Abstract
A major hurdle impeding the successful clinical development of drug candidates can be poor intestinal permeability. Low intestinal permeability may be enhanced by a prodrug approach targeting membrane transporters in the small intestine. Transporter specificity, affinity, and capacity are three factors in targeted prodrug design. The human apical sodium dependent bile acid transporter (SLC10A2) belongs to the solute carrier family (SLC) of transporters and is an important carrier protein expressed in the small intestine. In spite of appearing to be an excellent target for prodrug design, few studies have targeted human apical sodium dependent bile acid transporter (hASBT) to improve oral bioavailability. The review discusses bile acids including their chemistry and their absorptive disposition. Additionally, hASBT-mediated prodrug targeting is discussed, including QSAR, in-vitro models for hASBT assay, and the current progress in utilizing hASBT as a drug delivery target.
Keywords: bile acids, prodrugs, hASBT, QSAR, SLC10A2, cell culture, transporters
Introduction
Membrane transport proteins play important roles in the influx and efflux of various nutrients, metabolic substances and cell signaling molecules in the cell. Based on the sequencing of the human genome, approximately 500 to 1200 genes encode transport proteins 1,2. Transporters tend to be multifunctional and often play vital roles in translocating endogenous substances such as sugars, lipids, amino acids, bile acids, steroids, and hormones across biological membranes; they also influence the disposition and toxicity of drugs 3-5. Significant progress has been made in the discovery and characterization of transporters, including development of in silico models to predict their interaction with substrates and inhibitors 6-8. For example, pharmacophore-based models have been proposed for several influx transporters, such as peptide transporter (Pept1), nucleoside transporters (ENT family), OCT family, and the OATP family of proteins 9-12.
Despite this progress, the design of prodrugs to target transporters appears less developed. An example of a prodrug approach to target an intestinal transporter to enhance oral absorption is valacyclovir 13. The bioavailability of acyclovir was enhanced 2-fold to 3-fold via the oral administration of its valine ester (valacyclovir), which is a substrate for the small intestinal peptide transporter 14. However, the historical discovery of drugs whose intestinal absorption is mediated by Pept1 has generally been serendipitous 9. Two scenarios can be envisioned in the future exploitation of uptake transporters to enhance oral bioavailability. The prodrug approach can involve coupling a drug candidate to a natural substrate for a transporter. The advantage of such an approach is apparently low potential for toxicity, owing to the use of endogenous substrates. The second approach involves what has been referred to as “substrate mimicry”, wherein the three-dimensional drug structure resembles natural substrates either by design or by serendipity 15.
Bile Acids Transport and Enterohepatic Recirculation
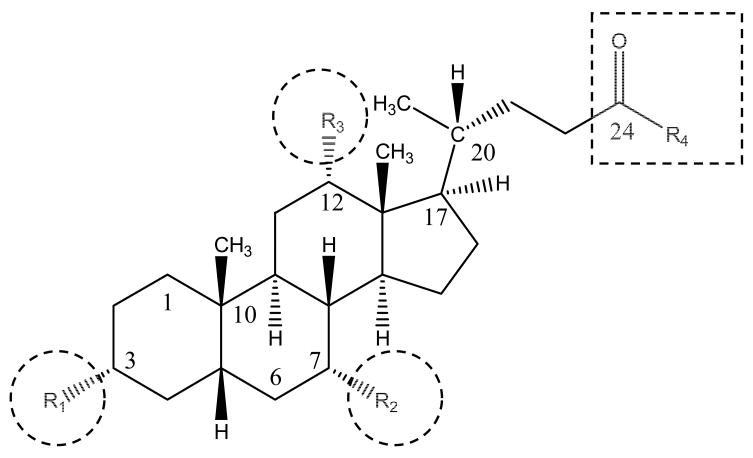
An understanding of bile acid structure and disposition may facilitate prodrug design that targets hASBT. Figure 1 illustrates the general structures of bile acids in humans. R1, R2 and R3 indicate hydroxyl groups while R4 represents a free carboxylic acid (unconjugated bile acids) or glycine or taurine substituent (conjugated bile acids). Table 1 describes 15 native bile acids and the structural differences among these primary and secondary bile acids.
Figure 1.
Structure of native bile acids. Bile acids differ in hydroxylation pattern (R1, R2, and R3 – indicated by circles) and in amino acid conjugation pattern at C-24 position (R4 – indicated by square). Substituent at R-4 is either a free carboxylic acid or conjugate of glycine or taurine. The steroidal hydroxyl groups and the C-24 carboxylate represent convenient sites where drugs can be conjugated directly or indirectly via a linker.
Table 1.
Bile acid nomenclature. Primary bile acids are formed in the hepatocyte. Secondary bile acids are derived from primary bile acids. All hydroxyl (-OH) groups are in the α-position, except when noted to be in the β-position. Positions R2, R3, and R4 are illustrated in Figure 1.
| Bile acid | R2 (C-7) | R3 (C-12) | R4 (C-24) | |
|---|---|---|---|---|
| Primary Bile acids | Cholate | -OH | -OH | -OH |
| Glycocholate | -NHCH2COOH | |||
| Taurocholate | -NH(CH2)2SO3H | |||
| Chenodeoxycholate | -OH | -H | -OH | |
| Glycochenodeoxycholate | -NHCH2COOH | |||
| Taurochenodeoxycholate | -NH(CH2)2SO3H | |||
| Secondary Bile acids | Deoxycholate | -H | -OH | -OH |
| Glycodeoxycholate | -NHCH2COOH | |||
| Taurodeoxycholate | -NH(CH2)2SO3H | |||
| Lithocholate | -H | -H | -OH | |
| Glycolithocholate | -NHCH2COOH | |||
| Taurolithocholate | -NH(CH2)2SO3H | |||
| Ursodeoxycholate | -OH (β) | -H | -OH | |
| Glycoursodeoxycholate | -NHCH2COOH | |||
| Tauroursodeoxycholate | -NH(CH2)2SO3H | |||
Cholate and chenodeoxycholate, the primary bile acids in humans, are synthesized in hepatocytes from cholesterol. Secondary bile acids are formed via bacterial metabolism in the small intestine. Conjugation of both primary and secondary bile acids occurs in the hepatocyte prior to excretion from the hepatocyte. In humans, cholate and chenodeoxycholate are the primary bile acids. Secondary bile acids are derived from cholate and chenodeoxycholate via bacterial 7-dehydroxylation or 7-epimerization. For example, deoxycholate is derived via 7-dehydroxylation of cholate, while ursodeoxycholate is formed via the epimerization of the 7-α hydroxyl group of chenodeoxycholate.
The principal bile acids in human bile are mainly conjugated cholic acid and chenodeoxycholic acid 16. Very small amounts of deoxycholate and ursodeoxycholate conjugates, and trace lithocholate conjugates, are also present. Conjugation increases bile acid polarity and lowers passive transport, such that bile acid re-absorption is controlled by intestinal bile acid transporters. This situation facilitates high intraluminal bile acid concentration to aid lipid absorption. Conjugation also improves bile acid solubility, providing resistance to precipitation in the presence of high of calcium ion concentration in gall bladder 17.
The enterohepatic recirculation of bile acids is a complex process and involves numerous transport proteins, including: the sodium-dependent hepatocyte bile salt uptake system NTCP (SLC10A1); hepatocellular bile salt export pump (BSEP, ABCB11); the apical sodium-dependent bile salt transporter (ASBT, SLC10A2); the organic anion transporting polypeptides OATP-C (SLC21A6), OATP8 (SLC21A8) and OATP-A (SLC21A3); and the multidrug resistance protein MRP3 (ABCC3). ASBT and NTCP are each members of the SLC10 family of solute carrier proteins and require sodium co-transport for their activity. ASBT is expressed on the apical membrane of enterocytes in the terminal ileum and mediates the reabsorption of bile acids from the ileum. In addition to ASBT, additional anion exchange mechanisms operate in the jejunum to reabsorb bile acids 18. NTCP is expressed in hepatocytes and localized on the basolateral (sinusoidal) domain of hepatocytes to reabsorb bile acids from the portal circulation. ASBT and NTCP serve to transport bile acids from the small intestine into portal circulation and from the portal circulation into the hepatocyte. The bile acid pool in humans is about 3-5 g, resulting in a turnover of 12-18 g of bile acid each day 16. In spite of this repeated cycling, the loss of bile in the feces is less than 0.5 g per day, reflecting the tremendous capacity and efficiency of hASBT 19.
Upon their translocation into the enterocytic cytoplasm by hASBT, bile acids bind to the 14 kD soluble cytoplasmic protein named the ileal bile acid binding protein (iBABP); iBABP is also known as Gastrotropin 20,21. iBABP shuttles bile acids across the cytosol to the basolateral membrane 19. It has been suggested that iBABP specifically interacts with hASBT, further supporting its role in bile acid transport 22. Regarding the transport of bile acids across the basolateral membrane of enterocytes, Dawson et al. reported that the heteromeric organic solute transporter Ostα-Ostβ is an ileal basolateral bile acid transporter 23. Based on expression and transport properties of the transporter complex in various tissues and using hASBT co-transfected cells, Ostα-Ostβ may be the major mechanism for ileal basolateral bile acid transport 23. Other possible mechanisms across the basolateral membrane include a truncated version of ASBT referred to as t-Asbt 24 and the multidrug resistance-associated protein 3 (MRP3, ABCC3) 19. The role of t-ASBT in basolateral transport was observed only in rats. Meanwhile, the importance of MRP3 is also controversial with conflicting reports about the contribution of MRP3 19,25.
Biology of ASBT
ASBT was first cloned in the laboratory of Paul Dawson (Wake Forest University) from hamster ileum; subsequently it has been cloned from rat, mouse, rabbits, and humans 26-30. ASBT consists of 348 amino acids (347 in rabbits) with an observed molecular weight of 43 kDa which differs from a predicted molecular weight of 38 kDa due to glycosylation 28. The ASBT gene is localized on chromosome 13q33. It exhibits 35% identity and 63% amino acid sequence similarity with its liver orthologue NTCP 31. The transporter is electrogenic, coupled with sodium in a 2:1 sodium: bile acid stoichiometry. hASBT presumably functions as a monomer, although some evidence suggests the existence of a dimer as well 32. hASBT is expressed at high levels in the terminal ileum, renal proximal tubules and biliary epithelium 33,34. Inherited mutations in hASBT results in primary bile acid malabsorption syndrome, suggesting that hASBT is the primary mechanism for intestinal reabsorption of bile acids 28,35.
Structural information on hASBT has been restricted to its primary sequence and membrane topology. While precise information about the substrate binding domains of hASBT are not available yet, its structural and functional determinants have been studied using various biophysical methods 36-40. These studies have indicated that hASBT exhibits a seven transmembrane (7 TM) topology similar to NTCP. Biochemical studies using photo affinity labeling and enzymatic digestion indicate that the substrate-binding domain of hASBT is localized to the seventh transmembrane domain and the C-terminus at 56-67 amino acids 36,40,41. Further, Zhang et al. have suggested the existence of four distinct binding sites based on a three-dimensional structure of hASBT developed in silico using homology modeling 37.
In Vitro Models for Functional Assessment of hASBT-mediated Transport
The comprehensive characterization of carrier-mediated transport of solutes requires assay methodologies that delineate the contribution of various pathways, including the role of an individual transporter. An additional practical consideration is the throughput capacity of the developed assay. High throughput absorption screens developed for early ADMET screening can be adapted for the rational design and evaluation of prodrugs to target transporters. An example is a cell culture model to study PepT1-mediated transport of peptides and peptide-based drugs.
In the development of cell-based transporter models, two approaches have been utilized. The first approach involves the use of primary or native cell systems that express the transporter of interest. For example, the human colon adenocarcinoma Caco-2 cell line endogenously expresses peptide transporters and has been utilized to evaluate prodrugs aimed at various peptide transporters 42. A potential limitation of such an approach is the relatively low transporter expression level, along with the expression of other confounding transporters 43. The second approach involves the use of transiently or stably transfected cell lines to over-express the transporter. Transfection generally offers high transporter expression, which increases the dynamic range of the assay by limiting influence of passive flux 44,45.
There is a paucity of cell-based transport assays for hASBT, compared to the several assay models for other, more widely studied transporters, such as Pept1 43. The development of cell-based assay systems for peptide transporters has aided in the understanding of the substrate requirements of peptide transporters. Such assays have also lead to the development of predictive three-dimensional QSAR models to rationalize the interaction of substrates and non-substrates with the transporter 46,47. Previously, in vitro cell-based assays for ASBT typically employed non-polarized cells, such as transiently transfected COS7 cells and stably transfected CHO cells 33. These models allow for uptake assessment, but are not competent monolayers and do not allow for transepithelial transport. Caco-2 cells have also been used for assessment of hASBT function, but the level of expression is low and not consistent 48,49. Sun et al. have previously developed a stably transfected hASBT-MDCK model for the study of sorting of hASBT to the apical membrane 50. However, this model exhibited poor monolayer integrity. Doubly transfected systems with rat Asbt and a basolateral transporter have also been attempted 25. We have recently developed a stably transfected hASBT-MDCK monolayer assay with suitable monolayer integrity 51. Such a monolayer model has been beneficial in allowing the measurement of prodrug transport, including quantification of intact prodrug in the receiver compartment. Additionally, the analytical requirements for a monolayer assay are generally less complex since the sample matrix is a protein free buffer compared to cell lysate in case of uptake.
Bile Acid Derivatives as “Trojan Horses” for Delivery of Therapeutics
Transporter specificity, affinity, and capacity are three critical factors in selecting a transporter for prodrug targeting. Favorable attributes include broad substrate specificity and high affinity for efficient absorption. Transport capacity, dictated both by expression level and intrinsic transporter capacity or turnover, also impacts extent of absorption enhancement. hASBT is saturable and exhibits high capacity and high affinity for native bile acids, suggesting hASBT to be a potential prodrug target 51,52.
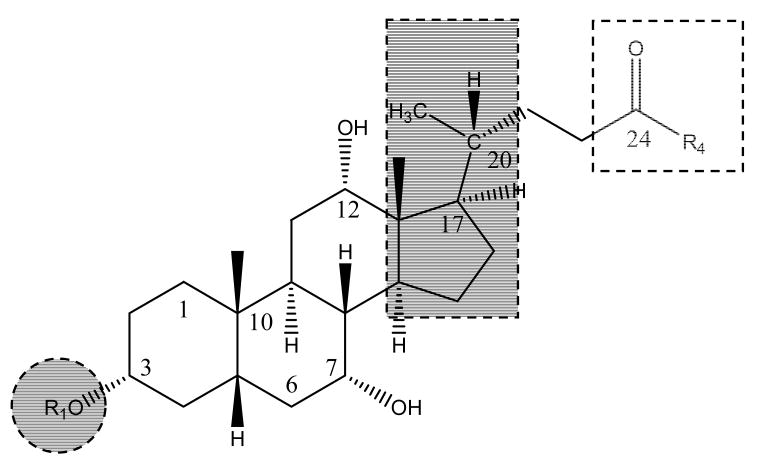
Interestingly, hASBT has received less emphasis than NTCP, in terms of targeting, perhaps reflecting the importance of the liver as a therapeutic target 53. NTCP appears to exhibit a broader substrate specificity than hASBT, including the ability to transport a wide range of cholephillic compounds and various drugs 29. NTCP has been evaluated in previous studies for targeted delivery of therapeutics to the liver 54. Examples include the targeted delivery of antisense nucleotides, cytostatic drugs, and HMG Co A reductase inhibitors 54-56. The compound of interest was usually coupled to a native bile acid that is recognized by the transporter. Figure 2 illustrates the structure of potential bile acid derivatives, including sites of drug attachment or integration with bile acid. Drugs can be attached in the C-3 region of the steroidal ring or through the C-24 carboxylate. Additionally, the C-17 region has also been exploited for integrating drug entities.
Figure 2.
Structure of potential bile acid derivatives. Drugs may be attached/integrated at the C-3 region (shaded circle) or the C-17 region (shaded rectangle) or via the C-24 carboxylate (dashed square). Table 2 lists examples for each of these scenarios.
Compared to NTCP, few studies have targeted ASBT to enhance systemic drug bioavailability. To enhance the transport of renin inhibitory heptapeptide ditekiren, the parent compound was conjugated to either cholic acid or taurocholic acid via a spacer. Although conjugates exhibited hASBT inhibition, they were not transported by hASBT 57. Similarly, the absorption of the C-24 dipeptide bile acid conjugate cholylglycyltyrosine was low compared to taurocholic acid 58. Swaan et al. evaluated the transport of peptides of varying length that were conjugated at the 24-position of cholic acid via an amide linkage 59. While conjugation of peptides to bile acids did not directly enhance absorption via hASBT-mediated transport, it lead to lower metabolic lability 54,59. Bhat et al. recently evaluated the potential of using steroidal pyrazoles as drug carriers for hASBT 60. Pyrazole rings were fused to the bile acid skeleton at the C2:C3 carbons and drug entities were subsequently attached via the pyrazole ring. In general, steroidal pyrazoles exhibited good binding affinity for NTCP, but lower binding affinity for hASBT, suggesting that these modified bile acid analogues are recognized by the bile acid transporters. However, they exhibited poor substrate affinity for hASBT.
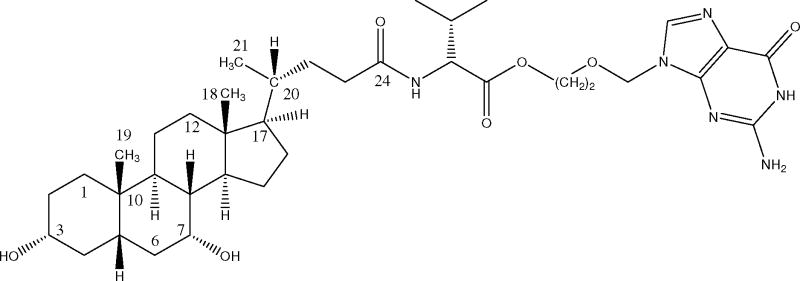
Currently, there are very few examples exemplifying enhanced systemic bioavailability of poorly absorbed drugs via prodrug targeting hASBT. Table 2 lists examples where drug has been conjugated to native bile acids to modulate disposition. The only example to our knowledge wherein an unambiguous enhancement in oral bioavailability was attained via prodrug that targeted ASBT is acyclovir valylchenodeoxycholate 61, which is illustrated in Fig. 3. Acyclovir valylchenodeoxycholate is a conjugate of valacyclovir and chenodeoxycholate. Acyclovir is an anti-viral drug with an oral bioavailability of only 20% due to low acyclovir intestinal permeability 62. Acyclovir valylchenodeoxycholate was designed to assess potential for hASBT targeting as against PepT1 targeting. Valacyclovir (L-valine ester prodrug of acyclovir) is an prodrug of acyclovir that has improved the oral bioavailability of acyclovir with human oral bioavailability of 54% 14,63. Valacyclovir is a substrate for the PepT1 intestinal transporter, with a Ki=4.08 mM in PepT1 expressing Xenopus laevis oocytes 64 and Ki=1.10 mM in stable lines of CHO/PepT1 13. Compared to PepT1, hASBT has a more favorable micromolar affinity profile 51,61. Acyclovir valylchenodeoxycholate possesses a favorable affinity for hASBT (Ki=36 μM), which was comparable to even the native bile acid cholic acid (Ki=25 μM). In rats, acyclovir valylchenodeoxycholate increased acyclovir oral bioavailability two-fold, based on urinary excretion of acyclovir after oral administration of the acyclovir conjugate versus oral administration of acyclovir. While a two-fold enhancement in acyclovir bioavailability from 25% to 48% in rats is notable and while hASBT appear to possess better affinity for substrates than does PepT1, the PepT1 prodrug valacyclovir increases acyclovir bioavailability 3-fold to 54% in humans 65. A possible explanation for the only two-fold increase in oral acyclovir bioavailability by acyclovir valylchenodeoxycholate, in spite of the prodrug's more favorable in vitro uptake properties, is prodrug hydrolysis in the stomach and proximal intestine. ASBT is located in the distal segment of the small intestine (i.e. ileum), such that a bile acid conjugate must exhibit a degree of hydrolytic stability in order to reach ASBT intact. It should be noted that, in spite of these promising ASBT results, our current understanding of hASBT substrate requirements is limited.
Table 2.
Examples of attempts to enhance disposition via prodrug derrivatives
| Position | Compound | Bile Acid | Nature of cargo | Active transport | Reference |
|---|---|---|---|---|---|
| C-3 | Ditekiren | Cholic acid | Peptide | No | 57 |
| Ditekiren | Taurocholic acid | Peptide | No | 57 | |
| Chlorambucil | Cholic acid | Small molecule | Yesa | 55,71 | |
| HR 780 | Cholic acid | Small molecule | Yesa | 72 | |
| Antisense oligonucleotides | Cholic acid | Oligonucleotide | No | 56,73 | |
| Naproxen | Pyrazole fused cholic acid | Small molecules | Yesb | 60 | |
| C-17 | HMG Co A reductase inhibitor | Modified Cholic acid | Hybrid molecule | - | 72,74 |
| C-24 | Acyclovir | Cholic acid | Small molecule | Yes | 61 |
| Peptides | Cholic acid | Peptides | No | 59 |
Assessed through enhanced biliary excretion
Indirect assessment via ion current measurement
Figure 3.
Structure of acyclovir valylchenodeoxcholate. Acyclovir was conjugated to the native bile acid chenodeoxycholate via a valine linker. This prodrug lead to increased oral absorption, compared to parent molecule administration.
Structure-Transport Requirements for hASBT
In the absence of a high resolution crystal structure for hASBT, little is known about the interaction of hASBT with its substrates. There is a general lack of information about the spatial requirements for substrate binding to and transport by hASBT 40,41,66. Most studies were conducted using ex-vivo tissue or organ preparations, or uptake studies using non-polarized cell culture models 29,33,67.
Lack and Weiner were the first to describe a basic structure transport relationship for the intestinal bile acid transport using the rat everted sac model 67. The Lack model suggests the following generalizations 68 :
Trihydroxy bile acids are better transported than dihydroxy bile acids. No one hydroxyl position on the steroid ring is necessary. Among themselves, the dihydroxy bile acids are equally well transported.
A single negative charge on the side chain is apparently required.
For inhibition, there is an inverse relationship between number of steroid hydroxyl groups and inhibitor affinity.
Lack's model reflects one of the very few translocation-based models to describe the substrate requirements of hASBT. However, the assay system utilized tissue and organ preparations from animals, which do not delineate the contribution of hASBT from other confounding factors, including other transporters, passive transport, and system hydrodynamics.
Recent studies have employed brush border vesicles and cell-based transport assays, which directly measure hASBT interaction with substrate. However, the majority of studies were inhibition studies, reflecting a focus to develop non-absorbable hASBT inhibitors as a means of lowering plasma cholesterol. Kramer et al. described a 3-D pharmacophore model for ASBT with five chemical features: one hydrogen bond donor, one hydrogen bond acceptor, and three hydrophobic features 69. They suggested the following structure-activity relationship for the native bile acids, which differed from the Lack model 29.
Two hydroxy groups at position 3, 7, or 12 are optimal whereas the presence of three hydroxy groups decreased affinity.
Vicinal hydroxy groups at positions 6 and 7 or a shift of the 7-hydroxy group to the 6-position significantly decreased the affinity.
One hydroxy group either in position 6 or 7 mediates affinity to the hASBT whereas the concomitant presence of two hydroxyl groups at 6 and 7 positions significantly diminish the affinity to ASBT.
Dawson and colleagues employed hASBT-COS cells to characterize the transport characteristic of several native bile acids 33. They reported that glyco-dihydroxy bile acids exhibit the greatest affinity for hASBT, which seems to contradict Lack's claim that trihydroxy bile acids are best transported. Using Caco-2 monolayers, Swaan et al. examined the transport of C-24 bile acid-peptide conjugates. Each provided a negative charge near C-24. They concluded that the C-24 side chain could be at least 14 Å in length to allow for translocation, and that large hydrophobic moieties increase binding to hASBT 66. They also observed cholic acid to have greater affinity (i.e. lower Kt) than taurocholate, which is in contrast to previous studies 70. In our previous work, prodrugs of acyclovir linked to bile acids via a valine linker were found to have high inhibition potency comparable to native bile acids. A negative charge at C-24 was not necessary for the transport of these conjugates 61.
Lack's generalizations about hASBT substrates have been found to be debatable and current studies have provided data that do not support the model. Interstudy comparisons indicate that a reliable relation between bile acid structure and transport parameters (e.g. Jmax and Kt) has not been elucidated, much less Lack's first generalization, concerning trihydroxy bile acids being better transported than dihydroxy bile acids. Kt values vary greatly between studies. We believe this situation reflects a lack of a comprehensive study of bile acid transport using a method that excludes confounding factors, such as other transporters.
The cloning of hASBT provides an opportunity to develop a selective and sensitive assay system to delineate hASBT substrate requirements. hASBT was stably transfected into MDCK to yield a competent, high-expression, stable assay for hASBT transport and inhibition studies 51. hASBT-mediated taurocholate permeability across hASBT-MDCK monolayers was almost 25-fold higher with sodium, than without sodium where hASBT is not functional. In the presence of sodium, taurocholate and mannitol permeabilities were 23.0×10-6 cm/sec and 2.60×10-6 cm/sec, respectively, indicating high hASBT functionality and monolayer integrity. Permeability values demonstrated low within day variability. Taurocholate uptake and inhibition kinetic parameters from hASBT-MDCK were similar to those obtained from hASBT-COS7 model, confirming hASBT functionality in hASBT-MDCK.
We have recently developed an hASBT-MDCK monolayer assay and this model was used for the systematic elucidation of hASBT-transport and hASBT-inhibition profiles of native bile acids in the absence of any confounding effects 52. Monohydroxy, dihydroxy, and trihydroxy bile acids were evaluated along with their glycine and taurine conjugates. Observations from this study include:
Glycine or taurine conjugation at C-24 enhanced the inhibitory potency of bile acids (i.e. Ki). This trend was consistent across all bile acids, including monohydroxy, dihydroxy and trihydroxy bile acids.
An inverse relationship was observed between number of steroidal hydroxyl groups and inhibitory potency, with monohydroxy bile acids being the most potent inhibitors. Chenodeoxycholate exhibited greater inhibition potency than ursodeoxycholate suggesting that C-7 α-OH is more favorable than C-7 β-OH.
Results from transport studies mirrored the trends from inhibition studies. Steroidal hydroxylation had a significant effect on transport affinity (i.e. Kt). Fewer hydroxyl groups promoted transport affinity towards hASBT.
Future Efforts
Future efforts should focus on coupling functional data and biophysical data to promote understanding the differences in transport affinity and inhibition potency of substrates and inhibitors, based upon specific interactions between the substrate and amino acid residues in the transport protein. Studies to date with native bile acids generally indicated that C-24 conjugation enhances hASBT substrate affinity. Future studies will benefit from a better insight into the structural features of the targeting moiety (i.e. bile acid) that favor interaction with hASBT and holds promise for the development of C-24 conjugated prodrugs. Since native conjugated bile acids exhibit a very narrow chemistry space around C-24 region, the influence of diverse C-24 chemistry space in enhancing or impeding bile acid interaction with hASBT remains a focus of future research. These studies should yield a better understanding of hASBT substrate requirements with application in the design of prodrugs to target hASBT.
Acknowledgments
This work was supported by National Institutes of Health grant DK67530.
List of Abbreviations
- hASBT
human apical sodium-dependent bile acid transporter
- SLC
solute carrier family
- MDCK
Madin-Darby canine kidney
- HBSS
Hanks balanced salt solution
- ABL
aqueous boundary layer
- TCA
taurocholic acid
- CDCA
chenodeoxycholic acid
References
- 1.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 2.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 3.Ayrton A, Morgan P. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica. 2001;31:469–497. doi: 10.1080/00498250110060969. [DOI] [PubMed] [Google Scholar]
- 4.Ho RH, Kim RB. Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther. 2005;78:260–277. doi: 10.1016/j.clpt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Kim RB. Transporters and Drug Discovery: Why, When, and How. Mol Pharm. 2006;3:26–32. doi: 10.1021/mp050084o. [DOI] [PubMed] [Google Scholar]
- 6.Stouch TR, Gudmundsson O. Progress in understanding the structure-activity relationships of P-glycoprotein. Adv Drug Deliv Rev. 2002;54:315–328. doi: 10.1016/s0169-409x(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 7.Gombar VK, Polli JW, Humphreys JE, Wring SA, Serabjit-Singh CS. Predicting P-glycoprotein substrates by a quantitative structure-activity relationship model. J Pharm Sci. 2004;93:957–968. doi: 10.1002/jps.20035. [DOI] [PubMed] [Google Scholar]
- 8.Crivori P, Reinach B, Pezzetta D, Poggesi I. Computational Models for Identifying Potential P-Glycoprotein Substrates and Inhibitors. Mol Pharm. 2006;3:33–44. doi: 10.1021/mp050071a. [DOI] [PubMed] [Google Scholar]
- 9.Ekins S, Johnston JS, Bahadduri P, D'Souza VM, Ray A, et al. In vitro and pharmacophore-based discovery of novel hPEPT1 inhibitors. Pharm Res. 2005;22:512–517. doi: 10.1007/s11095-005-2505-y. [DOI] [PubMed] [Google Scholar]
- 10.Chang C, Swaan PW, Ngo LY, Lum PY, Patil SD, et al. Molecular requirements of the human nucleoside transporters hCNT1, hCNT2, and hENT1. Mol Pharmacol. 2004;65:558–570. doi: 10.1124/mol.65.3.558. [DOI] [PubMed] [Google Scholar]
- 11.Chang C, Pang KS, Swaan PW, Ekins S. Comparative pharmacophore modeling of organic anion transporting polypeptides: a meta-analysis of rat Oatp1a1 and human OATP1B1. J Pharmacol Exp Ther. 2005;314:533–541. doi: 10.1124/jpet.104.082370. [DOI] [PubMed] [Google Scholar]
- 12.Bednarczyk D, Ekins S, Wikel JH, Wright SH. Influence of molecular structure on substrate binding to the human organic cation transporter, hOCT1. Mol Pharmacol. 2003;63:489–498. doi: 10.1124/mol.63.3.489. [DOI] [PubMed] [Google Scholar]
- 13.Han H, de Vrueh RL, Rhie JK, Covitz KM, Smith PL, et al. 5′-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm Res. 1998;15:1154–1159. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
- 14.Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, et al. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39:2759–2764. doi: 10.1128/aac.39.12.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang EY, Knipp GT, Ekins S, Swaan PW. Structural biology and function of solute transporters: implications for identifying and designing substrates. Drug Metab Rev. 2002;34:709–750. doi: 10.1081/dmr-120015692. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann AF, Mysels KJ. Bile acid solubility and precipitation in vitro and in vivo: the role of conjugation, pH, and Ca2+ ions. J Lipid Res. 1992;33:617–626. [PubMed] [Google Scholar]
- 18.Amelsberg A, Schteingart CD, Ton-Nu HT, Hofmann AF. Carrier-mediated jejunal absorption of conjugated bile acids in the guinea pig. Gastroenterology. 1996;110:1098–1106. doi: 10.1053/gast.1996.v110.pm8612999. [DOI] [PubMed] [Google Scholar]
- 19.St-Pierre MV, Kullak-Ublick GA, Hagenbuch B, Meier PJ. Transport of bile acids in hepatic and non-hepatic tissues. J Exp Biol. 2001;204:1673–1686. doi: 10.1242/jeb.204.10.1673. [DOI] [PubMed] [Google Scholar]
- 20.Vodenlich AD, Jr, Gong YZ, Geoghegan KF, Lin MC, Lanzetti AJ. Identification of the 14 kDa bile acid transport protein of rat ileal cytosol as gastrotropin. Biochem Biophys Res Commun. 1991;177:1147–1154. doi: 10.1016/0006-291x(91)90659-u. [DOI] [PubMed] [Google Scholar]
- 21.Oelkers P, Dawson PA. Cloning and chromosomal localization of the human ileal lipid-binding protein. Biochim Biophys Acta. 1995;1257:199–202. doi: 10.1016/0005-2760(95)00098-w. [DOI] [PubMed] [Google Scholar]
- 22.Kramer W, Wess G, Bewersdorf U, Corsiero D, Girbig F, et al. Topological photoaffinity labeling of the rabbit ileal Na+/bile-salt-cotransport system. Eur J Biochem. 1997;249:456–464. doi: 10.1111/j.1432-1033.1997.00456.x. [DOI] [PubMed] [Google Scholar]
- 23.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazaridis KN, Tietz P, Wu T, Kip S, Dawson PA, et al. Alternative splicing of the rat sodium/bile acid transporter changes its cellular localization and transport properties. Proc Natl Acad Sci U S A. 2000;97:11092–11097. doi: 10.1073/pnas.200325297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zelcer N, Saeki T, Bot I, Kuil A, Borst P. Transport of bile acids in multidrug-resistance-protein 3-overexpressing cells co-transfected with the ileal Na+-dependent bile-acid transporter. Biochem J. 2003;369:23–30. doi: 10.1042/BJ20021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. Journal of Biological Chemistry. 1994;269:1340–1347. [PubMed] [Google Scholar]
- 27.Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, et al. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. Journal of Clinical Investigation. 1995;95:745–754. doi: 10.1172/JCI117722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong MH, Oelkers P, Dawson PA. Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem. 1995;270:27228–27234. doi: 10.1074/jbc.270.45.27228. [DOI] [PubMed] [Google Scholar]
- 29.Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, et al. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res. 1999;40:1604–1617. [PubMed] [Google Scholar]
- 30.Saeki T, Matoba K, Furukawa H, Kirifuji K, Kanamoto R, et al. Characterization, cDNA cloning, and functional expression of mouse ileal sodium-dependent bile acid transporter. Journal of Biochemistry. 1999;125:846–851. doi: 10.1093/oxfordjournals.jbchem.a022358. [DOI] [PubMed] [Google Scholar]
- 31.Dawson PA, Oelkers P. Bile acid transporters. Curr Opin Lipidol. 1995;6:109–114. doi: 10.1097/00041433-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Weinman SA, Carruth MW, Dawson PA. Bile acid uptake via the human apical sodium-bile acid cotransporter is electrogenic. J Biol Chem. 1998;273:34691–34695. doi: 10.1074/jbc.273.52.34691. [DOI] [PubMed] [Google Scholar]
- 33.Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, et al. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274:G157–169. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- 34.Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, et al. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest. 1997;100:2714–2721. doi: 10.1172/JCI119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2) J Clin Invest. 1997;99:1880–1887. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallen S, Fryklund J, Sachs G. Inhibition of the human sodium/bile acid cotransporters by side-specific methanethiosulfonate sulfhydryl reagents: substrate-controlled accessibility of site of inactivation. Biochemistry. 2000;39:6743–6750. doi: 10.1021/bi000577t. [DOI] [PubMed] [Google Scholar]
- 37.Zhang EY, Phelps MA, Banerjee A, Khantwal CM, Chang C, et al. Topology scanning and putative three-dimensional structure of the extracellular binding domains of the apical sodium-dependent bile acid transporter (SLC10A2) Biochemistry. 2004;43:11380–11392. doi: 10.1021/bi049270a. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee A, Ray A, Chang C, Swaan PW. Site-directed mutagenesis and use of bile acid-MTS conjugates to probe the role of cysteines in the human apical sodium-dependent bile acid transporter (SLC10A2) Biochemistry. 2005;44:8908–8917. doi: 10.1021/bi050553s. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee A, Swaan PW. Membrane Topology of Human ASBT (SLC10A2) Determined by Dual Label Epitope Insertion Scanning Mutagenesis. New Evidence for Seven Transmembrane Domains. Biochemistry. 2006;45:943–953. doi: 10.1021/bi052202j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallen S, Bjorquist A, Ostlund-Lindqvist AM, Sachs G. Identification of a region of the ileal-type sodium/bile acid cotransporter interacting with a competitive bile acid transport inhibitor. Biochemistry. 2002;41:14916–14924. doi: 10.1021/bi0205404. [DOI] [PubMed] [Google Scholar]
- 41.Kramer W, Girbig F, Glombik H, Corsiero D, Stengelin S, et al. Identification of a ligand-binding site in the Na+/bile acid cotransporting protein from rabbit ileum. Journal of Biological Chemistry. 2001;276:36020–36027. doi: 10.1074/jbc.M104665200. [DOI] [PubMed] [Google Scholar]
- 42.Eriksson AH, Elm PL, Begtrup M, Nielsen R, Steffansen B, et al. hPEPT1 affinity and translocation of selected Gln-Sar and Glu-Sar dipeptide derivatives. Mol Pharm. 2005;2:242–249. doi: 10.1021/mp050015+. [DOI] [PubMed] [Google Scholar]
- 43.Herrera-Ruiz D, Knipp GT. Current perspectives on established and putative mammalian oligopeptide transporters. J Pharm Sci. 2003;92:691–714. doi: 10.1002/jps.10303. [DOI] [PubMed] [Google Scholar]
- 44.Bhardwaj RK, Herrera-Ruiz D, Sinko PJ, Gudmundsson OS, Knipp G. Delineation of human peptide transporter 1 (hPepT1)-mediated uptake and transport of substrates with varying transporter affinities utilizing stably transfected hPepT1/Madin-Darby canine kidney clones and Caco-2 cells. J Pharmacol Exp Ther. 2005;314:1093–1100. doi: 10.1124/jpet.105.087148. [DOI] [PubMed] [Google Scholar]
- 45.Lentz KA, Polli JW, Wring SA, Humphreys JE, Polli JE. Influence of passive permeability on apparent P-glycoprotein kinetics. Pharm Res. 2000;17:1456–1460. doi: 10.1023/a:1007692622216. [DOI] [PubMed] [Google Scholar]
- 46.Doring F, Will J, Amasheh S, Clauss W, Ahlbrecht H, et al. Minimal molecular determinants of substrates for recognition by the intestinal peptide transporter. J Biol Chem. 1998;273:23211–23218. doi: 10.1074/jbc.273.36.23211. [DOI] [PubMed] [Google Scholar]
- 47.Meredith D, Temple CS, Guha N, Sword CJ, Boyd CA, et al. Modified amino acids and peptides as substrates for the intestinal peptide transporter PepT1. Eur J Biochem. 2000;267:3723–3728. doi: 10.1046/j.1432-1327.2000.01405.x. [DOI] [PubMed] [Google Scholar]
- 48.Chandler CE, Zaccaro LM, Moberly JB. Transepithelial transport of cholyltaurine by Caco-2 cell monolayers is sodium dependent. Am J Physiol. 1993;264:G1118–1125. doi: 10.1152/ajpgi.1993.264.6.G1118. [DOI] [PubMed] [Google Scholar]
- 49.Hidalgo IJ, Borchardt RT. Transport of bile acids in a human intestinal epithelial cell line, Caco-2. Biochim Biophys Acta. 1990;1035:97–103. doi: 10.1016/0304-4165(90)90179-z. [DOI] [PubMed] [Google Scholar]
- 50.Sun AQ, Ananthanarayanan M, Soroka CJ, Thevananther S, Shneider BL, et al. Sorting of rat liver and ileal sodium-dependent bile acid transporters in polarized epithelial cells. Am J Physiol. 1998;275:G1045–1055. doi: 10.1152/ajpgi.1998.275.5.G1045. [DOI] [PubMed] [Google Scholar]
- 51.Balakrishnan A, Sussman DJ, Polli JE. Development of stably transfected monolayer overexpressing the human apical sodium-dependent bile acid transporter (hASBT) Pharm Res. 2005;22:1269–1280. doi: 10.1007/s11095-005-5274-8. [DOI] [PubMed] [Google Scholar]
- 52.Balakrishnan A, Wring SA, Polli JE. Interaction of Native Bile Acids with Human Apical Sodium Dependent Bile Acid Transporter (hASBT): Influence of Steroidal Hydroxylation Pattern and C-24 Conjugation. Pharm Res. 2006 doi: 10.1007/s11095-006-0219-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kramer W, Wess G, Schubert G, Bickel M, Girbig F, et al. Liver-specific drug targeting by coupling to bile acids. Journal of Biological Chemistry. 1992;267:18598–18604. [PubMed] [Google Scholar]
- 54.Kramer W, Wess G. Bile acid transport systems as pharmaceutical targets. Eur J Clin Invest. 1996;26:715–732. doi: 10.1111/j.1365-2362.1996.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 55.Kullak-Ublick GA, Glasa J, Boker C, Oswald M, Grutzner U, et al. Chlorambucil-taurocholate is transported by bile acid carriers expressed in human hepatocellular carcinomas. Gastroenterology. 1997;113:1295–1305. doi: 10.1053/gast.1997.v113.pm9322525. [DOI] [PubMed] [Google Scholar]
- 56.Petzinger E, Wickboldt A, Pagels P, Starke D, Kramer W. Hepatobiliary transport of bile acid amino acid, bile acid peptide, and bile acid oligonucleotide conjugates in rats. Hepatology. 1999;30:1257–1268. doi: 10.1002/hep.510300529. [DOI] [PubMed] [Google Scholar]
- 57.Kim DC, Harrison AW, Ruwart MJ, Wilkinson KF, Fisher JF, et al. Evaluation of the bile acid transporter in enhancing intestinal permeability to renin-inhibitory peptides. J Drug Target. 1993;1:347–359. doi: 10.3109/10611869308996094. [DOI] [PubMed] [Google Scholar]
- 58.Holzinger F, Schteingart CD, Ton-Nu HT, Eming SA, Monte MJ, et al. Fluorescent bile acid derivatives: relationship between chemical structure and hepatic and intestinal transport in the rat. Hepatology. 1997;26:1263–1271. doi: 10.1002/hep.510260526. [DOI] [PubMed] [Google Scholar]
- 59.Swaan PW, Hillgren KM, Szoka FC, Jr, Oie S. Enhanced transepithelial transport of peptides by conjugation to cholic acid. Bioconjug Chem. 1997;8:520–525. doi: 10.1021/bc970076t. [DOI] [PubMed] [Google Scholar]
- 60.Bhat L, Jandeleit B, Dias TM, Moors TL, Gallop MA. Synthesis and biological evaluation of novel steroidal pyrazoles as substrates for bile acid transporters. Bioorg Med Chem Lett. 2005;15:85–87. doi: 10.1016/j.bmcl.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 61.Tolle-Sander S, Lentz KA, Maeda DY, Coop A, Polli JE. Increased acyclovir oral bioavailability via a bile acid conjugate. Mol Pharm. 2004;1:40–48. doi: 10.1021/mp034010t. [DOI] [PubMed] [Google Scholar]
- 62.de Miranda P, Blum MR. Pharmacokinetics of acyclovir after intravenous and oral administration. J Antimicrob Chemother. 1983;12 B:29–37. doi: 10.1093/jac/12.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- 63.Jacobson MA. Valaciclovir (BW256U87): the L-valyl ester of acyclovir. J Med Virol. 1993 1:150–153. doi: 10.1002/jmv.1890410529. [DOI] [PubMed] [Google Scholar]
- 64.Balimane PV, Tamai I, Guo A, Nakanishi T, Kitada H, et al. Direct evidence for peptide transporter (PepT1)-mediated uptake of a nonpeptide prodrug, valacyclovir. Biochem Biophys Res Commun. 1998;250:246–251. doi: 10.1006/bbrc.1998.9298. [DOI] [PubMed] [Google Scholar]
- 65.Burnette TC, de Miranda P. Metabolic disposition of the acyclovir prodrug valaciclovir in the rat. Drug Metab Dispos. 1994;22:60–64. [PubMed] [Google Scholar]
- 66.Swaan PW, Szoka FC, Jr, Oie S. Molecular modeling of the intestinal bile acid carrier: a comparative molecular field analysis study. Journal of Computer-Aided Molecular Design. 1997;11:581–588. doi: 10.1023/a:1007919704457. [DOI] [PubMed] [Google Scholar]
- 67.Lack L, Weiner IM. Intestinal bile salt transport: structure-activity relationships and other properties. Am J Physiol. 1966;210:1142–1152. doi: 10.1152/ajplegacy.1966.210.5.1142. [DOI] [PubMed] [Google Scholar]
- 68.Lack L. Properties and biological significance of the ileal bile salt transport system. Environmental Health Perspectives. 1979;33:79–90. doi: 10.1289/ehp.793379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baringhaus KH, Matter H, Stengelin S, Kramer W. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. II. A reliable 3D QSAR pharmacophore model for the ileal Na(+)/bile acid cotransporter. Journal of Lipid Research. 1999;40:2158–2168. [PubMed] [Google Scholar]
- 70.Kagedahl M, Swaan PW, Redemann CT, Tang M, Craik CS, et al. Use of the intestinal bile acid transporter for the uptake of cholic acid conjugates with HIV-1 protease inhibitory activity. Pharm Res. 1997;14:176–180. doi: 10.1023/a:1012044526054. [DOI] [PubMed] [Google Scholar]
- 71.Kramer W, Wess G, Schubert G, Bickel M, Girbig F, et al. Liver-specific drug targeting by coupling to bile acids. J Biol Chem. 1992;267:18598–18604. [PubMed] [Google Scholar]
- 72.Kramer W, Wess G, Enhsen A, Bock K, Falk E, et al. Bile acid derived HMG-CoA reductase inhibitors. Biochim Biophys Acta. 1994;1227:137–154. doi: 10.1016/0925-4439(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 73.Starke D, Lischka K, Pagels P, Uhlmann E, Kramer W, et al. Bile acid-oligodeoxynucleotide conjugates: synthesis and liver excretion in rats. Bioorg Med Chem Lett. 2001;11:945–949. doi: 10.1016/s0960-894x(01)00048-8. [DOI] [PubMed] [Google Scholar]
- 74.Wess G, Kramer W, Han XB, Bock K, Enhsen A, et al. Synthesis and biological activity of bile acid-derived HMG-CoA reductase inhibitors. The role of 21-methyl in recognition of HMG-CoA reductase and the ileal bile acid transport system. J Med Chem. 1994;37:3240–3246. doi: 10.1021/jm00046a007. [DOI] [PubMed] [Google Scholar]