Abstract
Purpose
We used total body irradiation (TBI) as conditioning for cord blood transplantation studies in pigtailed macaques. In these studies, different doses of TBI were explored to obtain optimal myelosuppression with acceptable radiation-related side effects.
Methods
Four macaques received TBI ranging from 800 to 1320 cGy, followed by standard post-transplant care. Hematopoietic recovery was monitored by CBC and donor contribution by VNTR analysis.
Results
Animals receiving 800 or 1020 cGy TBI tolerated the irradiation well with autologous recovery of neutrophils within 24 days. At a dose of 1200 cGy, neither autologous recovery nor extramedullary toxicity was observed. A fourth animal received 1320 cGy of TBI and suffered significant toxicity necessitating termination of the study.
Conclusions
We conclude that previously considered myeloablative doses of TBI allowed for autologous recovery in the pigtailed macaque, and that a dose of 1200 cGy may be most appropriate, providing both myeloablation and acceptable nonhematopoietic toxicities.
Keywords: Conditioning, immunosuppression, monkeys, stem cell transplantation, radiation
Introduction
Studies involving myeloablative conditioning in nonhuman primates began in the 1950s and 1960s during the development of preclinical bone marrow transplantation models. From these studies, important information was generated regarding hematopoietic recovery after irradiation and irradiation-related toxicities; these findings had a profound impact, leading to the work pioneered by E. Donnall Thomas and colleagues [22] in human bone marrow transplantation.
Much of the work involving the effects of radiation and stem cell transplantation in monkeys was pioneered by studies in the 1960s and 1970s. These studies were designed with a variety of different endpoints; some were designed to determine the highest dose of radiation tolerated by nonhuman primates in the absence of hematopoietic rescue, while others were implemented to study hematopoietic recovery following stem cell transplant or to test different transplant regimens. Studies involving irradiation with no subsequent cell transplant include those by Crouch et al. [8], Van Putten, [24] and Broerse et al. [6]. Van Putten [24] showed that the maximum tolerated radiation dose in rhesus macaques (Macaca mulatta) was 624 cGy, while Crouch et al. [8] found that six of six rhesus macaques receiving 650 cGy of radiation died, with a survival time ranging from 12 to 16 days and a mean survival time of 14.5 days. Similarly, Broerse et al. [6] found that the LD50 of rhesus monkeys after x-ray exposure was 525 cGy.
Later studies examined the ability of cells to engraft lethally irradiated monkeys after different cell preparation techniques. Van Putten [24] investigated the effects of freezing and thawing on bone marrow cells used for autologous transplantation of rhesus macaques; these animals received supralethal radiation doses of at least 770 cGy. Using autologous baboon CD34+ cells, Berenson et al. [4] reported hematopoietic repopulation after high-dose TBI of 920 cGy. Donahue et al. [9] utilized 1000 cGy TBI for conditioning in rhesus macaques while studying retroviral mediated gene transfer. Likewise, Kluge et al. [14] conditioned rhesus macaques using 1000 cGy TBI prior to transplantation studies comparing retroviral transduction and engraftment ability of cells cultured under serum and serum-free conditions. We have previously shown that macaques have a lower radiation tolerance than baboons (Papio cynocephalus), and that a 1020-cGy conditioning regimen well tolerated by baboons leads to complications in macaques [5].
Other studies tested the effects of different transplant regimens (i.e. cell rescue or cytokine stimulation) on engraftment kinetics after high-dose TBI. Early cell rescue experiments following high-dose TBI by Van Bekkum et al. [3] demonstrated that rhesus macaques conditioned with 760 cGy TBI were able to engraft allogeneic bone marrow cells in over 95% of cases. Similar experiments by Broerse et al. [6] concluded that rhesus monkeys could be protected by autologous bone marrow transplantation after receiving doses up to 860 cGy of TBI. Studies carried out by Monroy et al. [18] combined cytokine support with cellular rescue and studied the ability of GM-CSF to increase hematopoietic recovery after autologous stem cell transplantation in rhesus macaques following 900 cGy TBI. Studies by Wagemaker et al. [25] investigated the efficacy of thrombopoietin in stem cell transplantation following 800 cGy TBI in rhesus macaques. Ageyama et al. [2] studied autologous hematopoietic stem cell transplantation in the cynomolgus monkey (Macaca fascicularis), using a high-dose conditioning regimen consisting of 1000 to 1100 cGy of TBI prior to infusion of autologous cells. Studies in our lab comparing intravenous infusion and intramarrow injection of stem cells in baboons have used a TBI dose of 1020 cGy to achieve myeloablation [13].
Thus, nonhuman primate transplantation models utilizing baboon, rhesus macaques, and cynomolgus macaques primarily use high-dose preparative regimens of TBI in the range of 650 to 1100 cGy. Studies with no subsequent stem cell rescue have determined that doses in the range of 525 cGy to 650 cGy are lethal (see Table 1). Here we report that, in the pigtailed macaque, doses up to 1020 cGy are not myeloablative and suggest that 1200 cGy results in myeloablation with acceptable nonhematopoietic toxicity.
Table 1.
Historical review of high-dose radiation nonhuman primate studies detailed in text.
| Study Type | Year | Author(s) | TBI Dose | Non-Human Primate Studied | Study Details |
|---|---|---|---|---|---|
| Without hematopoietic rescue | 1961 | Crouch et al. | 650 cGy | Macaca mulatta | 6 of 6 monkeys died after TBI of 650 cGy |
| 1965 | Van Putten | 624 cGy | Macaca mulatta | Highest survivable dose was 624 cGy | |
| 1978 | Broerse et al. | 525 cGy | Macaca mulatta | LD50 for irradiated monkeys was 525 cGy | |
| With hematopoietic rescue | 1965 | Van Putten | 770 cGy | Macaca mulatta | Effects of cell freezing/thawing on engraftment |
| 1969 | Van Bekkum et al. | 760 cGy | Macaca mulatta | Engraftment of allogeneic bone marrow cells | |
| 1978 | Broerse et al. | 860 cGy | Macaca mulatta | Engraftment of autologous cells | |
| 1987 | Monroy et al. | 900 cGy | Macaca mulatta | Effects of GM-CSF after autologous transplant | |
| 1988 | Berenson et al. | 920 cGy | Papio cynocephalus | Engraftment of autologus cells | |
| 1992 | Donahue et al. | 1000 cGy | Macaca mulatta | Retroviral gene transfer | |
| 1998 | Wagemaker et al. | 800 cGy | Macaca mulatta | Effects of thrombopoietin after transplant | |
| 2002 | Ageyama et al. | 1000–1100 cGy | Macaca fascicularis | Engraftment of autologous cells | |
| 2002 | Kluge et al. | 1000 cGy | Macaca mulatta | Effects of serum versus serum-free conditions | |
| 2004 | Bielefeldt-Ohmann et al. | 1020 cGy | Macaca nemestrina | Radiation sensitivity of macaques versus baboons | |
| 2007 | Jung et al. | 1020 cGy | Papio cynocephalus | Intravenous versus intramarrow injection of cells |
Materials and Methods
Animal housing and care
All pig-tailed macaques (Macaca nemestrina) were housed at the University of Washington National Primate Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. (We have previously shown that pigtailed macaque CD34+ cells are more highly permissive to transduction by lentiviral vectors than are cells of either the rhesus macaque or the baboon [23]; thus, we elected to use pigtailed macaques as our nonhuman primate model as they offer more promise for future gene therapy studies.) Experimental protocols were approved by the Institutional Animal Care and Use Committee. All monkeys used in this study were female, between the ages of 6.3 years and 8.4 years, with a weight at time of radiation of 5.4 to 5.8 kg. Because of the nature of the haploidentical cord blood transplant setting (i.e. umbilical cord blood cells from the fetus were transplanted into the mother), only female monkeys were used in this study.
For 3 days prior to transplant, animals received oral tacrolimus (FK-506) at a dose necessary to maintain serum trough levels between 10 and 15 ng/mL. Beginning on day 0 (the day of cord blood transplant), the animals received intravenous tacrolimus, also at a dose to maintain serum trough levels between 10 and 15 ng/mL (usually between 0.1 and 0.2 mg/kg/day). Intravenous methotrexate was administered at a dose of 15 mg/m2 on day 1, and 10 mg/m2 on days 3, 6, and 11. From day 0 through engraftment (absolute neutrophil count > 500/μL), the animals received intravenous G-CSF at 100 μg/kg/day and standard supportive care, including antibiotics, electrolytes, fluids, and transfusions. Daily complete blood counts were used to determine hematopoietic recovery.
Cord blood harvest and transplant
Approximately 1 week prior to delivery date, routine hysterotomy was performed through a ventral midline incision of the abdomen cranial to the pelvic rim. The uterus was exteriorized and the placental discs were located by palpation; the uterus was then incised along the longitudinal direction of the muscle between the discs. Umbilical cord blood and circulating fetal blood were collected by catheter placed into the carotid artery of the fetus, and saline was flushed through a catheter placed into the jugular vein to ensure maximal collection of cells. The fetus and placenta were then removed, the uterus was closed with absorbable sutures, and the abdomen was closed with a multi-layered closure. The blood product was red blood cell-depleted and cryopreserved for approximately 1 month while the dam recovered from the surgery. On the day of transplant, the cells were thawed and infused into the dam via either intravenous injection (macaque 05105) or intra-marrow injection (macaques 05094, A02175, and 05082). Cell doses administered ranged from 5.70×106 to 2.98×107 cells/kg (see Table 2).
Table 2.
Summary of sex, age, TBI dose, weights, follow-up time, and outcome for each macaque studied.
| Animal Number |
Sex | Age | TBI Dose |
Nucleated Cell Dose |
Time to ANC > 500/ul |
Weight at Date of TBI |
Weight at End of Study (Percent Weight Loss) |
Follow- Up Time |
Outcome | Bone Marrow Pathology Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| (yr) | (cGy) | (cells/kg) | (days) | (kg) | (kg) | (days) | ||||
| 05105 | Female | 6.3 | 800 | 5.70×106 | 24 | 5.4 | 4.8 (11.1%) | 29 | Autologous Recovery | |
| 05094 | Female | 7.2 | 1020 | 2.13×107 | 20 | 5.6 | 5.0 (10.7%) | 48 | Autologous Recovery | |
| A02175 | Female | 8.0 | 1200 | 1.60×107 | did not recover | 5.8 | 4.6 (20.7%) | 29 | Euthanized | Aplastic, no evidence of hematopoiesis |
| 05082 | Female | 8.4 | 1320 | 2.98×107 | did not recover | 5.6 | 5.6 (0%) | 19 | Euthanized | Aplastic, no evidence of hematopoiesis |
Abbreviations: TBI = total body irradiation, ANC = absolute neutrophil count.
Total body irradiation
All primates received fractionated total body irradiation on the two days prior to transplant. The TBI was administered as a total of four equivalent doses (morning and afternoon on two consecutive days) from a 6 MV X-ray beam of a single-source linear accelerator located at the Fred Hutchinson Cancer Research Center South Lake Union Facility. The animals underwent a training process to sit calmly in a specially modified cage. The cage provided clear access for the irradiation, while gently restricting excess movement by limiting space. The dose was administered at a rate of 7 cGy/min delivered as a midline tissue dose. The instrument was calibrated weekly to maintain accuracy.
Assessment of chimerism
Twice weekly following transplant, chimerism was assessed by analyzing the variable number of tandem repeats (VNTR) specific to both the donor and recipient DNA. By identifying these unique locations in the genome of the donor and the recipient, we were able to determine the percentage of hematopoietic activity contributed by the donor cells [11]. Briefly, a PCR reaction was performed using empirically tested primers designed to recognize polymorphic repeats that discriminate between the donor and recipient. The PCR reaction consisted of 0.5 μM each of forward and reverse primers, 10% 10X PCR buffer with magnesium chloride, 200 μM deoxyribonucleotide mix, Platinum Taq DNA polymerase (0.5 μL per reaction), and 60 ng of genomic sample DNA. A pre-screen determined the primer pairs that were most appropriate for each mother/child pair. For macaques 05105 and 05094, the following pair was used: 5′-[6-FAM]-TCC-TGA-CAT-TCC-TAG-GGT-GA-3′ and 5′-AAA-ACA-AAT-ATG-GCT-CTA-TCT-ATC-G-3′. For macaques A02175 and 05082, the following pair was used: 5′-[6-FAM]-ACA-GAA-GTC-TGG-GAT-GTG-GA-3′ and 5′-GCC-CAA-AAA-GAC-AGA-CAG-AA-3′. The samples were amplified, and Gene Mapper software was used to analyze the relative contributions of donor- and recipient-specific peak areas and to estimate percent donor chimerism.
Results
Effect of TBI dose on initial neutrophil and platelet decline
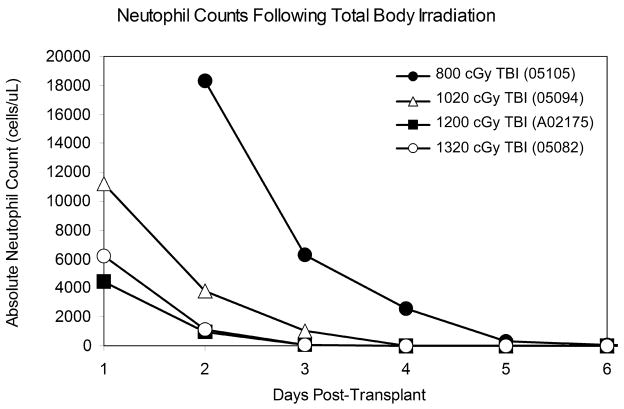
As part of our cord blood studies we have transplanted four animals. Three of the four animals involved in this study tolerated the TBI well with standard care; the fourth animal (05082) suffered from radiation-related toxicity and was euthanized on day 19. The TBI doses for the four animals were as follows: 800 cGy (macaque 05105), 1020 cGy (macaque 05094), 1200 cGy (macaque A02175), and 1320 cGy (macaque 05082). As expected, a precipitous decline in cell counts was observed beginning a few days after irradiation in all cases (Figure 1). Pancytopenia continued for approximately 3 weeks following radiation in macaques 05105 (800 cGy) and 05094 (1020 cGy). The study involving macaque A02175 (1200 cGy) was terminated at day 29, and the study involving macaque 05082 (1320 cGy) was terminated at day 19 without neutrophil recovery in either case.
Figure 1.
The period of follow-up for macaques 05105 and 05094 lasted until full neutrophil recovery was observed (see below); the follow-up for macaque A02175 ended at day 29. In each case, neutropenia (ANC < 500/μL) was observed within 5 days of transplant (see Figure 1). In each of the four animals studied, platelet counts dropped below 30,000/μL between 6 and 9 days after irradiation. Animals were transfusion-dependent throughout the course of the study; transfusions were usually given when platelet counts dropped below about 10,000/μL or warranted due to clinical indications. No evidence of platelet recovery was seen in any of the animals by termination of the study.
Effect of TBI dose on neutrophil recovery
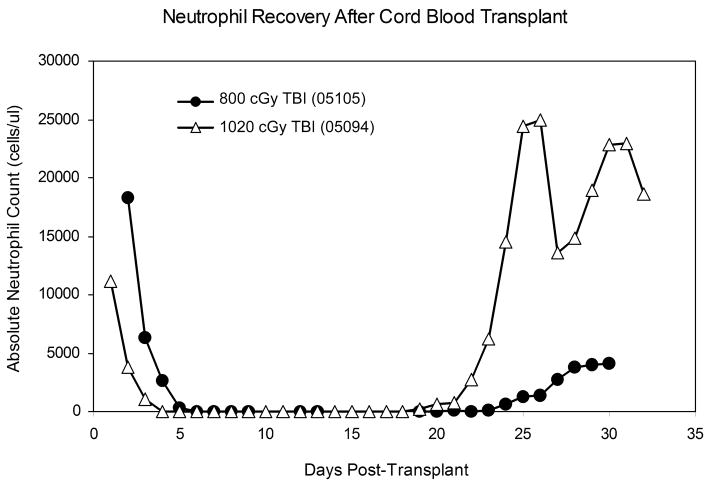
Macaque 05105 (800 cGy) showed neutrophil recovery (ANC > 500/μL) at day 24 following transplant, and macaque 05094 (1020 cGy) recovered neutrophils at day 20 following transplant (Figure 2). Macaques A02175 and 05082 were euthanized on days 29 and 19, respectively, with no signs of neutrophil recovery.
Figure 2.
Donor contribution to hematopoiesis following transplantation
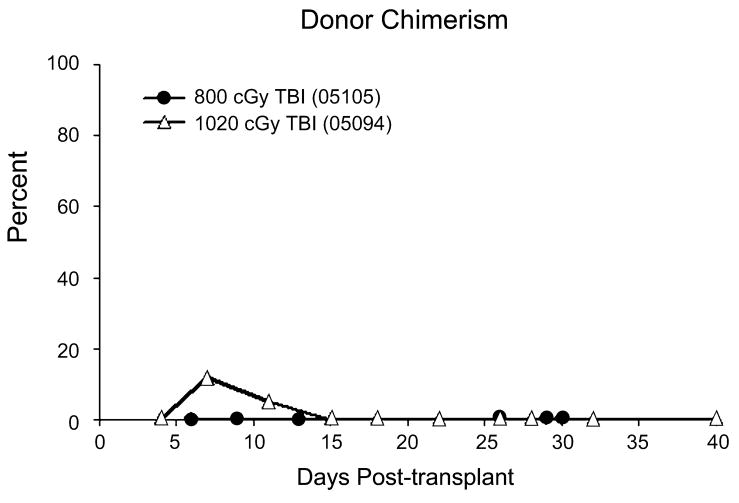
VNTR confirmed that the cells repopulating the marrow of macaques 05105 and 05094 were autologous and not donor-derived (see Figure 3). Macaque 05105 showed 0% donor engraftment throughout the course of the experiment. Macaque 05094 showed a brief period of transient donor engraftment between 1 and 2 weeks post-transplant. When 05094 was euthanized on day 48, there was no detectable donor contribution.
Figure 3.
Pathology
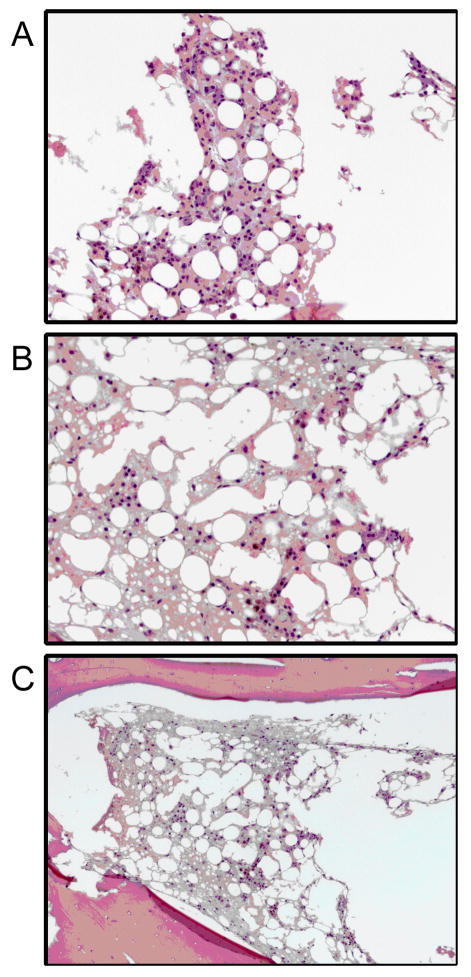
Samples from macaques A02175 and 05082, which were both euthanized before any sign of hematopoietic recovery by CBC, were analyzed on the basis of bone marrow pathology. Pathology on macaque A02175, which received 1200 cGy TBI, revealed nearly complete hematopoietic ablation. Marrow was found to be devoid of all developing hematopoietic cell lines; marrow space was replaced by free erythrocytes, edema fluid, hemosiderin laden histiocytes, and plasma cells. In addition, samples were analyzed from macaque 05082, which received 1320 cGy of TBI and was euthanized on day 19. Pathology confirmed that the marrow from this animal was aplastic with no identifiable hematopoiesis. The few cells present were primarily plasma cells with a few macrophages scattered throughout (Figure 4). This confirms that the doses of 1200 cGy and 1320 cGy did result in full ablation of the marrow.
Figure 4.
Extrahematopoietic toxicities of TBI
Macaque 05082 was euthanized after indications of sepsis. This animal became neutropenic on day 3 after transplant (Figure 1) and was euthanized on day 19 with no signs of hematopoietic recovery. Necropsy on this animal revealed multi-organ hemorrhage in the gastrointestinal tract, integument, urinary tract, cardiovascular/pulmonary system, adrenal glands, and central nervous system. At the time of necropsy on day 19, bone marrow was devoid of hematopoietic cells. Cultures from the blood and bile of this animal revealed Klebsiella oxytoca, an extended spectrum beta-lactamase producer resistant to all penicillins, cephalosporins, and aztreonam. However, in the other three animals, extrahematopoietic toxicities were minor and manageable with standard care. Symptoms included decreased appetite, minimal vomiting, and weight loss. Weight loss can most likely be attributed to decreased appetite.
Discussion
Here we present TBI dose-response data obtained from four Macaca nemestrina subjects. Among the four pigtailed macaques studied, we have shown that radiation doses of less than 1200 cGy do not result in complete myeloablative conditioning. A dose of 1200 cGy resulted in complete myeloablation with an observation interval of 29 days. A dose of 1320 cGy resulted in complete myeloablation but also caused excessive extrahematopoietic toxicities; the most notable of these were GI complications. Thus, based on the outcome of the limited number of animals we have presented here, we propose 1200 cGy as an acceptable and well-tolerated TBI dose in pigtailed macaques.
In most of the earlier nonhuman primate studies, the follow-up time of the subjects was brief; for example, all control animals in the Crouch et al. study [8] died between 12 and 16 days post-radiation. These animals died from complications resulting from excessive diarrhea and weight loss, hemorrhage, and infection. Perhaps with the improved post-transplant supportive care now available (which was not available in the 1960s), these animals would have survived long enough for recovery of autologous cells. When van Bekkum and colleagues pioneered the primate bone marrow transplant studies in the 1960s, they did not have the advantage of the superior antibiotics and growth factors that today’s researchers and clinicians have. For example, the antibiotic regimen in the Crouch et al. [8] studies consisted of intramuscular injections of penicillin, streptomycin, terramycin, and chloramphenicol used alternately. Chloramphenicol has fallen out of use since these studies due to the risk of aplastic anemia. More recent studies use improved, more potent, antibiotics, antivirals, and antifungals. For example, our studies have utilized the cocktail of ceftazadime, vancomycin, gentamicin, acyclovir, and fluconazole; many of these drugs were not in common usage or were just coming into use during the time of these early studies. Likewise, the growth factors available today, such as G-CSF, were not available to researchers of the 1950s and 1960s. This powerful cytokine was cloned in 1986 [19] and has only been used routinely within the last 10 years. Thus, with the current supportive care and improvements in facilities, conditions for hematopoietic stem cell transplantations are now very similar between nonhuman primates and human patients; for example, 1200 cGy of TBI is routinely used for patients undergoing hematopoietic cell transplantation.
We began the studies presented here with a radiation dose of 800 cGy, based on previous studies in an autologous transplantation setting suggesting a higher sensitivity of macaques to irradiation compared to baboons [5]. However, with our current supportive regimen and hyperfractionated TBI, we were able to increase the dose to 1200 cGy. To put these doses into perspective, clinical hematopoietic transplantation using bone marrow and/or mobilized peripheral blood, TBI doses of 1200 cGy are often considered “myeloablative” [12,15,17,20]. In fact, certain preparative regimens have used doses up to 1320 cGy in adults [7], and children (who are more radiation-tolerant than adults) often receive myeloablative doses as high as 1440 cGy [10,16].
We did not see any evidence of engraftment of the cord blood cells in any of the animals at the time of necropsy; therefore, it is not likely that these cells had a significant effect on hematopoietic recovery. In addition, there does not appear to be a correlation between dose of transplanted cells and whether or not the animal demonstrated autologous recovery. When considering the two animals that achieved autologous recovery, it is interesting to note that the dose of TBI given to 05094 was higher than that given to 05105 (1020 cGy as compared to 800 cGy), yet neutrophil recovery occurred 4 days earlier. Macaque 05094 received a cell dose that was nearly 4 times higher than the dose given to macaque 05105. Yet macaque 05094 recovered autologous neutrophils faster, suggesting that a higher cell dose at transplant did not provide significant competition to the autologous cells in terms of a graft-versus-host effect.
Each of the four animals used in this study was female, and each had given birth by C-section between 1.5 and 3 months before irradiation and transplant. In each case, examination by veterinary staff prior to irradiation confirmed that the animal had fully recovered from surgery and was not suffering from any effects related to the state of her parity. We cannot eliminate the possibility that these subjects may have responded differently to TBI than either male subjects or nulliparous subjects. However, early studies comparing radiation sensitivity of women of various parity states showed that parity does not appear to have a significant effect on radiation sensitivity [1,21]. Thus, we have no reason to believe that our findings cannot reliably be extrapolated to either male macaques or nulliparous female macaques.
It is also important to mention that the requirement for high-dose TBI is more crucial in cord blood transplant than in bone marrow transplant. We have extensive experience studying pigtailed macaques in the bone marrow transplant setting, and we have found that a TBI dose of 1020 cGy is acceptable in this setting. Due to the high number of cells transplanted in a bone marrow graft and the relatively rapid neutrophil and platelet recovery, there is lesser demand for full, extended myeloablation, and we have been able to pursue a less-aggressive conditioning regimen. Cord blood transplantation, however, is characterized by a low cell dose and a prolonged period of pancytopenia; thus, it becomes imperative that the recipient receives full ablation to prevent autologous recovery in the interim period between transplant and engraftment. Therefore, the data presented here is of most value in the field of cord blood transplantation.
We have presented data on only four animals, and we understand that this study is limited. However, given the constraints of research involving large animal models such as nonhuman primates, it is outside the realm of feasibility to perform these studies on large numbers of subjects. Though the results shown here represent data from a limited number of animals, we believe that there is important information to be gleaned from these studies about the level of myelosuppression and immunosuppression of Macaca nemestrina in response to varying doses of irradiation. Furthermore, the findings detailed here have important implications in the field of nonhuman primate transplantation models, as we have illustrated that TBI regimens utilizing formerly “myeloablative” doses of radiation may be more appropriately termed “myelosuppressive” with today’s post-radiation supportive care.
Acknowledgments
We would like to thank the staff of the University of Washington National Primate Research Center for experimental support and care of the animals. We would also like to thank Helen Crawford and Bonnie Larson for assistance with the preparation of the manuscript.
This work is supported by NIH grants HL84346 and HL53750, DHHS, Bethesda, MD.
References
- 1.Adams EE, Brues AM, Adams EE, Brues AM. Breast Cancer in Female Radium Dial Workers First Employed Before 1930. J Occup Med. 1980;22:583–587. [PubMed] [Google Scholar]
- 2.Ageyama N, Hanazono Y, Shibata H, Ohto K, Ono F, Nagashima T, Ueda Y, Donahue RE, Hasegawa M, Ozawa K, Yoshikawa Y, Terao K. Safe and Efficient Methods of Autologous Hematopoietic Stem Cell Transplantation for Biomedical Research in Cynomolgus Monkeys. Comparative Medicine. 2002;52:445–451. [PubMed] [Google Scholar]
- 3.Balner H, Dicke KA, van Putten LM, van Bekkum DW. Experimental Aspects of Bone Marrow Transplantation in Primates. Transplant Proc. 1969;1:25–30. [PubMed] [Google Scholar]
- 4.Berenson RJ, Andrews RG, Bensinger WI, Kalamasz D, Knitter G, Buckner CD, Bernstein ID. Antigen CD34+ Marrow Cells Engraft Lethally Irradiated Baboons. J Clin Invest. 1988;81:951–955. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bielefeldt-Ohmann H, Gough M, Durning M, Kelley S, Liggitt HD, Kiem H-P. Greater Sensitivity of Pigtailed Macaques (Macaca Nemstrina) Than Baboons to Total Body Irradiation. Journal of Comparative Pathology. 2004;131:77–86. doi: 10.1016/j.jcpa.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Broerse JJ, van Bekkum DW, Hollander CF, Davids JA. Mortality of Monkeys After Exposure to Fission Neutrons and the Effect of Autologous Bone Marrow Transplantation. International Journal of Radiation Biology and Related Studies in Physics, Chemistry nad Medicine. 1978;34:253–264. doi: 10.1080/09553007814550841. [DOI] [PubMed] [Google Scholar]
- 7.Brunstein CG, Setubal DC, Wagner JE. Expanding the Role of Umbilical Cord Blood Transplantation (Review) Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- 8.Crouch BG, van Putten LM, van Bekkum DW, de Vries MJ. Treatment of Total-Body X-Irradiated Monkeys With Autologous and Homologous Bone Marrow. J Natl Cancer Inst. 1961;27:53–65. [PubMed] [Google Scholar]
- 9.Donahue RE, Kessler SW, Bodine D, McDonagh K, Dunbar C, Goodman S, Agricola B, Byrne E, Raffeld M, Moen R, Bacher J, Zsebo KM, Nienhuis AW. Helper Virus Induced T Cell Lymphoma in Nonhuman Primates After Retroviral Mediated Gene Transfer. J Exp Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duerst RE, Horan JT, Liesveld JL, Abboud CN, Zwetsch LM, Senf ES, Constine LS, Raubertas RF, Passarell JA, DiPersio JF. Allogeneic Bone Marrow Transplantation for Children With Acute Leukemia: Cytoreduction With Fractionated Total Body Irradiation, High-Dose Etoposide and Cyclophosphamide. Bone Marrow Transplant. 2000;25:489–494. doi: 10.1038/sj.bmt.1702181. [DOI] [PubMed] [Google Scholar]
- 11.Ginsburg D, Antin JH, Smith BR, Orkin SH, Rappeport JM. Origin of Cell Populations After Bone Marrow Transplantation. Analysis Using DNA Sequence Polymorphisms. J Clin Invest. 1985;75:596–603. doi: 10.1172/JCI111736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobsohn DA, Hewlett B, Ranalli M, Seshadri R, Duerst R, Kletzel M. Outcomes of Unrelated Cord Blood Transplants and Allogeneic-Related Hematopoietic Stem Cell Transplants in Children With High-Risk Acute Lymphocytic Leukemia. Bone Marrow Transplant. 2004;34:901–907. doi: 10.1038/sj.bmt.1704681. [DOI] [PubMed] [Google Scholar]
- 13.Jung CW, Beard BC, Morris JC, Neff T, Beebe K, Storer BE, Kiem H-P. Hematopoietic Stem Cell Engraftment: a Direct Comparison Between Intramarrow and Intravenous Injection in Nonhuman Primates. Exp Hematol. 2007;35:1132–1139. doi: 10.1016/j.exphem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Kluge KA, Bonifacino AC, Sellers S, Agricola BA, Donahue RE, Dunbar CE. Retroviral Transduction and Engraftment Ability of Primate Hematopoietic Progenitor and Stem Cells Transduced Under Serum-Free Versus Serum-Containing Conditions. Mol Ther. 2002;5:316–322. doi: 10.1006/mthe.2002.0544. [DOI] [PubMed] [Google Scholar]
- 15.Konuma T, Ooi J, Takahashi S, Tomonari A, Tsukada N, Kato S, Kasahara S, Uchimaru K, Iseki T, Tojo A, Asano S. Myeloablative Unrelated Cord Blood Transplantation for Adult Acute Myeloid Leukemia Patients With 11q23 Abnormalities. Eur J Haematol. 2008;80:545–548. doi: 10.1111/j.1600-0609.2008.01057.x. [DOI] [PubMed] [Google Scholar]
- 16.Kornguth DG, Mahajan A, Woo S, Chan KW, Antolak J, Ha CS. Fludarabine Allows Dose Reduction for Total Body Irradiation in Pediatric Hematopoietic Stem Cell Transplantation. Int J Radiat Oncol Biol Phys. 2007;68:1140–1144. doi: 10.1016/j.ijrobp.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Massenkeil G, Nagy M, Neuburger S, Tamm I, Lutz C, le Coutre P, Rosen O, Wernecke KD, Dorken B, Arnold R. Survival After Reduced-Intensity Conditioning Is Not Inferior to Standard High-Dose Conditioning Before Allogeneic Haematopoietic Cell Transplantation in Acute Leukaemias. Bone Marrow Transplant. 2005;36:683–689. doi: 10.1038/sj.bmt.1705123. [DOI] [PubMed] [Google Scholar]
- 18.Monroy RL, Skelly RR, MacVittie TJ, Davis TA, Sauber JJ, Clark SC, Donahue RE. The Effect of Recombinant GM-CSF on the Recovery of Monkeys Transplanted With Autologous Bone Marrow. Concise Report. Blood. 1987;70:1696–1699. [PubMed] [Google Scholar]
- 19.Nagata S, Tsuchiya M, Asano S, Kaziro Y, Yamazaki T, Yamamoto O, Hirata Y, Kubota N, Oheda M, Nomura H, Ono M. Molecular Cloning and Expression of CDNA for Human Granulocyte Colony-Stimulating Factor. Nature. 1986;319:415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- 20.Novitzky N, Thomas V, Hale G, Waldmann H. Myeloablative Conditioning Is Well Tolerated by Older Patients Receiving T-Cell-Depleted Grafts. Bone Marrow Transplant. 2005;36:675–682. doi: 10.1038/sj.bmt.1705119. [DOI] [PubMed] [Google Scholar]
- 21.Rafnsson V, Tulinius H, Jonasson JG, Hrafnkelsson J, Rafnsson V, Tulinius H, Jonasson JG, Hrafnkelsson J. Risk of Breast Cancer in Female Flight Attendants: a Population-Based Study (Iceland) Cancer Causes Control. 2001;12:95–101. doi: 10.1023/a:1008983416836. [DOI] [PubMed] [Google Scholar]
- 22.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous Infusion of Bone Marrow in Patients Receiving Radiation and Chemotherapy. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 23.Trobridge GD, Beard BC, Gooch C, Wohlfahrt M, Olsen P, Fletcher J, Malik P, Kiem H-P. Efficient Transduction of Pigtailed Macaque Hemtopoietic Repopulating Cells With HIV-Based Lentiviral Vectors. Blood. 2008;111:5537–5543. doi: 10.1182/blood-2007-09-115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Putten LM. Quantitative Aspects of the Storage of Bone Marrow Cells for Transplantation. Eur J Cancer. 1965;1:15–22. doi: 10.1016/0014-2964(65)90075-7. [DOI] [PubMed] [Google Scholar]
- 25.Wagemaker G, Neelis KJ, Hartong SC, Wognum AW, Thomas GR, Fielder PJ, Eaton DL. The Efficacy of Recombinant Thrombopoietin in Murine and Nonhuman Primate Models for Radiation-Induced Myelosuppression and Stem Cell Transplantation (Review) Stem Cells. 1998;16:375–386. doi: 10.1002/stem.160375. [DOI] [PubMed] [Google Scholar]