Abstract
Aims
To estimate the cost-effectiveness of 10 mg rosuvastatin daily for older patients with systolic heart failure in the Controlled Rosuvastatin Multinational Study in Heart Failure (CORONA) trial.
Methods and results
This within trial analysis of CORONA used major cardiovascular (CV) events as the outcome measure. Resource use was valued and the costs of hospitalizations, procedures, and statin use compared. Cost-effectiveness was estimated as cost per major CV event avoided. There were significantly fewer major CV events in the rosuvastatin group compared with the placebo group (1.04 vs. 1.20 per patient; difference 0.164; 95% CI: 0.075–0.254, P < 0.001). The average cost of CV hospitalizations and procedures was significantly lower for those receiving rosuvastatin (£1531 vs. £1769; difference £238; 95% CI: £73–403, P = 0.005); the additional cost of the statin resulted in significantly higher total costs for the rosuvastatin group (£1769 vs. £2072; difference £303; 95% CI: £138–468, P < 0.001). Overall, rosuvastatin was found to cost £1840 (95% CI: £562–6028) per major CV event avoided.
Conclusion
This economic analysis showed that a significant reduction in major CV events with rosuvastatin led to significantly reduced costs of CV hospitalizations and procedures. The reduction in associated costs for major CV events was found to offset partially (by 44%) the cost of rosuvastatin treatment in patients with systolic heart failure.
Keywords: Cost-effectiveness, Heart failure, Statins, Multinational trial
Introduction
Heart failure is a major public health problem. Incidence, prevalence, and risk have been found to be high in both Europe and the United States.1,2 The Rotterdam study1 found that a male (female) aged 55 years old has 33.0% (28.5%) chance of developing heart failure during their remaining lifetime; while the Framingham Heart Study2 reported a lifetime risk at age 50 of 20.9% for men and 20.5% for women. The Rotterdam study further showed that the prevalence of heart failure increased with time and age; in 1998, 0.9% of subjects aged 55–64 had heart failure, compared with 17.4% of those aged 85 years or over. The combination of an ageing population in most western countries, a population which has the greatest incidence of heart disease, and improved survival after acute myocardial infarction (MI) is, therefore, likely to further increase the prevalence of heart failure.3
This increasing clinical burden is expected to be matched with an increasing financial burden. Using a prevalence-based approach the economic burden of heart failure in the UK was estimated to be £905.3 million in 2000, a 26% increase on 1995 estimates, and equivalent to 1.91% of total NHS expenditure.4 The major contributor to this financial burden is hospitalization, as such there is potential to reduce the burden if admissions to hospital and durations of hospital stay can be reduced. Indeed, it has been estimated that reducing length of stay by 1 day or reducing re-admissions by 50% could generate annual cost savings of between £50 and £120 million.4
HMG-CoA reductase inhibitors or ‘statins’ have been found to be beneficial in people with or at risk of cardiovascular (CV) disease, and importantly they have also been shown to be cost effective.5 However, existing statin trials generally excluded patients with heart failure leading to uncertainty about their potential role in that condition.6
The Controlled Rosuvastatin Multinational Study in Heart Failure (CORONA) trial was the first large-scale, prospective, randomized, placebo-controlled study to determine the effect of statins on clinical outcomes in patients with heart failure.7,8 This paper estimates the cost-effectiveness of a daily dose of 10 mg of rosuvastatin during the study treatment period.
Methods
Clinical trial
The CORONA trial assessed the effects of rosuvastatin on mortality and morbidity in patients with chronic systolic heart failure. Patients were allocated in a ratio of 1:1 to 10 mg rosuvastatin or matching placebo. Patients were recruited at 378 centres in 21 countries. The trial was approved by the Ethics Committee at each of the participating hospitals, and patients provided written informed consent. An economic analysis plan was drafted in advance of the data being released. The plan was developed using a slice of treatment-blinded trial data.
Details of the trial have been reported elsewhere,7 but in short patients aged 60 years or older with chronic symptomatic (NYHA functional class II–IV) heart failure of ischaemic aetiology and with an ejection fraction of ≤0.40 (≤0.35 if NYHA class II) were included. Patients already on a statin (or other lipid lowering drug) or considered by their own doctor to need (or have a contraindication to) a statin were not randomized. Lipid lowering treatment could not be stopped to make a patient eligible for inclusion. Furthermore, patients who had been treated with a statin previously were not eligible for randomization until 6 months after withdrawal of the statin.
The primary trial outcome was the composite of CV mortality, non-fatal MI, or non-fatal stroke, analysed as time to the first event. The secondary outcomes were all-cause mortality, any coronary event, CV mortality, and number of hospitalizations (for CV causes, unstable angina, and worsening heart failure).
Endpoint classification
All serious adverse events were classified by an independent Endpoint Committee (Appendix 1), such that no pre-selection exclusion of potential clinical events was undertaken by the investigators at a site level. In contrast to most previous trials, the adjudication comprised all possible non-CV and CV events. The Endpoint Committee used all available information such as relevant case report forms, reports of serious adverse events, copies of hospital discharge summaries, ECGs, and enzymes (if an acute MI was suspected or reported), physicians’ records, discharge letters, police reports, death certificates, and autopsy reports, blinded to subject identifiers. For each hospitalization one main reason was given for admission according to a pre-specified Endpoint Classification Manual. If a pre-specified complication (a new MI, new stroke, or unstable angina) occurred during the hospitalization, and was not the reason for admission, these were also adjudicated and recorded, as were pre-specified procedures (percutaneous transluminal coronary intervention (PCI), coronary artery bypass graft (CABG), and cardiac transplant). In addition if a non-fatal stroke occurred outside hospital this was also adjudicated and recorded.
Hospitalization was defined as care at an acute care hospital lasting ≥24 h. Cardiovascular causes were classified as cardiac causes (aggravated heart failure, acute MI, unstable angina, or other cardiac with diagnosis in full text) or non-cardiac (ischaemic, haemorrhagic or unclassifiable stroke, or other non-cardiac with diagnosis in full text). Non-CV causes were given in full text by the Endpoint Committee. Hospitalization for heart failure required documentation that worsening heart failure was the principal reason for hospitalization and, if competing reasons were judged to be of equal importance, heart failure was given preference. Deaths were classified in a similar way.
Health outcomes
Since the total number of major CV events was of greater interest than the primary endpoint (patient-based time to first event) from an economic perspective, the outcome used in the cost-effectiveness analysis was the total number of combined major fatal and non-fatal CV events avoided (as opposed to patients having events). ‘Major CV events’ were defined as any of the endpoints described earlier, that is: CV hospitalizations, additional CV events occurring while in hospital, CV events not requiring hospitalisation, and CV deaths. These events were aggregated for each individual (taking care to avoid double counting) and the total and mean number of events per patient were compared between the treatment and placebo groups.
Resource use and cost
The cost of health care comprises resource use counts and the unit cost of these resources. A range of resource use components were routinely collected during the trial. These include hospitalizations (the nature of the hospitalization and the length of stay), procedures, and medications. Primary care costs, over-the-counter medications, and patient costs were not collected. The perspective employed in the economic evaluation was that of the health care sector, i.e. direct costs to the patient and potential productivity costs to society were not estimated.
The index country for the cost analysis was the UK; all costs used 2005/06 as the base year (no inflationary adjustment was required). NHS Reference Costs9 were employed to value all hospitalizations and procedures, and while the costs of the more specific hospitalizations (MI, stroke, heart failure, and unstable angina) were easily identified, there were numerous (and many rare) CV and non-CV hospitalizations which required valuation. In order to cost accurately these events, two members of the Independent Endpoint Committee assigned ICD-10 codes to all CV and non-CV hospitalizations (OPCS-4 codes were used when hospitalizations explicitly involved procedures). These codes were then mapped to HRG (Healthcare Resource Group) codes using HRG4 Reference Cost Grouper software10 which were subsequently assigned a cost from the National Schedule of Reference Costs for NHS Trusts.9 Appendix 2 presents an example of the main unit costs that were employed in the analysis, the National Schedule (non-elective inpatient stay) gives the full list.9 The same unit costs were employed whether the patient was discharged alive, or died during the hospitalization, as the reference costs are estimated from finished consultant episodes (FCE), which includes those discharged alive as well as those who died during a hospitalization.
The total number of hospital admissions for each specific type of inpatient stay is presented and compared across the treatment groups, as is length of hospital stay. NHS Reference Costs use an average length of stay (set nationally), that is the unit costs are per event not per diem. As this is a multinational study that employs UK specific costs, event costs were employed rather than per diem costs.
The unit costs for each procedure (PTCA, CABG, or cardiac transplant) were used in preference to the unit cost of an associated hospitalization as the reference cost for a procedure includes both these elements (the intervention and inpatient stay). Multiple procedures (e.g. two PTCAs) were conservatively costed as a single procedure.
Concomitant medications were recorded at each study visit during the trial. Doses were only recorded for heart failure specific medications (e.g. loop-diuretics, thiazide diuretics, beta-blockers, ACE-inhibitors, AT1-receptor blockers, aldosterone antagonists, and digitalis) at baseline.
The discontinuation of study medication (and compliance more generally) was checked at each follow-up visit and this information was used to estimate the number of days on rosuvastatin. The daily cost of 10 mg of rosuvastatin (in 2006) was £0.64.11
Combining unit costs and resource use counts provides an estimate of the cost of each component (CV hospitalization, procedures, medication), as well as the total cost per patient in each arm of the trial. The difference in costs (and significance) between the two groups was estimated for all categories of cost (CV and non-CV hospitalizations and procedures) and total cost.
Statistical and cost-effectiveness analysis
All analyses were carried out using all patients randomized using the principle of intention-to-treat (ITT). The ITT population was defined as all patients who received a bottle of a study drug. All analyses were performed using STATA10 (StataCorp, TX, USA).
The cost-effectiveness of statins within the trial period was estimated by combining the estimates of cost and effect as described earlier in an incremental cost-effectiveness ratio (ICER). The 95% confidence interval for this ratio was estimated using Fieller's Theorem, a technique that includes any correlation between cost and outcome.12 Cost-effectiveness acceptability curves (CEACs), which allow decision-makers to assess the overall probability of a particular threshold, were also estimated.13 A CEAC shows combinations of ceiling values for the ICER and the probabilities that the ICER falls below any given ceiling ratio based on the observed size and variance of differences in cost and events in the trial. All ICERs are appropriately discounted using the current discount rate for the United Kingdom (3.5%) to adjust for differential timing of costs and effects.14 Where costs and effects are reported separately these are undiscounted (unless otherwise stated).
The cost of statin monitoring, to assess patient stability, was undertaken as sensitivity analysis. Statin monitoring was assumed to include a liver test15 at the initial prescribing visits, and a further liver test and accompanying primary care visit, valued as a GP visit,16 6 weeks later.
Results
A total of 5011 patients were randomized in 21 countries; 2514 were assigned to receive rosuvastatin and 2497 to receive placebo. Both groups had similar characteristics at baseline (Table 1). The mean follow-up time was 907 days in the placebo group and 910 days in the treatment group. The average number of days on the study medication was 822 in the placebo group and 839 in the rosuvastatin group (difference 17 days; 95% CI: −3.7–38; P = 0.11).
Table 1.
Baseline characteristics (means and proportions)
| Placebo, n = 2497 | Rosuvastatin, n = 2514 | P-value | |
|---|---|---|---|
| Age | 72.7 | 72.7 | 0.99 |
| Females | 24 | 2 | 0.95 |
| NYHA class | 0.61 | ||
| II | 37 | 37 | |
| III | 62 | 61 | |
| IV | 1.6 | 1.4 | |
| Ejection fraction | 0.31 | 0.31 | 0.94 |
| BMIa | 27 | 27 | 0.53 |
| Blood pressure | |||
| Systolicb | 129 | 129 | 0.52 |
| Diastolicb | 76 | 76 | 0.12 |
| Heart rate | 72 | 72 | 0.61 |
| Current smokerc | 8.3 | 8.9 | 0.41 |
| Medical history | |||
| MI | 60 | 60 | 0.87 |
| Past or current angina | 72 | 73 | 0.71 |
| CABG | 17 | 17 | 0.62 |
| PTCA/PCI | 11 | 12 | 0.66 |
| Hypertension | 63 | 63 | 0.95 |
| Diabetes | 29 | 30 | 0.90 |
| Current atrial fibrillation | 23 | 24 | 0.51 |
| Stroke | 12 | 13 | 0.87 |
| Aortic aneurysm | 3.3 | 2.7 | 0.20 |
| Intermittent claudication | 13 | 13 | 0.96 |
| Pacemaker | 12 | 10 | 0.08 |
| Implantable cardioverter-defibrillator | 2.6 | 2.9 | 0.51 |
NYHA, New York Heart Association; BMI, body mass index; MI, myocardial infarction; CABG, coronary-artery bypass grafting; PTCA, percutaneous transluminal coronary angioplasty; PCI, percutaneous coronary intervention.
Sample size variations: a2492, 2505; b2497, 2513; c2494, 2513.
Cardiovascular hospitalizations, procedures and deaths, the primary economic outcome measure of major CV events, and non-CV hospitalizations are presented in Table 2. Overall, there were significantly fewer CV hospitalizations in the treatment group (2191 vs. 2562; P < 0.001), and significantly fewer hospitalizations for any cause (CV and non-CV) (3685 vs. 4068; P = 0.006). There was no difference in the number of non-CV hospitalizations (1494 vs. 1506). The number of other CV events including: additional CV events occurring while in hospital; CV events not requiring hospitalization; CV deaths; and CV procedures were similar in the two treatment groups. When all CV events were combined, there were significantly fewer major CV events in the rosuvastatin group compared with the placebo group (2613 vs. 3006; P < 0.001). The average number of major CV events per patient was 1.20 in the placebo group, compared with 1.04 in the intervention group (P < 0.001), such that there were 13.7% (95% CI: 6.5–20.3%, P < 0.001) fewer major CV events in the rosuvastatin group. When considering fatal and non-fatal major CV events separately, there was a 2.7% (95% CI: −7.3–12.4%, P = 0.59) reduction in deaths, and 16.4% (95% CI: 8.0–23.9%, P < 0.001) fewer non-fatal major CV events in the rosuvastatin group.
Table 2.
Cardiovascular and non-cardiovascular events
| Placebo n = 2497 | Rosuvastatin n = 2514 | P-valuea | |
|---|---|---|---|
| Hospitalizations (number of admissions)b | |||
| Stroke | 103 | 86 | |
| Worsening heart failure | 1298 | 1108 | 0.012 |
| MI | 142 | 109 | |
| Unstable angina | 90 | 73 | 0.28 |
| Non-cardiac CV | 336 | 296 | |
| Other CV | 593 | 519 | |
| All CV hospitalizationsi | 2562 | 2191 | <0.001 |
| Hospitalizations (number of patients) | |||
| Stroke | 94 | 81 | |
| Worsening heart failure | 669 | 622 | |
| MI | 131 | 96 | |
| Unstable angina | 71 | 64 | |
| Non-cardiac CV | 231 | 226 | |
| Other CV | 406 | 394 | |
| All CV hospitalizations | 1164 | 1104 | |
| Additional events while in hospital (not reason for hospitalization)ii | |||
| Stroke | 10 | 9 | |
| Acute MI | 19 | 28 | |
| Unstable angina | 3 | 3 | |
| Additional events not requiring hospitalizationiii | |||
| Stroke | 5 | 3 | |
| CV deaths | |||
| Worsening heart failure deaths | 191 | 193 | 0.97 |
| MI deaths | 9 | 15 | 0.23 |
| Sudden deaths | 327 | 316 | 0.58 |
| Other CV deaths | 6 | 8 | |
| Non-cardiac CV deaths | 60 | 49 | |
| Total CV deaths | 593 | 581 | 0.43 |
| CV deaths while hospitalized | 186 | 202 | |
| CV deaths not while hospitalizediv | 407 | 379 | |
| Procedures | |||
| CABG | 28 | 32 | |
| PCI | 93 | 93 | |
| Cardiac transplant | 3 | 1 | |
| Major CV eventsc | |||
| Total numberi+ii+iii+iv | 3006 | 2613 | <0.001 |
| Average number (per patient) | 1.204 | 1.039 | <0.001 |
| Non-CV hospitalizations (number of admissions) | 1506 | 1494 | |
| Non-CV hospitalizations (number of patients) | 840 | 839 | |
| All hospitalizations (number of CV and non-CV admissions) | 4068 | 3685 | 0.006 |
| All hospitalizations (number of patients)d | 1523 | 1489 | |
CV, cardiovascular; MI, myocardial infarction; CABG, coronary-artery bypass grafting; PCI, percutaneous coronary intervention.
aP-values are only presented for the pre-specified trial endpoints.
bDetailed analysis of hospitalizations revealed a number of duplicate and simultaneous hospitalizations, these were investigated and corrected and as such the number of hospitalizations reported in this table differ slightly from those reported in the clinical trial paper.8
cMajor CV events are the summation of (i) CV hospitalizations, (ii) additional CV events while in hospital, (iii) events not requiring hospitalization, and (iv) CV deaths not while in hospital for a CV cause.
dThe number of patients hospitalized for CV causes and non-CV causes sums to more than the number of patients hospitalized for all causes, as patients could be hospitalized for both CV and non-CV causes during the course of the trial.
Table 3 presents descriptive statistics for the mean length of stay for each category of hospitalization. There was no significant difference between the two groups.
Table 3.
Length of stay (days) by type of hospitalization, mean (standard error), median (interquartile range)
| Placebo | Rosuvastatin | P-value | |
|---|---|---|---|
| Stroke | 15.0 (1.4), 11 (7,18) | 13.5 (1.4), 10 (5, 16) | 0.47 |
| Worsening heart failure | 12.6 (0.3), 9.5 (6, 15) | 11.9 (0.3), 9 (6, 15) | 0.09 |
| MI | 14.8 (1.2), 11 (7, 19) | 12.9 (1.0), 11 (6, 17) | 0.24 |
| Unstable angina | 12.2 (1.6), 9.5 (6, 14) | 13.3 (1.0), 12 (7, 18) | 0.58 |
| Non-cardiac CV | 12.8 (1.0), 8 (5, 12) | 13.2 (0.9), 8 (4, 15) | 0.77 |
| Other CV | 8.7 (0.4), 5 (3, 10) | 9.2 (0.4), 6 (3, 11) | 0.37 |
| Non CV | 11.2 (0.3), 8 (4, 14) | 11.2 (0.3), 8 (4, 14) | 0.88 |
CV, cardiovascular; MI, myocardial infarction.
Because the mean number of concomitant oral medications prescribed during the trial was similar in the two treatment groups [14.0 (range: 0–119) in the placebo group and 13.5 (range: 0–103) in the rosuvastatin group; difference 0.4; 95% CI: − 0.13–1.01; P = 0.14] these were neither valued nor included in the estimation of total cost.
Table 4 presents the results of the cost analysis. The average cost of CV hospitalizations for those receiving rosuvastatin was found to be significantly less than those receiving the placebo (£1288 vs. £1517; difference £229; 95% CI: £96–362; P = 0.001). The summation of CV hospitalization and procedure costs gave a similar estimate; the average cost in the rosuvastatin group was again significantly lower (£1531 vs. £1769; difference £238; 95% CI: £73–403; P = 0.005). This equates to a 13.4% (95% CI: 4.4–21.7%, P = 0.004) reduction in CV hospitalization and procedure costs. The cost of rosuvastatin was estimated to be £540 per patient for the duration of the trial, therefore, the cost savings from fewer CV hospitalizations and procedures (£238) was outweighed by the cost of treatment. Table 4 shows that combining the cost of CV hospitalizations with the cost of procedures and adding the cost of the statin resulted in a significantly higher average cost for those in the rosuvastatin group (£2072 vs. £1769; difference £303; 95% CI: £138–468; P < 0.001).
Table 4.
Average cost per patient (£sterling, 2005/06 prices)
| Placebo (std. error) | Rosuvastatin (std. error) | Difference (95% CI) | P-value | |
|---|---|---|---|---|
| Cost components | ||||
| Cost of CV hospitalization [a] | 1517 (51) | 1288 (44) | −229 (−362, −96) | 0.001 |
| Cost of procedures [b] | 252 (33) | 243 (27) | −9 (−92, 75) | 0.84 |
| Cost of non-CV hospitalizations [c] | 739 (31) | 726 (29) | −13 (−97, 70) | 0.76 |
| Cost of statin [d] | - | 540 (5) | 540 (531, 550) | <0.001 |
| Total costs | ||||
| Total cost excluding statin costs and non-CV hospitalization [a+b] | 1769 (64) | 1531 (54) | −238 (−403, −73) | 0.005 |
| Total cost excluding statin costs [a+b+c] | 2508 (75) | 2257 (65) | −251 (−446, −57) | 0.011 |
| Total cost excluding non-CV hospitalization [a+b+d] | 1769 (64) | 2072 (54) | 303 (138, 468) | <0.001 |
| Total cost [a+b+c+d] | 2508 (75) | 2798 (65) | 289 (95, 484) | 0.004 |
CV, cardiovascular.
A comparison of this cost with the improvement in effect (that is an increase in the number of major CV events avoided) gave an ICER of £1840 (95% CI: £562–6028), i.e. rosuvastatin cost £1840 for each major CV event avoided (Table 5). When the costs of non-CV hospitalizations were included, the cost per major CV event was slightly lower (£1759) but the confidence interval was wider (95% CI: £398–6092). When the cost of statin monitoring was included, the cost difference increased and the ICER became £2090 (95% CI: £729–6576) per major CV event avoided. Given cardiac transplants are rare and expensive events and it is likely to be chance that the placebo group had three times as many as the rosuvastatin group (Table 3), further sensitivity analysis was conducted that excluded these from the estimation. Table 5 shows that the exclusion of these also resulted in a slightly higher ICER of £1987 (95% CI: £705–6230).
Table 5.
Cost, effect, and cost-effectiveness (cost per major CV event avoided) (£sterling, 2005/06 prices)
| Rosuvastatin vs. placebo | Point estimate (95% CI) | ICER (95% CI)a |
|---|---|---|
| Incremental effectb | 0.164 (0.075, 0.254) | |
| Incremental total cost (CV hospitalization, procedure and Tx costs) | £303 (138, 467) | £1840 (562, 6028) |
| Incremental total cost, including non-CV hospitalization | £289 (95, 484) | £1759 (398, 6092) |
| Incremental total cost, including monitoring visits | £343 (179, 509) | £2090 (729, 6576) |
| Incremental total cost, excluding cardiac transplantations | £328 (176, 481) | £1987 (705, 6230) |
CV, cardiovascular; Tx, treatment; ICER incremental cost-effectiveness ratio.
a95% confidence interval for the ICER estimated using Fieller's Theorem.
bThis is the difference between an average of 1.204 major CV events per patient in the placebo group and 1.039 in the rosuvastatin group, see Table 2.
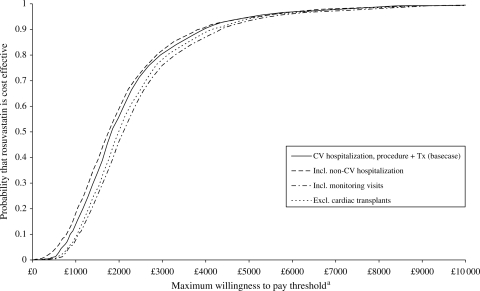
The extent of the uncertainty in these cost-effectiveness estimates is presented in Figure 1. It is evident in each CEAC that as the threshold increases, the curves asymptote to 1 suggesting that there is very little uncertainty in these estimates.
Figure 1.
Cost-effectiveness acceptability curves.
aMaximum willingness to pay to avoid one major CV event.
Discussion
This within trial analysis of CORONA showed that the use of rosuvastatin in older patients with ischaemic systolic heart failure resulted in a 14% reduction in major CV events which corresponds to a similar reduction in associated hospitalization and procedure costs compared to placebo. The additional cost of statin treatment, however, resulted in significantly higher total costs for those in the rosuvastatin group. As such the cost savings associated with hospitalizations and procedures for major CV events only offset partially the cost of rosuvastatin, so that treatment with rosuvastatin led to an ICER of £1840 per major CV event avoided.
Although this was a multinational study, we employed UK specific costs. A similar approach can be employed to estimate cost-effectiveness for other countries in the trial, using country-specific costs. However, we would expect to find few substantial differences because costs are merely weights for resource use.17,18 To control for differences in resource use between the trial population and practice in the index country, we employed unit costs for specific types of hospitalization and procedures, rather than per diem costs. This meant that our analysis is less sensitive to variations in length of hospital stay between countries and that our results should be more generalizable. There was no significant difference between lengths of stay in the two treatment groups.
Notably, this economic evaluation did not employ the trial primary outcome, which was a time to first event analysis, for a composite of death from CV causes, non-fatal MI, or non-fatal stroke. This outcome was used at the request of a regulatory authority to be in keeping with prior statin trials. It did not, however, include the most common and expensive non-fatal outcome in patients with heart failure, i.e. hospitalization for worsening heart failure. For the economic evaluation of CORONA, we felt it was important to examine the total burden of CV events in these patients, including hospitalization for worsening heart failure and other associated CV causes, as well as CV procedures. Our analyses showed the merit of undertaking an economic evaluation of what was a ‘non-conclusive’ clinical trial result, 8% observed relative risk reduction compared with 16% assumed relative risk reduction.
The estimated ICER is consistent with that reported in similar studies of statin treatment. For example, in the Heart Protection Study (HPS), 40 mg of simvastatin once daily in a group of patients which mainly excluded those with heart failure, the cost per major vascular event avoided was £11600; while in a subgroup of high-risk patients (individuals with a 42% 5 year risk of a major vascular event) an ICER of £4500 per major vascular event avoided was found.19 We estimate that the baseline risk for patients in our placebo arm was ∼17% per annum, nearly twice as high as the annual risk for high-risk patients in HPS (∼9%), this coupled with higher cost reduction in HPS (22% reduction in hospital costs compared with 13%) means that the HPS ICER of £4500 is approximately consistent with our ICER of £1840. Notably, in the HPS lifetime analysis, statin treatment for high-risk patients was found to be cost saving.5
However, it is important to emphasize that the patients in HPS were very different to those in CORONA, in that they had a much better prognosis than the CORONA patients (as indicated by the baseline risk) and there was also a significant difference in the number of fatal events in the HPS study, such that the two outcome measures, while similar, are not directly comparable. It is a limitation of the analysis that the composite endpoint used is a clinical outcome measure, rather than a quality adjusted life year (QALY) as favoured by economists. To appropriately estimate cost per QALY would require generic health-related quality of life data (like the EQ5D20) which were not collected in the trial, and also would necessitate extrapolation beyond the 3 year trial follow-up. As survival data were collected, it would be possible to estimate the cost per additional life year gained. However, it would not be informative to estimate the cost per additional life year gained since, as detailed in the trial paper,8 there is no significant difference in survival, such that any comparison of costs and outcomes would revert to a mere cost analysis, which would be uninformative as it would ignore the quality of life benefits of avoiding events.
As shown in HPS, it is important to consider the role of baseline risk and the sensitivity of cost-effectiveness estimates. Work is ongoing in this area; however, the methodology of heterogeneity issues is not trivial and it requires further understanding and development. Furthermore, the model that has been developed is based on a subsample of patients21 (due to missing data) while our economic analysis is applicable to the sample as a whole, such that further research is required.
In conclusion, although the primary outcome of the CORONA study was not met, this economic analysis shows that a significant reduction in major CV events with rosuvastatin led to significantly reduced costs of CV hospitalizations and procedures. The reduction in associated costs for major CV events was found to offset partially (by 44%) the cost of rosuvastatin treatment in patients with systolic heart failure.
Acknowledgements
The Health Economics Appraisal Team of the University of Glasgow and the CORONA Steering committee are responsible for the study design and the scientific execution of the health economic analysis of CORONA trial.
Appendix 1: The members of the CORONA study group
Executive Committee: P.D., Amphia Hospital, Breda, Netherlands; A.H., Dept. of Cardiology, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden (Chairman of the Executive Committee); Lennart Jansson, AstraZeneca, Mölndal, Sweden (Study team leader); J.K., Dept. of Cardiology, Rikshospitalet University Hospital, Oslo, Norway; Magnus Lindberg, AstraZeneca, Mölndal, Sweden, J.J.V.M., BHF Glasgow Cardiovascular Research Centre, University of Glasgow, UK; F.W., Dept. of Cardiology, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden; H.W., Nordic School of Public Health, Göteborg, Sweden (Independent biostatistician); J.W., Wallenberg Laboratory for Cardiovascular Research, Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden (Secretary of the Executive Committee).
Writing Committee: P.D., A.H., J.K., J.M. (Chairman of the Writing Committee), F.W., H.W., and J.W.
Steering Committee: Chairman J.K.; Co-chair P.D. and A.H. Members of the Steering Committee are the Executive Committee members and the following National Co-ordinating Investigators: Eduard Apetrei MD, PhD, V.B. MD, PhD, Michael Böhm MD, PhD, John GF Cleland MD, Jan H. Cornel MD, PhD, C.F. MD, Assen Goudev MD, PhD, Peer Grande MD, PhD, Lars Gullestad MD, PhD, Jaromir Hradec MD, PhD, FESC, A.J. MD, D.Sc., Gabriel Kamenský MD, PhD, Michel Komajda MD, Jerzy Korewicki MD, PhD, Timo Kuusi MD, PhD, François Mach MD, Vyacheslav Mareev MD, PhD, Naresh Ranjith MD, Maria Schaufelberger MD, PhD, Johan Vanhaecke MD, PhD, D.J.V. MD, PhD. The Steering Committee also includes one AstraZeneca monitor from each of the 21 participating countries (non-voting; for names see Kjekshus et al.7).
Affiliations for National Co-ordinating Investigators: Institute of Cardiology, Bucharest, Romania (E.A.); Dept. of Cardiology, Hospital Ramón y Cajal, Madrid, Spain (V.B.); Klinik für Innere Medizin III, Universitätsklinikum des Saarlandes, 66421 Homburg/Saar, Germany (M.B.); Dept. of Cardiology, University of Hull, Kingston upon Hull, Yorkshire, UK (J.G.F.C.); Dept of Cardiology, Medisch Centrum Alkmaar and member of the WCN, The Netherlands (J.H.C.); Dept. of Medicine, Heart failure Unit, S. Francisco Xavier Hospital, Faculty of Medical Sciences, New University of Lisbon, Portugal (C.F.); Dept. of Cardiology, Queen Giovanna University Hospital, Sofia, Bulgaria (A.G.); The Heart Center, Rigshospitalet, Copenhagen University, Denmark (P.G.); Dept. of Cardiology, Rikshospitalet University Hospital, Oslo, Norway (L.G.); 3rd Dept. of Medicine, University General Hospital, Charles University, Prague, Czech Republic (J.H.); Szent Janos Hospital, Budapest, Hungary (A.J.), Dept. of Non-invasive Cardiovascular Diagnostics, University Hospital Bratislava, Bratislava, Slovakia (G.K.); Dept. of Cardiology, Pitie Salpetriere Hospital, University Pierre et Marie Curie, Paris (M.K.); Dept. of Heart Failure and Transplantology, Institute of Cardiology, Warsaw, Poland (J.K.); Div. of Cardiology, Dept of Medicine, Helsinki University Hospital, Helsinki, Finland (T.K.); Cardiology Division, Geneva University Hospital, Switzerland (F.M.); Myasnikov Card. Research Institute, Moscow, Russia (V.M.); Coronary, Care Unit, R.K. Khan Hospital, University of KwaZula Natal, Nelson R. Mandela School of Medicine, Durban, South Africa (N.R.); Dept. of Acute and Cardiovascular Medicine, Sahlgrenska University Hospital Östra, Göteborg University, Göteborg, Sweden (M.S.); Dept. of Cardiology, University Hospital Gasthuisberg, Leuven, Belgium (J.V.); Dept. of Cardiology, University Medical Centre Groningen, The Netherlands (D.J.v.H.).
Investigators: A list of all investigators is available in the online version of the CORONA Design and Baseline publication at doi:10.1016/j.ejheart.2005.09.005.
Data and Safety Monitoring Board: Henry Dargie, Scottish Advanced Heart Failure Service, Glasgow Royal Infirmary, Glasgow, Scotland (Chairman); David DeMets, Dept. of Biostatistics and Medical Informatics, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA (DSMB biostatistician); Rory Collins, Clinical Trial Service Unit, University of Oxford, Oxford, UK; Jan Feyzi, Dept. of Biostatistics and Medical Informatics, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA (SDAC biostatistician); Barry Massie, Veterans Affairs Medical Center and University of California San Francisco, San Francisco.
Independent Endpoint Committee: Bengt-Olov Fredlund, Dept. of Emergency and Cardiovascular Medicine, Sahlgrenska University Hospital Östra, Göteborg University, Göteborg, Sweden; Mikael Holmberg, Dept. of Cardiology, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden; Katarina Saldeen, Dept. of Cardiology, Dept. of Cardiology, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden; Ola Samuelsson (Secretary), Dept. of Nephrology, Dept. of Cardiology, Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden and Karl Swedberg, Dept. of Emergency and Cardiovascular Medicine, Sahlgrenska University Hospital Östra, Göteborg University, Göteborg, Sweden (Chairman).
Appendix 2
Unit costs for major cardiovascular hospitalizations and procedures (£sterling, 2005/06 values)
| Resource | Unit cost |
|---|---|
| Hospitalizations | |
| Heart failure | 1694 |
| Unstable angina (ischaemic heart disease) | 1005 |
| Acute MI | 1695 |
| Ischaemic stroke | 2433 |
| Haemorrhagic stroke | 2237 |
| Unclassifiable (non-transient) stroke | 2433 |
| Arrhythmia | 1072 |
| Peripheral vascular disease | 1313 |
| Syncope or collapse | 951 |
| Other cardiac procedures | 1256 |
| Procedures | |
| PCI | 3401 |
| CABG | 8333 |
| Cardiac transplant | 32 113 |
| Monitoring costs | |
| Liver test | 5 |
| General practice clinic visit | 31 |
| Investigational product | |
| Rosuvastatin (10 mg—28 day tab) | 18 |
Funding
The work was funded by a grant from AstraZeneca, who also sponsored the CORONA trial.
Conflict of interest: A.H.B., H.W., P.D., A.H., J.K., A.J., F.W., D.J.v.V., C.F., and J.J.V.M. report receiving consulting or advisory board fees from AstraZeneca; A.H., J.K., A.J., D.J.v.V., V.B., and J.J.V.M. report receiving lecture fees from AstraZeneca; P.K.L., A.H.B., A.H., D.J.v.V., V.B., and J.J.V.M. report receiving research grants from AstraZeneca; F.W. reports having an equity interest in AstraZeneca; and J.W. reports being previously employed as a senior medical advisor at AstraZeneca. No other potential conflict of interest is reported.
References
- 1.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, MacIntyre K, Capewell S, McMurray JJ. Heart failure and the aging population: an increasing burden in the 21st century? Heart. 2003;89:49–53. doi: 10.1136/heart.89.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJ. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail. 2002;4:361–371. doi: 10.1016/s1388-9842(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 5.Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R. Lifetime cost effectiveness of simvastatin in a range of risk groups and age groups derived from a randomised trial of 20,536 people. BMJ. 2006;333:1145. doi: 10.1136/bmj.38993.731725.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krum H, McMurray JJ. Statins and chronic heart failure: do we need a large-scale outcome trial? J Am Coll Cardiol. 2002;39:1567–1573. doi: 10.1016/s0735-1097(02)01827-2. [DOI] [PubMed] [Google Scholar]
- 7.Kjekshus J, Dunselman P, Blideskog M, Eskilson C, Hjalmarson A, McMurray JV, Waagstein F, Wedel H, Wessman P, Wikstrand J. A statin in the treatment of heart failure? Controlled rosuvastatin multinational study in heart failure (CORONA): study design and baseline characteristics. Eur J Heart Fail. 2005;7:1059–1069. doi: 10.1016/j.ejheart.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Jánosi A, Kamensky G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 9.Department of Health. NHS reference costs 2005–06. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_062884 .
- 10.The NHS Information Centre. Casemix Service. http://www.ic.nhs.uk/our-services/standards-and-classifications/casemix/hrg4 .
- 11.British Medical Association & Royal Pharmaceutical Society of Great Britain. British National Formulary. London: BMJ; 2006. [Google Scholar]
- 12.Fieller EC. Some problems in interval estimation. J R Stat Soc Series B-Statist Methodol. 1954;16:175–185. [Google Scholar]
- 13.van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ. 1994;3:309–319. doi: 10.1002/hec.4730030505. [DOI] [PubMed] [Google Scholar]
- 14.HM Treasury. The Green Book: Appraisal and Evaluation in Central Government. London: TSO; 2007. [Google Scholar]
- 15.Healthcare Commission. Getting results: pathology services in acute and specialist trusts. http://www.healthcarecommission.org.uk/_db/_documents/Pathology_final_tagged.pdf . [Google Scholar]
- 16.Netten A, Curtis L. Unit Costs of Health and Social Care 2006. Canterbury: Personal Social Services Research Unit, University of Kent; 2006. [Google Scholar]
- 17.McMurray JJ, Andersson FL, Stewart S, Svensson K, Solal AC, Dietz R, Vanhaecke J, van Veldhuisen DJ, Ostergren J, Granger CB, Yusuf S, Pfeffer MA, Swedberg K. Resource utilization and costs in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2006;27:1447–1458. doi: 10.1093/eurheartj/ehl016. [DOI] [PubMed] [Google Scholar]
- 18.Reed SD, Anstrom KJ, Bakhai A, Briggs AH, Califf RM, Cohen DJ, Drummond MF, Glick HA, Gnanasakthy A, Hlatky MA, O'Brien BJ, Torti FM, Tsiatis AA, Willan AR, Mark DB, Schulman KA. Conducting economic evaluations alongside multinational clinical trials: toward a research consensus. Am Heart J. 2005;149:434–443. doi: 10.1016/j.ahj.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R. Cost-effectiveness of simvastatin in people at different levels of vascular disease risk: economic analysis of a randomised trial in 20,536 individuals. Lancet. 2005;365:1779–1785. doi: 10.1016/S0140-6736(05)63014-0. [DOI] [PubMed] [Google Scholar]
- 20.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 21.Wedel H, McMurray JJ, Lindberg M, Wikstrand J, Cleland JG, Cornel JH, Dunselman P, Hjalmarson A, Kjekshus J, Komajda M, Kuusi T, Vanhaecke J, Waagstein F. Predictors of fatal and non-fatal outcomes in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): incremental value of apolipoprotein A-1, high-sensitivity C-reactive peptide and N-terminal pro B-type natriuretic peptide. Eur J Heart Fail. 2009;11:281–291. doi: 10.1093/eurjhf/hfn046. [DOI] [PMC free article] [PubMed] [Google Scholar]