Abstract
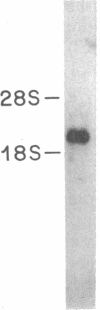
The 67-kDa calelectrin is the largest member of a family of Ca2+-binding proteins that associate with membranes and phospholipids in a Ca2+-dependent manner. Oligonucleotide probes based on peptide sequences obtained from purified bovine 67-kDa calelectrin were used to screen a human retina cDNA library, and the complete primary structure of human 67-kDa calelectrin was deduced by DNA sequence analysis. The protein consists of eight 68-amino acid repeats separated by linking sequences of variable lengths. It is highly similar to the human lipocortin I and II sequences, each of which contains four such repeats. The amino termini of the three proteins show no sequence similarity; however, in the repeated regions the proteins are 42-45% identical in sequence. Analysis of the 16 repeats from the three proteins provides insights into the structural basis for Ca2+-dependent phospholipid binding. These data place the calelectrins and the lipocortins into the same gene family and suggest that these proteins have similar functions and have evolved from a common ancestor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz C. E., Zaks W. J., Hamman H. C., Crane S., Martin W. H., Gould K. L., Oddie K. M., Parsons S. J. Identification of chromaffin granule-binding proteins. Relationship of the chromobindins to calelectrin, synhibin, and the tyrosine kinase substrates p35 and p36. J Biol Chem. 1987 Feb 5;262(4):1860–1868. [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- De B. K., Misono K. S., Lukas T. J., Mroczkowski B., Cohen S. A calcium-dependent 35-kilodalton substrate for epidermal growth factor receptor/kinase isolated from normal tissue. J Biol Chem. 1986 Oct 15;261(29):13784–13792. [PubMed] [Google Scholar]
- Geisow M. J., Fritsche U., Hexham J. M., Dash B., Johnson T. A consensus amino-acid sequence repeat in Torpedo and mammalian Ca2+-dependent membrane-binding proteins. Nature. 1986 Apr 17;320(6063):636–638. doi: 10.1038/320636a0. [DOI] [PubMed] [Google Scholar]
- Gerke V., Weber K. Calcium-dependent conformational changes in the 36-kDa subunit of intestinal protein I related to the cellular 36-kDa target of Rous sarcoma virus tyrosine kinase. J Biol Chem. 1985 Feb 10;260(3):1688–1695. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Boudreau M., Galyean R., Hunter T., Tack B. Association of the S-100-related calpactin I light chain with the NH2-terminal tail of the 36-kDa heavy chain. J Biol Chem. 1986 Aug 15;261(23):10485–10488. [PubMed] [Google Scholar]
- Huang K. S., Wallner B. P., Mattaliano R. J., Tizard R., Burne C., Frey A., Hession C., McGray P., Sinclair L. K., Chow E. P. Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp60v-src and of the epidermal growth factor receptor/kinase. Cell. 1986 Jul 18;46(2):191–199. doi: 10.1016/0092-8674(86)90736-1. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Creutz C. E. Cell biology. Consensus in exocytosis. Nature. 1986 Apr 17;320(6063):573–573. doi: 10.1038/320573a0. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Alu-Alu recombination deletes splice acceptor sites and produces secreted low density lipoprotein receptor in a subject with familial hypercholesterolemia. J Biol Chem. 1987 Mar 5;262(7):3354–3361. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moore P. B. 67 kDa calcimedin, a new Ca2+-binding protein. Biochem J. 1986 Aug 15;238(1):49–54. doi: 10.1042/bj2380049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. J., Gallagher C. J., Crumpton M. J. Cellular distribution of p68, a new calcium-binding protein from lymphocytes. EMBO J. 1984 May;3(5):945–952. doi: 10.1002/j.1460-2075.1984.tb01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris C. J., Tack B. F., Kristensen T., Glenney J. R., Jr, Hunter T. The cDNA sequence for the protein-tyrosine kinase substrate p36 (calpactin I heavy chain) reveals a multidomain protein with internal repeats. Cell. 1986 Jul 18;46(2):201–212. doi: 10.1016/0092-8674(86)90737-3. [DOI] [PubMed] [Google Scholar]
- Shadle P. J., Gerke V., Weber K. Three Ca2+-binding proteins from porcine liver and intestine differ immunologically and physicochemically and are distinct in Ca2+ affinities. J Biol Chem. 1985 Dec 25;260(30):16354–16360. [PubMed] [Google Scholar]
- Silva F. G., Sherrill K., Spurgeon S., Südhof T. C., Stone D. K. High-level expression of the 32.5-kilodalton calelectrin in ductal epithelia as revealed by immunocytochemistry. Differentiation. 1986;33(2):175–183. doi: 10.1111/j.1432-0436.1986.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. Calelectrins are a ubiquitous family of Ca2+-binding proteins purified by Ca2+-dependent hydrophobic affinity chromatography by a mechanism distinct from that of calmodulin. Biochem Biophys Res Commun. 1984 Aug 30;123(1):100–107. doi: 10.1016/0006-291x(84)90385-1. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Ebbecke M., Walker J. H., Fritsche U., Boustead C. Isolation of mammalian calelectrins: a new class of ubiquitous Ca2+-regulated proteins. Biochemistry. 1984 Mar 13;23(6):1103–1109. doi: 10.1021/bi00301a010. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C., Stone D. K. Purification of calelectrins. Methods Enzymol. 1987;139:30–35. doi: 10.1016/0076-6879(87)39072-x. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Walker J. H., Fritsche U. Characterization of calelectrin, a Ca2+-binding protein isolated from the electric organ of Torpedo marmorata. J Neurochem. 1985 Apr;44(4):1302–1307. doi: 10.1111/j.1471-4159.1985.tb08757.x. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Walker J. H., Obrocki J. Calelectrin self-aggregates and promotes membrane aggregation in the presence of calcium. EMBO J. 1982;1(10):1167–1170. doi: 10.1002/j.1460-2075.1982.tb00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner B. P., Mattaliano R. J., Hession C., Cate R. L., Tizard R., Sinclair L. K., Foeller C., Chow E. P., Browing J. L., Ramachandran K. L. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]