means ± SD; Hgb, hemoglobin; PCV, packed cell volume; RBC, red blood cell count; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate using MDRD equation.
Figure 1.
Frequency distribution of RBC and creatinine values in 64 patients with pure autonomic failure (PAF) and 75 control subjects.
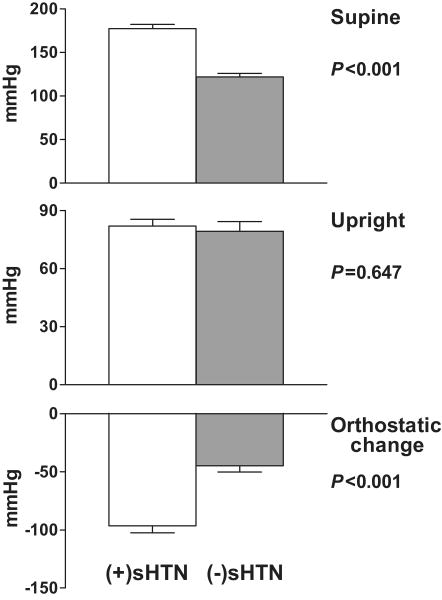
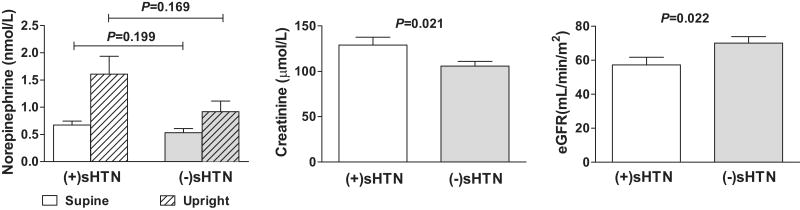
Forty-eight percent of the patients with PAF had sHTN. Those with sHTN also experienced a greater fall in systolic BP (SBP) and diastolic BP (DBP) during the posture study [(+)sHTN: -96/-39 ± 30/17 mmHg; (-)sHTN: -45/-23 ± 26/17; P<0.001 for change in SBP and P=0.004 for change in DBP; Figure 2]. As a result, standing BPs did not differ (P=0.647). Compared with (-)sHTN patients, (+)sHTN patients had a higher serum creatinine (133 ± 44 vs. 106 ± 27 μmol/L, P= 0.021) and a lower eGFR (57 ± 22 vs. 70 ± 20 mL/min/1.73m2, P= 0.022; Figure 3). No other laboratory test results, including the semi-quantitative urinary albumin data, differed significantly according to sHTN status [Hgb:8.2±0.9 vs. 8.4±0.9 mmol/L, P=0.576; BUN: 8.6 ± 2.9 vs. 7.8 ± 2.5 mmol/L, P= 0.219; upright plasma NE: 1.61±1.58 vs. 0.92±0.75 nmol/L, P=0.169; upright PRA: 0.4±0.5 vs. 0.9±1.5 μg/L/h, P=0.297 for (+)sHTN vs. (-)sHTN]. When patients with PAF were stratified by supine DBP or heart rate, orthostatic changes in BP or heart rate, upright BP or heart rate, or upright plasma NE (<1.0 nmol/L or ≥ 1.0 nmol/L), no differences in Hgb, PCV, RBC, BUN, creatinine, or eGFR were discovered.
Figure 2.
Systolic blood pressures in patients with pure autonomic failure stratified by those with and without supine hypertension (sHTN).
Figure 3.
Chemistry data in patients with pure autonomic failure stratified by those with and without supine hypertension (sHTN).
Discussion
This is the first study to evaluate renal function in patients with PAF. The principal new findings are that patients with PAF have elevations in serum creatinine and BUN and a reduction in eGFR when compared with a similarly-aged, healthy population. We also confirm earlier findings of anemia and low plasma renin activity, consistent with lack of sympathetic stimulation to the kidney. The lower eGFR and higher creatinine in the subgroup of patients having a supine SBP≥150 mmHg further suggest that diminished renal function in PAF may be related to supine hypertension. Our results not only underscore the importance of controlling supine hypertension in patients with PAF, but they also indicate that these patients should be considered at increased risk for developing chronic kidney disease.
Our hypothesis of decreased renal function in patients with PAF was based on results from observational studies in other autonomic disorders that suggest that autonomic neuropathy contributes to a loss of renal function. In five patients with congenital DBH deficiency, an extremely rare disorder characterized by absent sympathetic noradrenergic activity5, 6, BUN ranged from 5.7 mmol/L in a 17 year old to 21.1 mmol/L in a 54 year old. Respective creatinines were 62 μmol/L and 239 μmol/L4. Perhaps the most thoroughly characterized example of kidney disease associated with autonomic dysfunction is the renal failure that occurs as a complication of FD. Thirty-nine percent of the FD population has an eGFR less than 60 mL/min at age 20 years3, and serum creatinine and BUN increase with age.
Biaggioni proposed that sympathetic neuropathy in PAF extends to the kidneys7. Sympathetic mechanisms in the kidney are involved in regulation of sodium homeostasis, renin release and erythropoietin synthesis, all of which are impaired in patients with PAF8, 9, 18, 19. Renal end-organ damage was suggested in the initial report of PAF by Bradbury and Eggleston, wherein all three cases had high normal BUN levels20. More recently, 30% of 100 patients with orthostatic hypotension, including 11 with PAF, had serum creatinine >115 μmol/L, a prevalence similar to that in a group of hypertensive patients21. Our findings of elevated serum creatinine and BUN, reduced eGFR, and albuminuria provide additional support for an association between sympathetic deprivation and impaired renal function in patients with PAF.
The direct cause of the loss of renal function in patients with PAF cannot be determined from our data, but orthostatic hypotension and supine hypertension likely contribute to the risk. Although it is possible that the rise in BUN and creatinine in DBH deficiency relates in part to a dopamine-mediated renal toxicity, both patients with PAF and those with DBH deficiency experience severe orthostatic hypotension and syncope. Doppler flow measurements indicate that the postural fall in BP in patients with FD is accompanied by a decrease in renal perfusion which may lead to renal damage. Patients with FD also often have supine hypertension, and hypertension is a known risk factor for kidney disease22. Approximately 50% of our patients with PAF experienced high BPs when supine, consistent with previous reports23, 24. Supine hypertension is often not treated aggressively in PAF for fear of worsening orthostatic hypotension and syncope. Furthermore, the influence of supine hypertension on the prognosis of patients with PAF is unclear, although it has been associated with target organ damage in the form of left ventricular hypertrophy2. We found that creatinine was significantly higher and eGFR was significantly lower in the patients with supine hypertension compared with those without supine hypertension. Supine hypertension in PAF has been attributed to increased vascular resistance25. An increase in renal vascular resistance could contribute to renal hypoperfusion and eventually a reduction in GFR. Our results suggest that supine hypertension, orthostatic hypotension, or possibly BP lability may negatively influence renal function. Effects on kidney function in PAF could relate to an inability to regulate renal blood flow in response to systemic changes in pressure, i.e., impaired autoregulation, as a result of decreased sympathetic innervation of the kidney. The enhanced pressor and depressor responses to a variety of agents, including water26-29, may further increase the risk of end-organ damage in patients with autonomic failure.
Although these findings are not definitive evidence of impaired renal function in PAF, they demonstrate that longitudinal studies are warranted. Ambulatory BP measurements can be used in patients with PAF to determine the association between loss of renal function and the severity and total duration of hypotensive and hypertensive episodes, as well as the variability of pressure within a 24-hour period. Preliminary data suggest that patients with supine hypertension who do not lower their BP during the night (non-dippers) tend to have a higher serum creatinine and lower eGFR (30, Luis Okamoto, unpublished data, 2009).
The decrease in renal function in congenital disorders of autonomic failure suggests that autonomic dysfunction precedes the renal dysfunction. However, early and follow-up evaluations in patients with PAF are needed to definitively demonstrate which is the primary deficit in this disorder, as well as the relationship between changes in renal function biomarkers and anemia. The frequent occurrence of anemia in PAF is related to decreased erythropoietin production in the kidney and underscores the potential for important interactions between the hematologic and renal systems in PAF.
An important limitation of this study is its retrospective design. Further, we did not recruit our control group but used a cohort derived from Vanderbilt's de-identified electronic medical records. Although our data were consistent with previously published chemistry values in the elderly10, 11, 31-33, other biomarkers of renal function, such as quantitation of urinary protein, were not available. We were also unable to control for medications taken prior to admission that might have contributed to impaired renal function or anemia. At some point, patients may have received treatment that had a bearing on e.g. hemoglobin. We believe, however, that our data indicate an impairment of renal function in patients with PAF. Future prospective studies are needed that would include serial measures of GFR. Detailed evaluation of renal blood flow might provide additional information about the cause of renal problems in PAF.
Perspectives
Our data indicate that patients with PAF may have somewhat compromised renal function that is associated with the supine hypertension that commonly occurs in this patient population. Despite the difficulties inherent in treating hypertension in patients whose autonomic disorder results in profound orthostatic hypotension, our results stress that failure to manage the hypertension may put the patients at increased risk of developing renal failure.
Acknowledgments
We would like to acknowledge the assistance of Shannon Carter and Melissa Basford with identifying a control dataset from the Synthetic Derivative.
Sources of Funding: This research was supported in part by NIH grants P01 HL056693, and R01 HL071784, the Vanderbilt CTSA grant UL1 RR024975, and the Paden Center. The Vanderbilt University Medical Center's Synthetic Derivative is supported by institutional funding and by the Vanderbilt CTSA grant.
Footnotes
Conflicts of Interest: The authors have reported no conflicts of interest.
Reference List
- 1.Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125–126. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 2.Vagaonescu TD, Saadia D, Tuhrim S, Phillips RA, Kaufmann H. Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet. 2000;355:725–726. doi: 10.1016/S0140-6736(99)05320-9. [DOI] [PubMed] [Google Scholar]
- 3.Elkayam L, Matalon A, Tseng CH, Axelrod F. Prevalence and severity of renal disease in familial dysautonomia. Am J Kidney Dis. 2006;48:780–786. doi: 10.1053/j.ajkd.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Garland E, Raj SR, Biaggioni I, Black BK, Robertson D. A hyperdopaminergic nephropathy in DBH deficiency. Clin Auton Res. 2005;15:321. [Google Scholar]
- 5.Man in 't Veld AJ, Boomsma F, Moleman P, Schalekamp MA. Congenital dopamine-β-hydroxylase deficiency: A novel orthostatic syndrome. Lancet. 1987;1:183–187. doi: 10.1016/s0140-6736(87)90002-x. [DOI] [PubMed] [Google Scholar]
- 6.Robertson D, Goldberg MR, Hollister AS, Onrot J, Wiley R, Thompson JG, Robertson RM. Isolated failure of autonomic noradrenergic neurotransmission: Evidence for impaired beta-hydroxylation of dopamine. N Engl J Med. 1986;314:1494–1497. doi: 10.1056/NEJM198606053142307. [DOI] [PubMed] [Google Scholar]
- 7.Biaggioni I. The sympathetic nervous system and blood volume regulation: lessons from autonomic failure patients. Am J Med Sci. 2007;334:61–64. doi: 10.1097/MAJ.0b013e318065c03b. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox CS, Aminoff MJ, Slater JD. Sodium homeostasis in patients with autonomic failure. Clin Sci Mol Med. 1977;53:321–328. doi: 10.1042/cs0530321. [DOI] [PubMed] [Google Scholar]
- 9.Biaggioni I, Robertson D, Krantz S, Jones M, Haile V. The anemia of primary autonomic failure and its reversal with recombinant erythropoietin. Ann Intern Med. 1994;121:181–186. doi: 10.7326/0003-4819-121-3-199408010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Eknoyan G, Levin NW. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 11.Giorno R, Clifford JH, Beverly S, Rossing RG. Hematology reference values. Analysis by different statistical technics and variations with age and sex. Am J Clin Pathol. 1980;74:765–770. doi: 10.1093/ajcp/74.6.765. [DOI] [PubMed] [Google Scholar]
- 12.Hale WE, Stewart RB, Marks RG. Haematological and biochemical laboratory values in an ambulatory elderly population: an analysis of the effects of age, sex and drugs. Age Ageing. 1983;12:275–284. doi: 10.1093/ageing/12.4.275. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 14.Mosqueda-Garcia R. Evaluation of autonomic failure. In: Robertson D, Biaggioni I, editors. Disorders of the Autonomic Nervous System. London, United Kingdom: Harwood Academic Press; 1995. pp. 25–59. [Google Scholar]
- 15.Robertson D. Assessment of autonomic function. In: Baughman KL, Green BM, editors. Manual for House Officers. Baltimore, MD: Williams and Wilkins; 1981. pp. 86–131. [Google Scholar]
- 16.Lakhal K, Ehrmann S, Runge I, Legras A, Dequin PF, Mercier E, Wolff M, Regnier B, Boulain T. Tracking hypotension and dynamic changes in arterial blood pressure with brachial cuff measurements. Anesth Analg. 2009;109:494–501. doi: 10.1213/ane.0b013e3181a8d83a. [DOI] [PubMed] [Google Scholar]
- 17.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 18.Biaggioni I, Garcia F, Inagami T, Haile V. Hyporeninemic normoaldosteronism in severe autonomic failure. J Clin Endocrinol Metab. 1993;76:580–586. doi: 10.1210/jcem.76.3.7680352. [DOI] [PubMed] [Google Scholar]
- 19.Hoeldtke RD, Streeten DH. Treatment of orthostatic hypotension with erythropoietin. N Engl J Med. 1993;329:611–615. doi: 10.1056/NEJM199308263290904. [DOI] [PubMed] [Google Scholar]
- 20.Bradbury S, Eggleston C. Postural hypotension: A report of three cases. American Heart Journal. 1925;1:73–86. [Google Scholar]
- 21.Ejaz AA, Haley WE, Wasiluk A, Meschia JF, Fitzpatrick PM. Characteristics of 100 consecutive patients presenting with orthostatic hypotension. Mayo Clin Proc. 2004;79:890–894. doi: 10.4065/79.7.890. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Prevalence of chronic kidney disease and associated risk factors--United States, 1999-2004. MMWR Morb Mortal Wkly Rep. 2007;56:161–165. [PubMed] [Google Scholar]
- 23.Biaggioni I, Robertson RM. Hypertension in orthostatic hypotension and autonomic dysfunction. Cardiol Clin. 2002;20:291–301. doi: 10.1016/s0733-8651(01)00005-4. [DOI] [PubMed] [Google Scholar]
- 24.Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I. The hypertension of autonomic failure and its treatment. Hypertension. 1997;30:1062–1067. doi: 10.1161/01.hyp.30.5.1062. [DOI] [PubMed] [Google Scholar]
- 25.Kronenberg MW, Forman MB, Onrot J, Robertson D. Enhanced left ventricular contractility in autonomic failure: assessment using pressure-volume relations. J Am Coll Cardiol. 1990;15:1334–1342. doi: 10.1016/s0735-1097(10)80023-3. [DOI] [PubMed] [Google Scholar]
- 26.Biaggioni I, Onrot J, Stewart CK, Robertson D. The potent pressor effect of phenylpropanolamine in patients with autonomic impairment. JAMA. 1987;258:236–239. [PubMed] [Google Scholar]
- 27.Jordan J, Shannon JR, Diedrich A, Black B, Robertson D, Biaggioni I. Water potentiates the pressor effect of ephedra alkaloids. Circulation. 2004;109:1823–1825. doi: 10.1161/01.CIR.0000126283.99195.37. [DOI] [PubMed] [Google Scholar]
- 28.Robertson D, Hollister AS, Carey EL, Tung CS, Goldberg MR, Robertson RM. Increased vascular beta2-adrenoceptor responsiveness in autonomic dysfunction. J Am Coll Cardiol. 1984;3:850–856. doi: 10.1016/s0735-1097(84)80264-8. [DOI] [PubMed] [Google Scholar]
- 29.Young TM, Mathias CJ. The effects of water ingestion on orthostatic hypotension in two groups of chronic autonomic failure: multiple system atrophy and pure autonomic failure. J Neurol Neurosurg Psychiatry. 2004;75:1737–1741. doi: 10.1136/jnnp.2004.038471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto LE, Gamboa A, Shibao C, Black BK, Diedrich A, Raj SR, Robertson D, Biaggioni I. Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension. 2009;53:363–369. doi: 10.1161/HYPERTENSIONAHA.108.124552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tietz NW, Shuey DF, Wekstein DR. Laboratory values in fit aging individuals--sexagenarians through centenarians. Clin Chem. 1992;38:1167–1185. [PubMed] [Google Scholar]
- 32.Jernigan JA, Gudat JC, Blake JL, Bowen L, Lezotte DC. Reference values for blood findings in relatively fit elderly persons. J Am Geriatr Soc. 1980;28:308–314. doi: 10.1111/j.1532-5415.1980.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 33.Baldwin JG., Jr True anemia: incidence and significance in the elderly. Geriatrics. 1989;44:33–36. [PubMed] [Google Scholar]