Abstract
Aims
We evaluated cardiac magnetic resonance imaging (CMR) as a non-invasive test for cardiac allograft rejection.
Methods and results
We performed CMR on 50 heart-transplant recipients. Acute rejection was confirmed in 11 cases by endomyocardial biopsy (EMB) and presumed in 8 cases with a recent fall in left-ventricular ejection fraction (LVEF) not attributable to coronary allograft vasculopathy. Control patients had both normal LVEF and no significant rejection on EMB. Cardiac magnetic resonance imaging evaluated myocardial function, oedema, and early and late post-Gadolinium-DTPA contrast enhancement. Patients with confirmed rejection demonstrated elevated early relative myocardial contrast enhancement (4.1 ± 0.3 vs. 2.8 ± 0.2, P < 0.001) and a trend to higher oedema suggested by higher relative myocardial intensity on T2-weighted imaging compared to controls (2.1 ± 0.1 vs. 1.7 ± 0.1, P = 0.1). With rejection defined as increased early contrast enhancement or myocardial oedema, the sensitivity and specificity of CMR compared with EMB were 100 and 73%, respectively. Eight patients with presumed rejection also had elevated early myocardial contrast enhancement compared with controls, (8.7 ± 1.9 vs. 2.8 ± 0.2, P < 0.05), which reduced following increased immunosuppression (8.7 ± 1.9 vs. 4.6 ± 1.2, P < 0.05). In these patients LVEF improved following increased immunosuppression (32 ± 5 vs. 46 ± 5%, P < 0.05).
Conclusion
Cardiac magnetic resonance imaging is a promising modality for non-invasive detection of cardiac allograft rejection.
Keywords: Cardiac transplantation, Rejection, Magnetic resonance imaging
Introduction
Cardiac transplantation offers improved quality of life and survival in patients with advanced heart failure.1,2 An ongoing challenge in transplant medicine is the need to balance the risk of allograft rejection against the inherent risks of immunosuppressive therapy.3 Despite the development of more specific anti-rejection drugs,4 regular surveillance for acute rejection with endomyocardial biopsy (EMB), at least for the first 12 months post-transplant is the current standard of care.5 While conflicting data exist on the variability and accuracy of EMB,6 it remains the gold standard for the diagnosis of acute cellular rejection.
Recently the International Society for Heart and Lung Transplantation (ISHLT) has published revised guidelines,7 aimed at simplifying the evaluation of EMB as well as improving its efficacy. The new ISHLT grading system suggests four grades of acute cellular rejection: 0R (no rejection), 1R (mild rejection), 2R (moderate rejection), and 3R (severe rejection). While aggressive therapy with intravenous corticosteroids or other immunomodulatory agents is generally recommended for moderate rejection and above, mild rejection is usually managed conservatively, as the majority of such episodes resolve on follow-up EMB without increased immunosuppression.8 Despite this general consensus, interpretation of EMB findings can be problematic, especially in longer-term post-transplant patients in whom EMB is often suboptimal due to scarring from prior EMB procedures. In addition, EMB is an invasive procedure that has a risk of serious adverse events, including cardiac tamponade and death.9
A reliable non-invasive test for acute cellular rejection is therefore desirable not only in terms of reducing potential risks from EMB but also in its ability to complement current EMB regimes. Two important features discriminating moderate acute cellular rejection from lesser grades are the presence of multiple areas of myocardial necrosis as well as myocardial oedema. Cardiac magnetic resonance imaging (CMR) has been demonstrated in prior studies to identify both these features in the non-transplant population,10,11 with at least one study also demonstrating increased myocardial oedema in acute cardiac allograft rejection.12 Furthermore, multisequential CMR is an established non-invasive investigation for the diagnosis of acute myocarditis,13 a condition that has some histological similarity to acute cellular rejection.14
In this prospective study, we evaluated the efficacy of multisequential CMR in the diagnosis of acute cardiac allograft rejection.
Methods
Patient selection
Patients who had undergone cardiac transplantation were invited to participate in this study performed at the Alfred Hospital, Melbourne, Australia between June 2006 and November 2008. Patients were excluded if they had a known contra-indication to CMR (such as non-compatible metallic implant), claustrophobia, or were haemodynamically unstable. Patients with rapid atrial fibrillation as well as severe renal impairment (glomerular filtration rate <30 mL/min) were also excluded. All research was carried out with the approval of the Alfred Hospital Ethics Committee, and informed consent was obtained prior to patient enrolment.
Study patient groups
Nine patients had 11 cases of confirmed rejection (two patients had two separate episodes) defined by grade 2R or greater acute cellular rejection on EMB. One patient had two episodes of acute rejection 6 months apart; the second had two episodes of acute rejection 5 weeks apart. In both cases interval EMB showed resolution of acute rejection in between rejection episodes. Patients with presumed rejection (n = 8) had an unexplained fall in left-ventricular ejection fraction (LVEF) to below 40% with globally reduced function not attributable to cardiac allograft vasculopathy (CAV). In six patients EMB was performed but did not suggest significant rejection and in two patients EMB was not able to be performed due to technical factors. Patients with presumed rejection were treated with either intravenous methylprednisolone (six patients) or anti-thymocyte gammaglobulin (two patients) followed by increased maintenance immunosuppression. Improvement in LVEF was evaluated on follow-up CMR and CAV was excluded as a cause of graft dysfunction in all subjects in this group with coronary angiography. Control patients (n = 33) had a normal LVEF and all had undergone EMB, which demonstrated either no evidence of cellular rejection on EMB (grade 0) or mild cellular rejection (grade 1R). As the vast majority of grade 1R rejections are found to resolve on follow-up EMB without any specific intervention,8 and are therefore generally managed conservatively, we included these patients in the control group to evaluate the real world performance of CMR in the evaluation of acute rejection.
Control patients were scheduled for EMB in a routine setting for surveillance of rejection. Similarly, with respect to patients with confirmed rejection, one patient had presented with reduced LV function and EMB confirmed rejection, while the remainder had acute rejection diagnosed on routine surveillance biopsy. Conversely, all patients in the presumed rejection group had presented with an unexplained fall in LVEF, although none had acute heart failure at the time of CMR scanning.
Cardiac magnetic resonance protocol
We performed CMR on 50 heart transplant recipients on a clinical 1.5T CMR scanner (Signa HD 1.5T, GE Healthcare, Waukesha, WI, USA). All sequences, apart from early relative enhancement, were acquired during a breath-hold of 10–15 s. Patients in the acute rejection groups (either confirmed or presumed) all underwent CMR on the basis of clinical suspicion of acute rejection, and were enrolled at the time of CMR scanning. Patients in the control group underwent CMR scanning as part of our post-transplant clinical protocol, to monitor LVEF and record baseline CMR inflammatory measures. In the case of confirmed rejection, the mean time between EMB showing confirmed acute cellular rejection and CMR was 3.3 ± 1.2 days. All cases of presumed rejection and 8 out of 11 cases of confirmed rejection also underwent follow-up CMR scanning. This was performed following resolution of cellular rejection on repeat EMB in patients with confirmed rejection, and at least 3 weeks following treatment of presumed rejection. Analysis of CMR findings was performed on a dedicated CMR workstation by a CMR-trained clinician blinded to the biopsy data.
Assessment of left-ventricular size and function
Left-ventricular size and function were assessed by a balanced steady state free precession (FIESTA©, GE Healthcare, Waukesha WI, USA) pulse sequence (TR 3.8 ms, TE 1.6 ms, 30 phases, slice thickness 8 mm). All cine CMR sequences were performed in three standard short-axis slices (apical, mid, and basal), kept identical for each sequence throughout the CMR examination. In addition, long-axis slices corresponding to 2-, 3-, and 4-chamber views were acquired, planned from the short-axis slices. Left-ventricular volume and LVEF were calculated by the area–length method from biplane analysis (combining 4-chamber and 2-chamber views).
Cardiac magnetic resonance assessment of rejection
Early relative contrast enhancement was evaluated with an axial T1-weighted free-breathing spin echo sequence (repetition time 1R-to-R interval, echo time 20 ms, echo train length determined by R-to-R interval) applied for 4 min following a bolus Gadolinium-DTPA (0.1 mmol/kgBW Magnevist®, Schering, Germany). Four axial slices through the heart were acquired, and calculation of early relative contrast enhancement of the myocardium compared with skeletal muscle was performed as previously described.10 To reduce error, early relative contrast enhancement was calculated in all four axial slices and then averaged to obtain the final relative enhancement value.
Myocardial oedema was identified by increased relative signal intensity on a short inversion time inversion recovery (STIR) sequence. This sequence consisted of a black-blood, T2-weighted triple inversion recovery sequence (slice thickness 8 mm, repetition time 2xR-to-R interval; echo time 65 ms; inversion time 140 ms) using the body coil. Three standard short-axis slices identical to those described earlier were acquired. Relative myocardial STIR intensity was calculated on a slice by slice basis by dividing the signal intensity of myocardium by that of skeletal muscle, and then all three slices were averaged to obtain the final value.
Finally, late gadolinium-DTPA enhancement (LGE) was performed in the same short- and long-axis views as cine imaging 10 min after a second bolus of Gadolinium-DTPA (0.1 mmol/kgBW Magnevist®, Schering, Germany) using an inversion-recovery gradient echo technique (TR 7.1 ms; TE 3.1 ms; TI individually determined to null the myocardial signal, range 180–250 ms, slice thickness 8 mm, matrix 256 × 192, number of acquisitions = 2). The threshold for the presence of LGE was signal intensity greater than two standard deviations above that of remote myocardium.
Endomyocardial biopsy procedure, histological analysis, and management of acute rejection
Right-ventricular EMB was obtained by the transvenous right internal jugular approach. A minimum of four specimens were obtained for each patient, immediately fixed in formalin and then stained with haematoxylin and eosin. All specimens were reviewed by an expert pathologist who was blinded to clinical and CMR data and biopsies were then graded according to revised ISHLT guidelines. Routine assessment for humeral rejection was not performed.
Treatment of acute cellular rejection was at the discretion of the treating clinician. Typically, both confirmed and presumed rejection was treated with either an intravenous pulse of methylprednisolone or anti-thymocyte gammaglobulin, along with a subsequent increase in maintenance immunosuppressive therapy.
Statistics
All continuous data are expressed as mean ± standard error unless otherwise stated. Comparison between groups was made with Student's paired or unpaired t-tests or Mann Whitney rank sum test as appropriate, and comparisons of proportions were performed with Fisher's exact test. Comparison between multiple groups was made by ANOVA with post hoc analysis performed by the Student–Newman–Keuls Method. Diagnostic performance of CMR features of acute rejection was evaluated by calculating the area under the receiver operating characteristic (ROC) analysis curve, with selection of diagnostic thresholds that maximized diagnostic accuracy. For all comparisons, a two-tailed P-value <0.05 was considered significant. All calculations were made using a computerized statistical package (SPSS for Windows©, Release 15.0, SPSS, Inc.).
Results
Clinical and demographic characteristics
A total of 50 patients, including 17 patients with either confirmed or presumed rejection and 33 controls, underwent 68 CMR scans (Table 1). Patients in control and rejection groups were matched with respect to age and sex. The mean time post-heart transplantation was longer in the group with presumed rejection, perhaps indicative of a lower diagnostic efficacy of EMB in the late post-transplant period. As expected there was a lower mean LVEF in the presumed rejection group.
Table 1.
Clinical and demographic data
| Control (n = 33) | Confirmed rejection (n = 9) | Presumed rejection (n = 8) | |
|---|---|---|---|
| Age (years) | 50 ± 3 | 43 ± 6 | 45 ± 7 |
| Male sex (%) | 28/33 (85) | 8/9 (89) | 6/8 (75) |
| Days post-transplant | 802 ± 224 | 420 ± 261 | 2725 ± 528* |
| LVEF pre-treatment, % | 65 ± 1 | 56 ± 4 | 32 ± 5 |
| LVEF post-treatment, % | — | 56 ± 7 | 46 ± 5** |
| LVEDVI pre-treatment, mL | 85 ± 4 | 87 ± 10 | 109 ± 12 |
| LVEDVI post-treatment, mL | — | 82 ± 8 | 107 ± 16 |
P = NS for all comparisons unless otherwise stated.
*P = 0.007 compared with controls.
**P = 0.002 compared with pre-treatment LVEF.
Cardiac magnetic resonance changes in confirmed acute cellular rejection
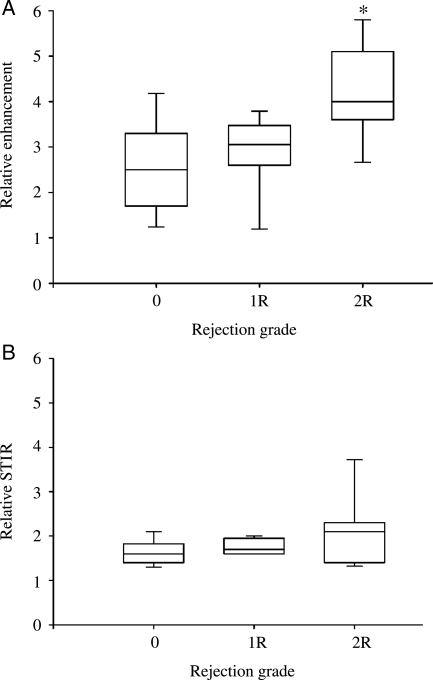
All patients with acute cellular rejection were grade 2R. There was a significant difference in early relative myocardial contrast enhancement according to biopsy grade (2.8 ± 0.3 in Grade 0 vs. 2.9 ± 0.3 in Grade 1R vs. 4.1 ± 0.3 in Grade 2R, P = 0.01 by ANOVA; Figure 1A). Post hoc analysis revealed significant differences between Grade 2R cases and both Grade 0 and Grade 1R cases (P < 0.05 for both comparisons). Compared with controls (Grades 0 and 1R), patients with confirmed rejection had significantly elevated early relative myocardial contrast enhancement (4.1 ± 0.3 for confirmed rejection vs. 2.8 ± 0.2 for controls, P = 0.001). As patients with confirmed rejection were almost exclusively within their first 15 months post-transplantation, we compared this group to a subgroup of controls (n = 23) also within 15 months post-transplantation and found a similar difference in early relative myocardial contrast enhancement (4.1 ± 0.3 for confirmed rejection vs. 3.0 ± 0.2 for time-matched controls, P = 0.007). There were no significant differences in relative STIR signal intensity across rejection grades (1.6 ± 0.1 vs. 1.8 ± 0.1 vs. 2.1 ± 0.2, P = NS; Figure 1B) although there was a non-significant trend to higher relative signal intensity in confirmed rejection compared to controls (2.1 ± 0.2 vs. 1.7 ± 0.1, P = 0.1). The patterns of increased signal intensity on early relative enhancement and STIR imaging, when present, were diffuse as previously described in patients with myocarditis.10
Figure 1.
Cardiac magnetic resonance in confirmed rejection. Box and whisker plots of early relative myocardial contrast enhancement (Relative enhancement, A) and myocardial oedema (Relative short inversion time inversion recovery, B) in patients with Grade 0, 1R, and 2R rejection (*P < 0.05 compared with Grade 0 and 1R by ANOVA).
Diagnostic performance of cardiac magnetic resonance in acute cellular rejection
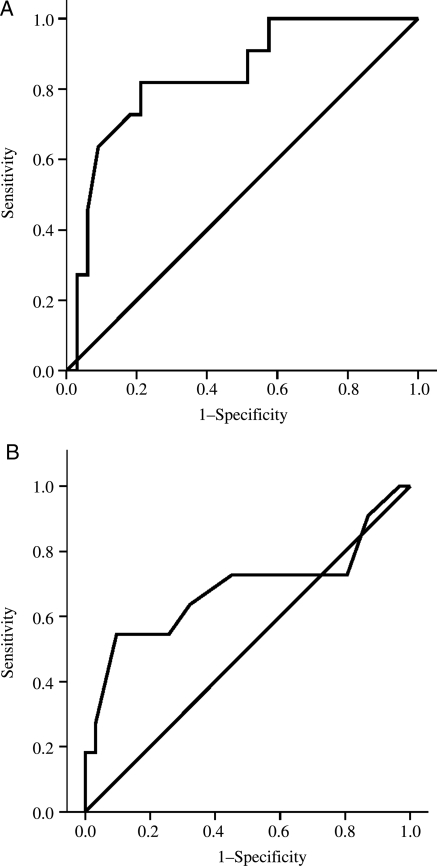
For early myocardial enhancement the area under the ROC analysis curve was 0.84 for the diagnosis of acute rejection (Figure 2A), and a cut-off of 3.5 yielded a sensitivity and specificity for confirmed rejection of 82% and 79%, respectively. The presence of elevated relative STIR intensity (>2.0) yielded only a modest area under the ROC analysis curve of 0.68 (Figure 2B), with a low sensitivity of 55% but a specificity of 90% for the diagnosis of acute rejection. A combined CMR criteria for acute rejection, based on the presence of either elevated early relative myocardial contrast enhancement (>3.5) or increased relative STIR intensity (>2.0) yielded and sensitivity and specificity for confirmed rejection of 100% and 73%, respectively. LGE was present in two out of 11 (18%) cases of confirmed rejection and 3/33 (9%) controls occurring in a patchy, mid-wall distribution, predominantly at the junction of the left and right ventricles (Figure 3A). There was no significant increase in the incidence of LGE in confirmed rejection compared with controls (P = NS, Fisher's exact test), and adding the presence of LGE into the CMR diagnostic criteria for acute rejection did not improve the diagnostic performance.
Figure 2.
Diagnostic performance of cardiac magnetic resonance. Receiver operating characteristic analysis curves representing the diagnostic performance of early relative myocardial contrast enhancement (Relative enhancement, A) and myocardial oedema (Relative short inversion time inversion recovery, B) in the diagnosis of confirmed acute cellular rejection.
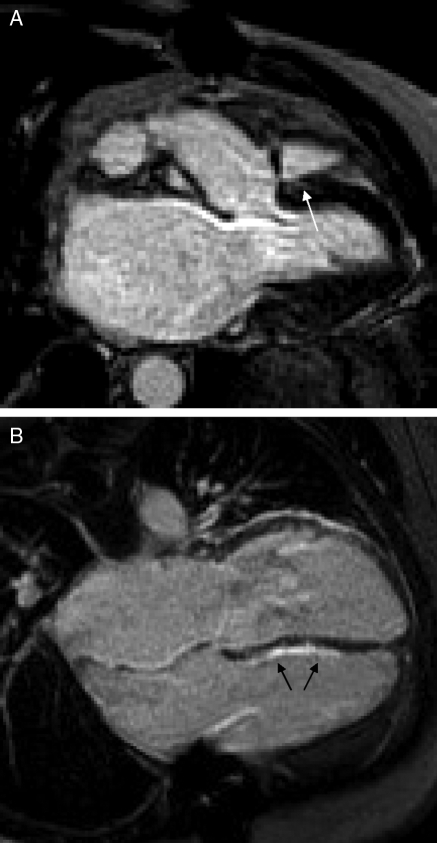
Figure 3.
Late gadolinium-DTPA enhancement images in cardiac transplantation. There is a small area of late gadolinium enhancement in the region of the right and left-ventricular junction of a control patient (A, white arrow). A long-term post-transplant patient with a non-diagnostic endomyocardial biopsy but presumed rejection has a large amount of late gadolinium enhancement in the right-ventricular aspect of the interventricular septum (B, black arrows). The area of bright signal intensity adjacent to the left-ventricular wall clearly extends across the atrioventicular groove, and is likely to represent epicardial fat.
Cardiac magnetic resonance changes in presumed rejection
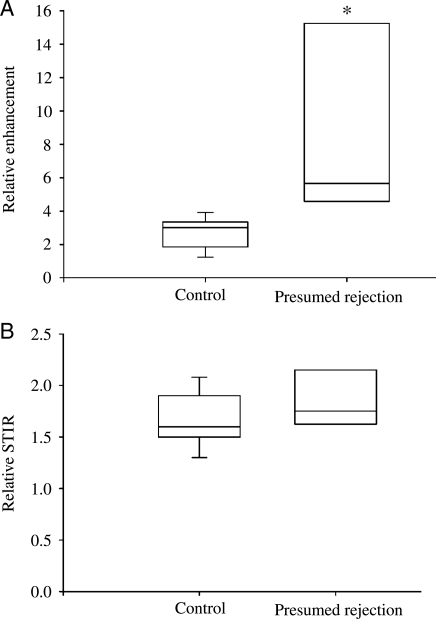
Eight heart-transplant patients developed significant LV dysfunction during routine clinical surveillance and subsequently underwent CMR scanning. All patients underwent coronary angiography as part of a yearly or 2 yearly surveillance programme. The mean time between coronary angiography and CMR scanning was 244 ± 90 days. Four patients did not have a recent (within 6 months) coronary angiogram. Of these, one had a dobutamine stress echo that was negative for ischaemia, and one had coronary angiography after CMR scanning that showed no significant disease. In the remaining two patients, the LV function normalized after increased immunosuppression, so repeat coronary angiography was not performed. CMR confirmed significant LV dysfunction, which was global in all cases (mean LVEF 32 ± 5%). Relative early myocardial contrast enhancement was elevated compared with controls (8.7 ± 1.9 vs. 2.8 ± 0.2, P=0.02; Figure 4A). Importantly, all patients with presumed rejection had relative myocardial enhancement above the threshold for acute rejection of 3.5 as defined earlier. As patients with presumed rejection were longer-term transplant patients (mean time post transplant of 2725 ± 528 days), we compared this group of patients to a cohort of longer-term (>15 months post-transplant) control patients (n = 9, mean time post-transplant 2625 ± 390 days) and found a similar difference in early relative myocardial contrast enhancement (8.7 ± 1.9 in presumed rejection vs. 1.8 ± 0.1 in time-matched controls, P = 0.01). Again there was only a non-significant trend towards higher relative STIR intensity in these patients (1.9 ± 0.1 vs. 1.7 ± 0.1, P = 0.2; Figure 4B). There was no correlation between STIR intensity and the degree of LVEF reduction, nor was there any correlation between the change in LVEF and the change in STIR intensity on repeat CMR examination. All eight patients with presumed rejection had LGE on CMR scanning, which was a significantly higher incidence than that of controls (P < 0.001, Fisher's exact test). The pattern of LGE was predominantly involving the right ventricular aspect of the interventricular septum (Figure 3B). This is perhaps not surprising, as multiple prior EMB procedures in these patients could be expected to result in endocardial fibrosis in this region.
Figure 4.
Cardiac magnetic resonance in presumed rejection. Box and whisker plots of early relative myocardial contrast enhancement (Relative enhancement, A) and myocardial oedema (Relative short inversion time inversion recovery, B) in patients with presumed rejection compared with controls (*P < 0.05).
Follow-up cardiac magnetic resonance scanning in confirmed and presumed rejection
The mean time between initial and follow-up CMR scanning in patients with confirmed rejection was 49 ± 8 days, with all patients demonstrating either Grade 0 or Grade 1R on EMB. Following increased immunosuppressive therapy, these patients demonstrated a significant reduction in early relative myocardial contrast enhancement (4.1 ± 0.3 pre-treatment vs. 2.9 ± 0.6 post-treatment, P < 0.05). There was a non-significant trend to reduced myocardial oedema following treatment (relative STIR intensity of 2.1 ± 0.1 pre-treatment vs. 1.7 ± 0.1 post-treatment, P = 0.3). There was no change in LVEF following treatment (58 ± 3% pre-treatment vs. 60 ± 4% post-treatment, P = NS).
Follow-up CMR scanning was performed in patients with presumed rejection a mean time of 91 ± 21 days after the initial CMR scan. Following increased immunosuppressive therapy there was a significant improvement in LVEF (32 ± 5 vs. 46 ± 5%, P = 0.002). Early relative myocardial contrast enhancement was reduced on follow-up CMR scanning (8.7 ± 1.9 vs. 4.6 ± 1.2, P = 0.02), but there was no change in myocardial relative myocardial STIR intensity on follow-up CMR scanning (1.9 ± 0.1 pre-treatment vs. 2.2 ± 0.2 post-treatment, P = NS).
Discussion
In this prospective study, we utilized multisequential CMR in the non-invasive diagnosis of acute rejection in cardiac transplant recipients. In patients with a diagnostic EMB, CMR was 100% sensitive for the presence of acute cellular rejection, with a specificity of 73%. In patients with non-diagnostic biopsies but presumed rejection based on clinical findings, we observed similar CMR findings to those in patients with confirmed rejection. Following increased immunosuppression in this group, there was significant improvement in LVEF, supporting the clinical diagnosis of presumed rejection. Our data therefore suggest that CMR may be of clinical benefit not only with respect to its potential to reduce the number of surveillance EMB procedures performed in patients following cardiac transplantation, but also as a diagnostic test in patients with an unexplained fall in LVEF in whom EMB is unavailable or non-diagnostic.
The most prominent CMR feature in those patients with confirmed rejection in our study was the presence of an elevated early relative myocardial contrast enhancement. Prior studies in acute myocarditis have demonstrated similar elevations in early relative myocardial contrast enhancement,10 with a comparable threshold for the diagnosis of acute myocarditis.13 While the mechanisms of elevated early relative myocardial contrast enhancement in myocarditis are incompletely defined, it is believed that expansion of the extracellular space secondary to acute necrosis and oedema, leading to contrast accumulation is a significant factor.15 As both necrosis and oedema are histological features of worsening degrees of acute cellular rejection on EMB,7 it is likely that the same mechanisms observed in acute myocarditis would contribute to the CMR changes we observed in acute cellular rejection. Increased myocardial uptake of contrast in the presence of myocardial necrosis has been recognized in heart-transplant recipients with acute rejection,16 although no study to our knowledge has evaluated the diagnostic performance of a multisequential CMR approach for the diagnosis of acute cardiac allograft rejection. On its own, an elevated early relative myocardial contrast enhancement above 3.5 yielded a sensitivity for the diagnosis of acute cellular rejection of 82%; when the presence of elevated relative intensity on T2-weighted (STIR) imaging was included in the CMR-based diagnostic criteria the sensitivity improved to 100%, with a minimal reduction in specificity. This compares favourably with other non-invasive methods for the detection of acute cellular rejection, including signal-averaged electrocardiography,17 echocardiography,18 and gene expression profiling,19 which have demonstrated sensitivities for the diagnosis of acute cardiac allograft rejection ranging from 70 to 90%.
Previously, higher levels of myocardial oedema suggested by T2 weighted imaging have been demonstrated in acute rejection in cardiac transplant recipients.12 Although we did not observe a significant increase in overall myocardial oedema in patients with cellular rejection, the diagnostic performance of CMR improved when the presence of elevated relative STIR intensity was included in the diagnostic criteria. From our data it would appear that the presence of myocardial oedema is a specific rather than a sensitive criterion for acute cellular rejection, perhaps consistent with its association with the most severe end of the spectrum of acute rejection.7 The use of a multisequential approach has previously been advocated in the CMR diagnosis of acute myocarditis, where the diagnostic performance of CMR is improved when oedema (STIR) and LGE imaging is added to post-contrast imaging.13 However, in contrast to myocarditis we did not find the presence of LGE a useful discriminator in the diagnosis of acute rejection. This is perhaps reflective of the microscopic size of areas of necrosis in acute cellular rejection on EMB, which would be too small to demonstrate on LGE imaging.
A major diagnostic dilemma in patients who are several years post-cardiac transplantation is the observation of an unexplained fall in LVEF in combination with a non-diagnostic EMB. In this group of patients EMB is hindered by the presence of endomyocardial fibrosis from prior biopsy sites and central venous access is often more difficult as a consequence of multiple prior cannulations. In addition, diffuse myocardial fibrosis is common in longer-term heart-transplant recipients,20 making acquisition of good samples of myocardium difficult. The development of a non-invasive test for acute rejection would therefore be particularly useful in the evaluation of such patients. Furthermore, the spatial resolution achieved with CMR cine imaging permits highly accurate assessment of ventricular volume and function. While nuclear cardiac gated blood pool imaging can also provide an accurate measure of LVEF, and is often used in routine screening for cardiac allograft dysfunction, the lack of ionizing radiation with CMR is an additional benefit in patients on long-term immunosuppressive therapy.
We did not include patients with suspected humoral rejection in our study, largely due to the difficulty in definitively diagnosing this clinical entity, as well as its relatively low incidence.21,22 It could be postulated that CMR might be useful in the diagnosis of humoral rejection, as myocardial oedema can be assessed with STIR imaging. However in our study we observed no correlation between the degree of LVEF reduction and STIR intensity in patients with presumed rejection, nor was there a correlation between the change in LVEF and change in STIR intensity on repeat CMR examination. Our study was not designed to evaluate changes in CMR STIR intensity in patients with humoral rejection, and further study focusing on this specific question is warranted. Although humoral rejection cannot be excluded as a cause for the reduction in LVEF in our cohort with presumed rejection, changes suggestive of humoral rejection were not present on EMB. In patients with presumed rejection, we also do not have a gold standard for acute rejection as such patients in our study were defined in part by the presence of a non-diagnostic biopsy. In addition, while all underwent surveillance coronary angiography, in some cases no recent angiographic data was available. While a number of factors may have contributed to the reduction in LVEF in patients with presumed rejection, these patients observed similar CMR changes to those with biopsy-proven cell-mediated rejection, which subsequently improved along with LVEF following increased immunosuppression targeted at cell-mediated rejection. Therefore the most likely explanation is that of unrecognized cell-mediated rejection.
Our data provide strong evidence for the utility of multisequential CMR in the non-invasive diagnosis of acute cellular rejection in cardiac transplant recipients. While invasive EMB should retain its place in the frontline of surveillance for cardiac allograft rejection, the integration of CMR into this process can help to reduce patient risk and discomfort as well as provide complementary information, especially in circumstances where EMB may be non-diagnostic.
Funding
This work was supported by an Institute Grant from the National Health and Medical Research Council of Australia as well as a Roche Cellcept Australia Research Grant. A.J.T. is supported by a National Heart Foundation Grant, Melbourne, Australia. G.V. is supported by a National Health and Medical Research Council/National Heart Foundation Post Graduate Research Scholarship, Melbourne, Australia. L.I. is supported by a National Health and Medical Research Council Post Graduate Research Scholarship, Melbourne, Australia. H.P. is supported by a research grant from Swiss National Science Foundation SNSF, Bern, Switzerland. D.M.K. is the recipient of a Wellcome Trust Senior Research Fellowship. J.C. is an employee of GE Healthcare Australia.
Conflict of interest: none declared.
Acknowledgements
This work was performed at the Alfred Heart Centre and Baker IDI Heart and Diabetes Research Institute, Melbourne, Australia.
References
- 1.Fisher DC, Lake KD, Reutzel TJ, Emery RW. Changes in health-related quality of life and depression in heart transplant recipients. J Heart Lung Transplant. 1995;14:373–381. [PubMed] [Google Scholar]
- 2.Fraund S, Pethig K, Franke U, Wahlers T, Harringer W, Cremer J, Fieguth HG, Oppelt P, Haverich A. Ten year survival after heart transplantation: palliative procedure or successful long term treatment? Heart. 1999;82:47–51. doi: 10.1136/hrt.82.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenfeld J, Miller GG, Shakar SF, Zolty R, Lowes BD, Wolfel EE, Mestroni L, Page RL, II, Kobashigawa J. Drug therapy in the heart transplant recipient: part I: cardiac rejection and immunosuppressive drugs. Circulation. 2004;110:3734–3740. doi: 10.1161/01.CIR.0000149745.83186.89. [DOI] [PubMed] [Google Scholar]
- 4.Hosenpud JD, Novick RJ, Breen TJ, Keck B, Daily P. The Registry of the International Society for Heart and Lung Transplantation: twelfth official report–1995. J Heart Lung Transplant. 1995;14:805–815. [PubMed] [Google Scholar]
- 5.White JA, Guiraudon C, Pflugfelder PW, Kostuk WJ. Routine surveillance myocardial biopsies are unnecessary beyond one year after heart transplantation. J Heart Lung Transplant. 1995;14:1052–1056. [PubMed] [Google Scholar]
- 6.Winters GL, McManus BM. Consistencies and controversies in the application of the International Society for Heart and Lung Transplantation working formulation for heart transplant biopsy specimens. Rapamycin Cardiac Rejection Treatment Trial Pathologists. J Heart Lung Transplant. 1996;15:728–735. [PubMed] [Google Scholar]
- 7.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Winters GL, Loh E, Schoen FJ. Natural history of focal moderate cardiac allograft rejection. Is treatment warranted? Circulation. 1995;91:1975–1980. doi: 10.1161/01.cir.91.7.1975. [DOI] [PubMed] [Google Scholar]
- 9.Baraldi-Junkins C, Levin HR, Kasper EK, Rayburn BK, Herskowitz A, Baughman KL. Complications of endomyocardial biopsy in heart transplant patients. J Heart Lung Transplant. 1993;12:63–67. [PubMed] [Google Scholar]
- 10.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–1809. doi: 10.1161/01.cir.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 12.Marie PY, Angioi M, Carteaux JP, Escanye JM, Mattei S, Tzvetanov K, Claudon O, Hassan N, Danchin N, Karcher G, Bertrand A, Walker PM, Villemot JP. Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black-blood magnetic resonance imaging sequence. J Am Coll Cardiol. 2001;37:825–831. doi: 10.1016/s0735-1097(00)01196-7. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 14.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ, Jr, Olsen EG, Schoen FJ, Myocarditis A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 15.Saeed M, Wendland MF, Takehara Y, Masui T, Higgins CB. Reperfusion and irreversible myocardial injury: identification with a nonionic MR imaging contrast medium. Radiology. 1992;182:675–683. doi: 10.1148/radiology.182.3.1535880. [DOI] [PubMed] [Google Scholar]
- 16.Almenar L, Igual B, Martinez-Dolz L, Arnau MA, Osa A, Rueda J, Palencia M. Utility of cardiac magnetic resonance imaging for the diagnosis of heart transplant rejection. Transplant Proc. 2003;35:1962–1964. doi: 10.1016/s0041-1345(03)00653-5. [DOI] [PubMed] [Google Scholar]
- 17.Valentino VA, Ventura HO, Abi-Samra FM, Van Meter CH, Price HL. The signal-averaged electrocardiogram in cardiac transplantation. A noninvasive marker of acute allograft rejection. Transplantation. 1992;53:124–127. doi: 10.1097/00007890-199201000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Vivekananthan K, Kalapura T, Mehra M, Lavie C, Milani R, Scott R, Park M. Usefulness of the combined index of systolic and diastolic myocardial performance to identify cardiac allograft rejection. Am J Cardiol. 2002;90:517–520. doi: 10.1016/s0002-9149(02)02525-0. [DOI] [PubMed] [Google Scholar]
- 19.Deng MC, Eisen HJ, Mehra MR, Billingham M, Marboe CC, Berry G, Kobashigawa J, Johnson FL, Starling RC, Murali S, Pauly DF, Baron H, Wohlgemuth JG, Woodward RN, Klingler TM, Walther D, Lal PG, Rosenberg S, Hunt S. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006;6:150–160. doi: 10.1111/j.1600-6143.2005.01175.x. [DOI] [PubMed] [Google Scholar]
- 20.Winters GL, Costanzo-Nordin MR. Pathological findings in 2300 consecutive endomyocardial biopsies. Mod Pathol. 1991;4:441–448. [PubMed] [Google Scholar]
- 21.Hammond EH, Yowell RL, Nunoda S, Menlove RL, Renlund DG, Bristow MR, Gay WA, Jr, Jones KW, O'Connell JB. Vascular (humoral) rejection in heart transplantation: pathologic observations clinical implications. J Heart Transplant. 1989;8:430–443. [PubMed] [Google Scholar]
- 22.Michaels PJ, Espejo ML, Kobashigawa J, Alejos JC, Burch C, Takemoto S, Reed EF, Fishbein MC. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]