Abstract
Indole-3-carbinol and its diindole condensation product 3-3′-diindolylmethane are dietary phytochemicals that have striking anticarcinogenic properties in human cancer cells. Molecular, cellular, physiological, and clinical studies have documented that both indole-3-carbinol and 3-3′-diindolylmethane have potent endocrine modulating activities through a myriad of mechanisms. The focus of this review is to discuss the evidence that directly links the anticancer actions of these two indole compounds to the control of steroid receptor and growth factor receptor signaling.
This minireview discusses recent evidence uncovering new mechanistic insights by which the anti-cancer dietary phytochemicals indole-3-carbinol and 3-3′-diindolylmethane mediate their potent endocrine modulating activities.
The selective modulation of cellular cascades triggered by nuclear and cell surface receptor signaling is integrally linked to the ability of cancer cells to maintain their growth and cell survival properties and to be resistant to apoptotic signals. For human cancers that directly depend upon activated steroid hormone and/or growth factor receptor systems for their proliferation, anticancer therapeutic strategies have been developed that directly target specific receptors or downstream signaling components. Notable examples include the treatment of steroid-sensitive reproductive cancer cells with steroid receptor antagonists or selective modulators (tamoxifen for breast cancer and flutamide for prostate cancer), and the development of therapeutics designed to disrupt growth factor receptor-activated signal transduction components, such as those used to inhibit ErbB2 tyrosine kinase activity (1,2).
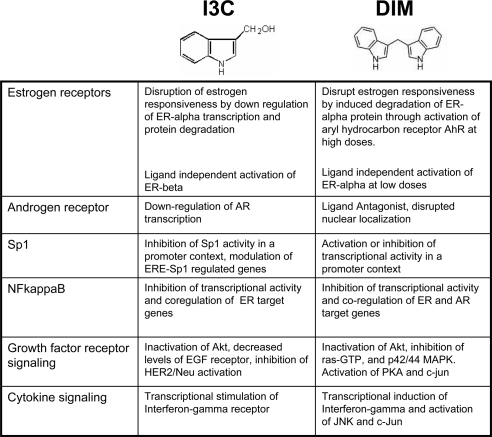
Epidemiological and physiological studies suggest that phytochemicals from vegetables and fruits represent a natural source of potential anticancer molecules (3) and that many of these bioactive phytochemicals also represent new classes of endocrine disruptors with a wide range of specific effects that depend upon the cellular phenotype and tissue origin. Two such phytochemicals are indole-3-carbinol (I3C), a natural compound derived by hydrolysis from glycobrassicin produced in Brassica cruciferous vegetables such as cabbage, broccoli, and Brussels sprouts, and its natural diindole condensation product 3–3′-diindolylmethane (DIM). The structures of I3C and DIM are shown in Fig. 1. Both indoles are promising chemotherapeutic and chemopreventative agents, and they exhibit potent anticarcinogenic properties in a wide range of human cancers such as lung, liver, colon, cervical, endometrial, prostate, and breast cancer (4,5,6,7). Pharmacokinetics studies, mostly with animal models, show that I3C and its bioactive acid condensation products DIM, indolo[3,2-b]carbazole, and 1-(3-hydroxymethyl)indolyl-3-indolylmethane are absorbed from the plasma within 1 h and a mixture of indoles accumulate in a variety of indole-sensitive tissues with the highest level in the liver (4). The accumulated tissue levels are high enough to activate the aryl hydrocarbon receptor and alter estrogen metabolism (4), suggesting that the organ-attainable level of specific indoles can account for their overall in vivo anticancer effects.
Figure 1.
Chemical structures of I3C and DIM and summary of their key modulatory effects on hormone responsiveness and receptor signaling in human cancer cells. EGF, Epidermal growth factor.
Emerging evidence has shown that the direct exposure of human cancer cells to I3C or DIM trigger distinct and complementary sets of transcriptional, cell signaling, enzymatic, and metabolic cascades that directly lead to the cell cycle arrest and/or apoptosis (4,6,8). Through this pleiotropic response, both indoles have potent effects in tumorigenic cells from many tissue types that include both steroid-sensitive and insensitive phenotypes that are representative of early- and late-stage reproductive cancers (5,6,8). Within the context of this dynamic antiproliferative environment, I3C and DIM can either inhibit or enhance the effectiveness of nuclear and growth factor receptor cascades (summarized in Fig. 1) in hormone-responsive cancers, suggesting that both indoles represent unique types of endocrine modulators.
I3C Modulation of Estrogen Receptor (ER) Expression and Signaling
There is compelling evidence, especially in human reproductive cancer cells, that I3C treatment selectively modulates ER expression or activity. A critical issue in understanding the ability of I3C to disrupt estrogen signaling is that estrogens mediate their cellular responses through two distinct intracellular receptors that are encoded by separate genes (9), ER-α and ER-β. In the cell systems tested to date, after activation by ligand binding, each ER regulates the transcription of unique but overlapping sets of target genes (10), although both receptors are capable of activating synthetic reporter plasmids driven by estrogen response elements (EREs). Each receptor subtype has distinct cellular and physiological functions and in human reproductive cancer cells, and a high ER-α:ER-β ratio correlates well with enhanced cellular proliferation, whereas predominance of functional ER-β over ER-α is associated with lower levels of proliferation (11,12,13). High levels of ER-β can antagonize ER-α signaling (14).
Several groups observed that treatment of human cancer cells with I3C disrupted estrogen responsiveness, which eventually led to the discovery that the indole reversal of estrogen-induced mitogenesis can be linked to a robust down-regulation of functional ER-α expression as well as the activation of ER-β. In human breast cancer cell lines that are estrogen sensitive, such as MCF-7 and T47D cells, I3C strongly down-regulated estrogen-induced proliferation, prevented the induction of estrogen-simulated target genes such as progesterone receptor, as well as inhibited the estrogen-induced reporter activities driven by an estrogen response element or ERE (15). Furthermore, I3C cooperates with tamoxifen to more effectively induce a G1 cell cycle arrest and ablated phosphorylation of the retinoblastoma protein (16). One study demonstrated that small interfering RNA knockdown of the breast cancer susceptibility gene-1 (BRCA1) in breast cancer cells attenuated the I3C-mediated cytoxicity and indole inhibition of estrogen-stimulated ER activity, whereas I3C treatment prevented the estrogen-induced BRCA1-dependent cell migration (17). I3C has also been shown to reverse estrogen-mediated cell survival responses. For example, in human cervical cancer cells, I3C disrupted the estrogen-mediated protection to genotoxic agents such as UVB, mitomycin C, and cisplatin (18), and also reversed the estrogen-induced expression of Bcl2 as part of its proapoptic response (19).
I3C does not bind to either ER subtype in MCF7 cells (16), and current evidence points to the loss of ER-α expression as the mechanism by which this indole ablates ER ERE-reporter plasmid activity and inhibits estrogen-dependent growth (15,20). In human breast cancer cells, I3C treatment down-regulates ER-α protein and transcript levels, which fits with an earlier observation that indole treatment decreased the level of phosphorylated ER-α although the total receptor levels were not reported (21). In transient transfections of human breast cancer cells, I3C strongly attenuated ER-α gene (esr1) promoter activity (15), although the precise indole-regulated transcription factors that mediate this response were not identified. It is known that activated ER-α protein autoregulates promoter activity of esr1 through the GATA family of transcription factors (22), which suggests that I3C could potentially ablate ER-α expression by disrupting this regulatory loop. Indeed, we have recently observed that I3C induces ER-α protein ubiquitination and degradation in a process that requires the aryl hydrocarbon receptor (AhR) and which leads to the loss of GATA3 expression and, in turn, down modulates esr1 promoter activity (Marconett, C. N., S. N. Sundar, K. M. Poindexter, T. R. Stueve, L. F. Bjeldanes, and G. L. Firestone, manuscript submitted). AhR is a member of the nuclear receptor gene family that binds to a wide range of ligands, including DIM, and plays a critical role in the phytochemical induced degradation of ER-α protein (23,24).
An intriguing effect of I3C in human breast cancer cells is the ligand-independent activation of ER-β under the antiproliferation conditions in which ER-α expression is inhibited (15). I3C was shown to increase recruitment of ER-β to a consensus ERE and weakly induce expression of the progesterone receptor gene (15). The biological significance is that indole treatment simultaneously enhances antiproliferative signaling through ER-β and down-regulates proliferative signaling through ER-α. The mechanism of indole activation of ER-β has not been established although conceivably I3C could disrupt or enhance known posttranslational modifications of ER-β that control its activity, such as its phosphorylation state (25).
DIM Effects on ER Signaling
Depending on the concentration and cell type, DIM, which is the major diinole condensation product of I3C, can induce estrogenic responses through the ligand-independent activation of ERs or disrupt estrogen responsiveness through its interaction with the AhR (24,26). In human breast and endometrial cancer cells, DIM exhibits its antiestrogenic effects at relatively high doses (50 μm), compared with lower doses (10 μm) in which DIM activates ER-α (27,28,29). It is well established that DIM is a ligand for the AhR, and this interaction was recently shown in human breast cancer cells to mediate the DIM-induced degradation of ER-α protein (24). In other cell types, association of the DIM-AhR complex with ER-α has also be shown to recruit transcriptional coregulators to the promoters of estrogen-responsive genes (30). Microarray gene expression profiling of MCF-7 cells revealed that DIM reverses the estrogen-regulated expression of many estrogen target genes, although for a subset of genes DIM enhanced the estrogen-regulated expression (31). The agonist, synergistic, or antagonistic effects of DIM on estrogen signaling are generally consistent with growth-inhibitory response of this indole. For example, DIM and 17-β estradiol down-regulated CXCR4, which is a cytokine required for breast cancer metastasis, as well as of the oncogenic Bcl6 protein responsible for p53 degradation in germinal cells leading to transformation (31).
Although DIM exhibits relatively low affinity for ER-α (29), this indole is as effective as 17β-estradiol in stimulating target gene expression as well as ERE-reporter plasmid activity in endometrial cancer cells (27,28). In both cell systems, DIM activates protein kinase A (PKA), which can phosphoryate ER-α, and an inhibition of PKA activity using the pharmacological inhibitor H89 prevented the estrogenic activity of DIM (28). It is worth noting that in human breast cancer cell lines, PKA is sufficient and necessary for DIM activation of ER-α, whereas, in the Ishikawa endometrial cancer cell line, both PKA and the p42/44 MAPK are required for this DIM response. In contrast to DIM, a triindole condensation product of I3C was shown to bind with high affinity to ER-α and activate expression of estrogen-responsive genes (32).
The DIM activation of ER-α under conditions in which this indole arrests the growth of human breast and endometrial cancer cells is seemingly paradoxical. However, one rationale is that signaling through ER-α induces differentiated properties of certain target cells, which is associated with an inhibition of proliferation. In Ishikawa human endometrial cancer cells, DIM activation of ER-α induces expression and secretion of TGF-α, and exposure of these cells to exogenous TGF-α strongly inhibits cell growth (27). Knockdown of TGF-α expression ablated the DIM-mediated growth arrest, showing that the DIM-induced estrogenic response of enhancing TGF-α expression mediates an arrest of cell growth (27). The physiological effects of an estrogenic dose of DIM as well as potential effects on ER-β are largely unresolved and warrant investigation.
Indole Disruption of Androgen Responsiveness by Targeting Androgen Receptor (AR) Expression and Activity
Androgen-activated AR transcriptional signaling is a critical risk determinant in transformation to cancerous prostate epithelium (33), and the development of androgen-resistant human prostate cancer can be associated with the ligand-independent phosphorylation and activation of AR (34). Evidence to date shows that both I3C and DIM disrupt androgen-dependent and -independent AR signaling in human prostate cancer cells at distinct cellular levels of regulation. The I3C cell cycle arrest of LNCaP human prostate cancer cells was accompanied by the down-regulation of prostate-specific antigen (35), which is an AR-regulated gene. A follow-up study demonstrated that I3C treatment causes a rapid ablation of AR transcript levels and down-regulation of AR promoter-driven reporter plasmid activity (36). The precise I3C-regulated transcriptional regulators of AR have not been identified, although small interfering RNA knockdown of BRCA1 partially reversed the antiandrogenic effects of I3C (17). It is interesting to note that in an in vitro yeast system designed to screen for steroid responsiveness using green fluorescent protein-linked reporter plasmids, I3C displayed significant antiandrogenic properties (7), which implicates I3C as a potent chemopreventive agent against prostate cancer because AR function is critical in the promotion stage of all prostate neoplasia. The affinity of I3C for AR is unknown, although current evidence suggests that the primary I3C effect is the transcriptional down-regulation of AR expression.
In contrast to I3C, DIM acts as a strong antagonist ligand of AR transcriptional activity, which is attributed to the direct binding of DIM to AR (37). Three-dimensional modeling of the DIM interaction with AR revealed that even though DIM is bulkier than natural androgens, the DIM interaction with AR is predicted to decrease the level of hydrogen bonding leading to a displaced helix 12 which is critical to AR nuclear translocation and activity (37). Thus, the AR can be considered a direct target protein for DIM.
B-DIM (BioResponse-DIM) is a longer lasting formulated version of DIM, which was shown to inhibit the growth of androgen-sensitive LNCaP cells and its androgen-refractory variant C4-2 cell line (38). B-DIM treatment stabilized glycogen synthase kinase-3β and induced the proteasome-mediated degradation of β-catenin, which can function in the coregulation of AR activity. B-DIM was also shown to down-regulate AR transcripts by decreasing the recruitment of the FOXO3A transcription factor to the AR promoter in LNCaP human prostate cancer cells (39). Consistent with these effects of B-DIM, methylene-substituted derivatives of DIM (C-DIMs) abrogate AR-mediated signaling by decreasing AR expression independent of the agonist effects of these derivatives on peroxisome proliferator-activated receptor-γ (40).
Effects of I3C and DIM on Estrogen and Androgen Metabolism that Alters Physiological Signaling
Cellular and physiological studies have shown that I3C and DIM selectively alter the production of steroid metabolites that accounts for some of their in vivo endocrine disruption and tumor protection effects. Both indoles are potent inducers of steroid-metabolizing enzymes such as 2-hydroxylating cytochrome (cyp1A1) that converts circulating estradiol, a mitogenic estrogen, to a 2-hydroxy metabolite that correlates with lower risk of gynecological cancers (41). It has been reported that the DIM stimulation of cyp1A1 requires the AhR (42), implicating this nuclear receptor as a critical mediator of this DIM endocrine-disrupting activity. In a microarray analysis of human reproductive cancer cells, DIM enhanced expression of aldo-keto reductase family 1 member 3, which is responsible for reduction of estrone levels as well as inactivation of 5-α dihydrotestosterone leading to diminished promotion of early prostate cancer cells (31,43). Thus, DIM stimulates expression of a steroid-modifying enzyme that is produced at much reduced levels in breast and prostate tumors compared with the surrounding normal tissue.
In an earlier clinical study, I3C treatment of a normal group of healthy men and women altered plasma levels of circulating estrogen and also decreased urinary levels of estradiol, estrone, estriol, and 16-α hydroxyestrone (44). These down-regulated estrogen metabolites correlate positively with increased risk for a variety of reproductive cancers (41). In a clinical study involving postmenopausal women with a history of early-stage breast cancer, B-DIM significantly enhanced the urinary level of 2-hydroxyestrone (45), which is an estrogen metabolite that is associated with lowering breast cancer risk. This alteration in estrogen metabolism in humans was confirmed by another clinical study in which I3C exposure in women with systemic lupus erythematosus caused a significant reversal favoring increased levels of 2-hydroxyestrone over 16-hydroxyestrone, and correlating with inhibition of disease progression (46).
Indole Control of Transcription Factors that Are Mechanistically Linked to Hormone- and Growth Factor Receptor-Signaling Pathways
Specificity factor 1 (Sp1) and nuclear factor κ B (NFκB) are two ubiquitously expressed transcription factor families the activities of which are closely intertwined with steroid and growth factor receptor signaling and cancer cell growth (47,48). These transcription factors control expression of a wide variety of gene products that includes those involved in cell cycle control, differentiation, cell survival, and apoptosis (49,50). Several groups have reported that depending on the cell type, the anticancer effects of I3C and DIM can be accompanied by the regulation of Sp1 (8,51,52,53) or NFκB transcriptional activity (54), and it is tempting to consider that these transcriptional cascades also play a direct role in the endocrine disruption effects of these indoles.
Expression of many steroid receptor target genes can be influenced by the availability and activity of Sp1 at the target gene promoters (55), including specific estrogen-responsive genes in which ER-α and Sp1 interact at composite elements in the gene promoters. I3C treatment of human breast cancer cells suppresses the interactions of Sp1 with composite elements in the cyclin-dependent kinase-6 promoter (51) and the matrix metalloprotease 2 promoter (53), suggesting that this indole can blunt critical Sp1-promoter interactions in a way that could potentially disrupt estrogen signaling. Consistent with this concept, I3C down-regulates expression of several estrogen-responsive genes, such as the progesterone receptor, epidermal growth factor receptor, cathepsin D, and pS2, that contain composite ERE-Sp1 elements in their promoter (55). Also, I3C attenuates the estrogen stimulation of the Bcl2 antiapoptotic gene, in which expression is controlled by ER-α and Sp1 interactions at the Bcl2 gene promoter (19).
In contrast to I3C, DIM treatment increased nuclear binding of Sp1 to the p21 gene promoter in breast cancer cells (52). DIM enhances estrogen responsiveness at low concentrations and, because Sp1 can functionally interact with ER-α to stimulated gene transcription, it seems plausible that DIM can enhance its estrogenic effect through activation of Sp1. The precise mechanisms by which I3C or DIM controls Sp1 activity has not been established, although it is interesting to note that indole treatment results in the attenuation or stimulation of protein kinase activities, such as the ERK family (56), that are known to phosphorylate Sp1 (50,57).
Clinical studies have shown an inverse correlation between NFκB levels and either ER or AR expression in breast cancer and prostate cancer tissue, respectively (58,59). Also, ER-α competes with NFκB for specific sets of transcriptional coactivators (60). As such, the ability of I3C and DIM to decrease NFκB levels, and disrupt NFκB signaling and nuclear localization in reproductive cancer cells may potentially have profound endocrine disruption effects. One study has shown that NFκB signaling activates AR in the absence of ligand, and this effect has been proposed to lead to androgen independence and poor prognosis (59). The indole down-regulation of NFκB suggests that I3C- and DIM-based therapeutic strategies may therefore have the capacity to prevent progression to hormone independence.
Indole Control of Growth Factor and Cytokine Receptor Signaling Pathways
I3C and DIM have been shown to alter growth factor and cytokine receptor signaling by inhibiting or stimulating the expression and/or activity of receptor-activated signal transduction components, or by selectively altering expression of the receptors and their corresponding ligands. An important growth factor signaling cascade involves the phosphatidyl inositol 3-kinase mediated activation of Akt that initiates an intricate downstream cascade for control of cell growth and survival (61,62). Treatment with either I3C or DIM inhibits Akt activity in a variety of indole-sensitive cancer cells. Although the upstream regulators of Akt that are regulated by DIM are yet to be identified, I3C has been shown to decrease epidermal growth factor receptor levels (63), suggesting that this response may lead to a reduced phosphatidyl inositol 3 kinase-dependent activation of Akt that is observed in both breast and prostate cancer cells (54,64). The I3C down-regulation of epidermal growth factor receptor expression was shown to contribute to the apoptotic response in steroid-insensitive human breast cancer cells (65), whereas, in HER2/Neu overexpressing human breast cancer cells, DIM inhibited HER2/neu activation as part of its apoptotic effect (66). Beyond these studies, relatively little is known about specific effects of either I3C or DIM on expression or activation of the epidermal growth factor receptor gene family.
The indole-mediated loss in Akt activity causes specific alterations in a myriad of growth factor and hormone-signaling pathways including a decrease in the cellular levels of activated FOXO3a, which targets the AR gene promoter- and growth factor-responsive genes (39). Other receptor-activated signal transduction components regulated by I3C and DIM include protein kinases, G proteins, and downstream transcription factors (28,56,67). Intriguingly, within the same signaling cascade the effects of I3C and DIM can be distinct. For example, both I3C and DIM inhibit angiogenesis by disrupting vascular endothelial growth factor (VEGF) utilization and endothelial cell growth (56,68,69). However, I3C was shown to down-regulate VEGF expression by an inhibition of NFκB activity, whereas DIM attenuated VEGF receptor signaling by strongly down-regulating ERK1/2 activity. Ras-GTP content was abolished with DIM treatment, which, in turn, resulted in the reduced activities of Raf and MAPK kinase, culminating in the drop of ERK1/2 activation (56).
In human reproductive cancer cells and in vivo, DIM and I3C have striking effects on cytokines and their signaling pathways. I3C stimulates expression and promoter activity of the interferon γ receptor-1 in human breast cancer cells, which results in an enhanced signal transduction cascade (70). DIM stimulated interferon-γ gene expression in human breast cancer cells by activating the JNK and p38/MAPK pathways (67), whereas a combination of DIM and interferon-γ synergized to stimulate signal transducer and activator of transcription-1 activity (71). These DIM-regulated effects on interferon-γ signaling as well as on the level of granulocyte colony-stimulating factor and IL-12 have been proposed to play a crucial role in the immune enhancing response of DIM in vivo (72).
Perspectives
Dietary indoles elicit changes in cellular signaling pathways that project them as potent anticancer phytochemicals on many levels of the carcinogenic process. These indoles have been demonstrated to attenuate hormone-induced mitogenesis by altering circulating levels of key steroids, such as androgens and estrogens, as well as by down-regulating receptor expression and/or modulating receptor activities and intracellular signaling components. Studies to date have also unearthed the ability of indoles to diminish actions of key regulatory pathways, which, when deregulated, can lead to endocrine resistance. The finding that I3C triggers key antiproliferative responses in human breast cancer cells by the direct inhibition of elastase enzymatic activity (73) implicates the presence of specific indole target proteins that selectively mediate the ability of I3C and DIM to alter hormone receptor signaling. Indeed, DIM acts an activating ligand of AhR and an antagonist ligand of the AR, which accounts for a subset of the endocrine disruption activities of DIM. However, because of the wide range of cellular pathways that are regulated by I3C and DIM, an exciting future direction will be to identify additional indole targets that are critical for the I3C and DIM endocrine disruption of human cancer cells. This preclinical information will be critical to understand how dietary indoles may potentially inhibit promotion of initiated cells to occult cancers and prevent existent occult cancers from progression to endocrine-resistant states.
Acknowledgments
We thank Leonard F. Bjeldanes (Department of Nutritional Sciences and Toxicology, University of California at Berkeley) and Crystal Marconett (Department of Molecular and Cell Biology, University of California at Berkeley) for their helpful discussions and suggestions during the writing of this manuscript.
Footnotes
The writing of this review and the results of an unpublished study from our laboratory mentioned in the text were supported by the National Institute of Health Public Service Grant CA102360 from the National Cancer Institute.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 16, 2009
Abbreviations: AhR, Aryl hydrocarbon receptor; AR, androgen receptor; B-DIM, BioResponse-DIM; DIM, 3-3′-diindolylmethane; ER, estrogen receptor; ERE, estrogen response element; I3C, indole-3-carbinol; PKA, protein kinase A; VEGF, vascular endothelial growth factor.
References
- Daniele L, Sapino A 2009 Anti-HER2 treatment and breast cancer: state of the art, recent patents, and new strategies. Recent Pat Anticancer Drug Discov 4:9–18 [DOI] [PubMed] [Google Scholar]
- Hynes NE, Macdonald G 2009 ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol 21:177–184 [DOI] [PubMed] [Google Scholar]
- Hayes JD, Kelleher MO, Eggleston IM 2008 The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr 47 (Suppl 2):73–88 [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Ichikawa H 2005 Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 4:1201–1215 [DOI] [PubMed] [Google Scholar]
- Kim YS, Milner JA 2005 Targets for indole-3-carbinol in cancer prevention. J Nutr Biochem 16:65–73 [DOI] [PubMed] [Google Scholar]
- Weng JR, Tsai CH, Kulp SK, Chen CS 2008 Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett 262:153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee TF, Schoonen WG, Hamers AR, Bento MJ, Peijnenburg AA 2008 Screening of synthetic and plant-derived compounds for (anti)estrogenic and (anti)androgenic activities. Anal Bioanal Chem 390:1111–1119 [DOI] [PubMed] [Google Scholar]
- Firestone GL, Bjeldanes LF 2003 Indole-3-carbinol and 3-3′-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J Nutr 133:2448S–2455S [DOI] [PubMed] [Google Scholar]
- Enmark E, Gustafsson JA 1999 Oestrogen receptors—an overview. J Intern Med 246:133–138 [DOI] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS 2002 Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology 143:4172–4177 [DOI] [PubMed] [Google Scholar]
- Campbell-Thompson M, Lynch IJ, Bhardwaj B 2001 Expression of estrogen receptor (ER) subtypes and ERβ isoforms in colon cancer. Cancer Res 61:632–640 [PubMed] [Google Scholar]
- Roger P, Sahla ME, Mäkelä S, Gustafsson JA, Baldet P, Rochefort H 2001 Decreased expression of estrogen receptor β protein in proliferative preinvasive mammary tumors. Cancer Res 61:2537–2541 [PubMed] [Google Scholar]
- Shaaban AM, O'Neill PA, Davies MP, Sibson R, West CR, Smith PH, Foster CS 2003 Declining estrogen receptor-β expression defines malignant progression of human breast neoplasia. Am J Surg Pathol 27:1502–1512 [DOI] [PubMed] [Google Scholar]
- Monroe DG, Johnsen SA, Subramaniam M, Getz BJ, Khosla S, Riggs BL, Spelsberg TC 2003 Mutual antagonism of estrogen receptors α and β and their preferred interactions with steroid receptor coactivators in human osteoblastic cell lines. J Endocrinol 176:349–357 [DOI] [PubMed] [Google Scholar]
- Sundar SN, Kerekatte V, Equinozio CN, Doan VB, Bjeldanes LF, Firestone GL 2006 Indole-3-carbinol selectively uncouples expression and activity of estrogen receptor subtypes in human breast cancer cells. Mol Endocrinol 20:3070–3082 [DOI] [PubMed] [Google Scholar]
- Cover CM, Hsieh SJ, Cram EJ, Hong C, Riby JE, Bjeldanes LF, Firestone GL 1999 Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Res 59:1244–1251 [PubMed] [Google Scholar]
- Fan S, Meng Q, Auborn K, Carter T, Rosen EM 2006 BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br J Cancer 94:407–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Carter TH, Auborn KJ 2004 Apoptosis in cervical cancer cells: implications for adjunct anti-estrogen therapy for cervical cancer. Anticancer Res 24:2649–2656 [PubMed] [Google Scholar]
- Dong L, Wang W, Wang F, Stoner M, Reed JC, Harigai M, Samudio I, Kladde MP, Vyhlidal C, Safe S 1999 Mechanisms of transcriptional activation of bcl-2 gene expression by 17β-estradiol in breast cancer cells. J Biol Chem 274:32099–32107 [DOI] [PubMed] [Google Scholar]
- Wang TT, Milner MJ, Milner JA, Kim YS 2006 Estrogen receptor α as a target for indole-3-carbinol. J Nutr Biochem 17:659–664 [DOI] [PubMed] [Google Scholar]
- Ashok BT, Chen Y, Liu X, Bradlow HL, Mittelman A, Tiwari RK 2001 Abrogation of estrogen-mediated cellular and biochemical effects by indole-3-carbinol. Nutr Cancer 41:180–187 [DOI] [PubMed] [Google Scholar]
- Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M 2007 Positive cross-regulatory loop ties GATA-3 to estrogen receptor α expression in breast cancer. Cancer Res 67:6477–6483 [DOI] [PubMed] [Google Scholar]
- Safe S, Wormke M 2003 Inhibitory aryl hydrocarbon receptor-estrogen receptor α cross-talk and mechanisms of action. Chem Res Toxicol 16:807–816 [DOI] [PubMed] [Google Scholar]
- Okino ST, Pookot D, Basak S, Dahiya R 2009 Toxic and chemopreventive ligands preferentially activate distinct aryl hydrocarbon receptor pathways: implications for cancer prevention. Cancer Prev Res 2:251–256 [DOI] [PubMed] [Google Scholar]
- Driggers PH, Segars JH, Rubino DM 2001 The proto-oncoprotein Brx activates estrogen receptor β by a p38 mitogen-activated protein kinase pathway. J Biol Chem 276:46792–46797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, Safe S 2003 The aryl hydrocarbon receptor mediates degradation of estrogen receptor α through activation of proteasomes. Mol Cell Biol 23:1843–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong H, Firestone GL, Bjeldanes LF 2001 Cytostatic effects of 3,3′-diindolylmethane in human endometrial cancer cells result from an estrogen receptor-mediated increase in transforming growth factor-α expression. Carcinogenesis 22:1809–1817 [DOI] [PubMed] [Google Scholar]
- Leong H, Riby JE, Firestone GL, Bjeldanes LF 2004 Potent ligand-independent estrogen receptor activation by 3,3′-diindolylmethane is mediated by cross talk between the protein kinase A and mitogen-activated protein kinase signaling pathways. Mol Endocrinol 18:291–302 [DOI] [PubMed] [Google Scholar]
- Riby JE, Chang GH, Firestone GL, Bjeldanes LF 2000 Ligand-independent activation of estrogen receptor function by 3,3′-diindolylmethane in human breast cancer cells. Biochem Pharmacol 60:167–177 [DOI] [PubMed] [Google Scholar]
- Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S 2003 Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature 423:545–550 [DOI] [PubMed] [Google Scholar]
- Mulvey L, Chandrasekaran A, Liu K, Lombardi S, Wang XP, Auborn KJ, Goodwin L 2007 Interplay of genes regulated by estrogen and diindolylmethane in breast cancer cell lines. Mol Med 13:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riby JE, Feng C, Chang YC, Schaldach CM, Firestone GL, Bjeldanes LF 2000 The major cyclic trimeric product of indole-3-carbinol is a strong agonist of the estrogen receptor signaling pathway. Biochemistry 39:910–918 [DOI] [PubMed] [Google Scholar]
- Gelmann EP 2002 Molecular biology of the androgen receptor. J Clin Oncol 20:3001–3015 [DOI] [PubMed] [Google Scholar]
- Yeh S, Chang HC, Miyamoto H, Takatera H, Rahman M, Kang HY, Thin TH, Lin HK, Chang C 1999 Differential induction of the androgen receptor transcriptional activity by selective androgen receptor coactivators. Keio J Med 48:87–92 [DOI] [PubMed] [Google Scholar]
- Zhang J, Hsu B A JC, Kinseth B A MA, Bjeldanes LF, Firestone GL 2003 Indole-3-carbinol induces a G1 cell cycle arrest and inhibits prostate-specific antigen production in human LNCaP prostate carcinoma cells. Cancer 98:2511–2520 [DOI] [PubMed] [Google Scholar]
- Hsu JC, Zhang J, Dev A, Wing A, Bjeldanes LF, Firestone GL 2005 Indole-3-carbinol inhibition of androgen receptor expression and downregulation of androgen responsiveness in human prostate cancer cells. Carcinogenesis 26:1896–1904 [DOI] [PubMed] [Google Scholar]
- Le HT, Schaldach CM, Firestone GL, Bjeldanes LF 2003 Plant-derived 3,3′-diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem 278:21136–21145 [DOI] [PubMed] [Google Scholar]
- Chinnakannu K, Chen D, Li Y, Wang Z, Dou QP, Reddy GP, Sarkar FH 2009 Cell cycle-dependent effects of 3,3′-diindolylmethane on proliferation and apoptosis of prostate cancer cells. J Cell Physiol 219:94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH 2007 Regulation of FOXO3a/β-catenin/GSK-3β signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem 282:21542–21550 [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Safe S 2007 1,1-bis(3′-indolyl)-1-(p-substitutedphenyl)methanes inhibit growth, induce apoptosis, and decrease the androgen receptor in LNCaP prostate cancer cells through peroxisome proliferator-activated receptor γ-independent pathways. Mol Pharmacol 71:558–569 [DOI] [PubMed] [Google Scholar]
- Sepkovic DW, Bradlow HL 2009 Estrogen hydroxylation—the good and the bad. Ann NY Acad Sci 1155:57–67 [DOI] [PubMed] [Google Scholar]
- Chen I, McDougal A, Wang F, Safe S 1998 Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis 19:1631–1639 [DOI] [PubMed] [Google Scholar]
- Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K 2000 Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J 351:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michnovicz JJ, Adlercreutz H, Bradlow HL 1997 Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst 89:718–723 [DOI] [PubMed] [Google Scholar]
- Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF 2004 Pilot study: effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer 50:161–167 [DOI] [PubMed] [Google Scholar]
- McAlindon TE, Gulin J, Chen T, Klug T, Lahita R, Nuite M 2001 Indole-3-carbinol in women with SLE: effect on estrogen metabolism and disease activity. Lupus 10:779–783 [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M 2009 Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov 8:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Lockwood CJ 2002 Steroid hormones, endometrial gene regulation and the Sp1 family of proteins. J Soc Gynecol Investig 9:329–334 [PubMed] [Google Scholar]
- Deniaud E, Baguet J, Mathieu AL, Pagès G, Marvel J, Leverrier Y 2006 Overexpression of Sp1 transcription factor induces apoptosis. Oncogene 25:7096–7105 [DOI] [PubMed] [Google Scholar]
- Tan NY, Khachigian LM 2009 Sp1 Phosphorylation and its regulation of gene transcription. Mol Cell Biol 29:2483–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram EJ, Liu BD, Bjeldanes LF, Firestone GL 2001 Indole-3-carbinol inhibits CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter. J Biol Chem 276:22332–22340 [DOI] [PubMed] [Google Scholar]
- Hong C, Kim HA, Firestone GL, Bjeldanes LF 2002 3,3′-Diindolylmethane (DIM) induces a G(1) cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21(WAF1/CIP1) expression. Carcinogenesis 23:1297–1305 [DOI] [PubMed] [Google Scholar]
- Hung WC, Chang HC 2009 Indole-3-carbinol inhibits Sp1-induced matrix metalloproteinase-2 expression to attenuate migration and invasion of breast cancer cells. J Agric Food Chem 57:76–82 [DOI] [PubMed] [Google Scholar]
- Chinni SR, Sarkar FH 2002 Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC-3 cells. Clin Cancer Res 8:1228–1236 [PubMed] [Google Scholar]
- Safe S, Kim K, Kim K 2008 Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol 41:263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Firestone GL, Bjeldanes LF 2006 Inhibition of growth factor-induced Ras signaling in vascular endothelial cells and angiogenesis by 3,3′-diindolylmethane. Carcinogenesis 27:541–550 [DOI] [PubMed] [Google Scholar]
- Kim HS, Lim IK 2009 Phosphorylated extracellular signal-regulated protein kinases 1 and 2 phosphorylate Sp1 on serine59 and regulates cellular senescence via transcription of p21Sdi1/Cip1/Waf1. J Biol Chem 284:15475–15486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Eppenberger-Castori S, Eppenberger U, Benz CC 2005 The NFκB pathway and endocrine-resistant breast cancer. Endocr Relat Cancer 12 (Suppl 1):S37–S46 [DOI] [PubMed] [Google Scholar]
- Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L, Case TC, Ellwood-Yen K, Sawyers CL, Bhowmick NA, Blackwell TS, Yull FE, Matusik RJ 2008 The nuclear factor-κB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res 68:6762–6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzidis D, Gilmore TD 2005 Transcription factor cross-talk: the estrogen receptor and NF-κB. Trends Endocrinol Metab 16:46–52 [DOI] [PubMed] [Google Scholar]
- Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF 2008 The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets 8:187–198 [DOI] [PubMed] [Google Scholar]
- Renner O, Blanco-Aparicio C, Carnero A 2008 Genetic modelling of the PTEN/AKT pathway in cancer research. Clin Transl Oncol 10:618–627 [DOI] [PubMed] [Google Scholar]
- Moiseeva EP, Heukers R, Manson MM 2007 EGFR and Src are involved in indole-3-carbinol-induced death and cell cycle arrest of human breast cancer cells. Carcinogenesis 28:435–445 [DOI] [PubMed] [Google Scholar]
- Rahman KW, Sarkar FH 2005 Inhibition of nuclear translocation of nuclear factor-κB contributes to 3,3′-diindolylmethane-induced apoptosis in breast cancer cells. Cancer Res 65:364–371 [PubMed] [Google Scholar]
- Moiseeva EP, Almeida GM, Jones GD, Manson MM 2007 Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol Cancer Ther 6:3071–3079 [DOI] [PubMed] [Google Scholar]
- McGuire KP, Ngoubilly N, Neavyn M, Lanza-Jacoby S 2006 3,3′-diindolylmethane and paclitaxel act synergistically to promote apoptosis in HER2/Neu human breast cancer cells. J Surg Res 132:208–213 [DOI] [PubMed] [Google Scholar]
- Xue L, Firestone GL, Bjeldanes LF 2005 DIM stimulates IFNγ gene expression in human breast cancer cells via the specific activation of JNK and p38 pathways. Oncogene 24:2343–2353 [DOI] [PubMed] [Google Scholar]
- Chang X, Tou JC, Hong C, Kim HA, Riby JE, Firestone GL, Bjeldanes LF 2005 3,3′-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis 26:771–778 [DOI] [PubMed] [Google Scholar]
- Kunimasa K, Kobayashi T, Sugiyama S, Kaji K, Ohta T 2008 Indole-3-carbinol suppresses tumor-induced angiogenesis by inhibiting tube formation and inducing apoptosis. Biosci Biotechnol Biochem 72:2243–2246 [DOI] [PubMed] [Google Scholar]
- Chatterji U, Riby JE, Taniguchi T, Bjeldanes EL, Bjeldanes LF, Firestone GL 2004 Indole-3-carbinol stimulates transcription of the interferon γ receptor 1 gene and augments interferon responsiveness in human breast cancer cells. Carcinogenesis 25:1119–1128 [DOI] [PubMed] [Google Scholar]
- Riby JE, Xue L, Chatterji U, Bjeldanes EL, Firestone GL, Bjeldanes LF 2006 Activation and potentiation of interferon-γ signaling by 3,3′-diindolylmethane in MCF-7 breast cancer cells. Mol Pharmacol 69:430–439 [DOI] [PubMed] [Google Scholar]
- Xue L, Pestka JJ, Li M, Firestone GL, Bjeldanes LF 2008 3,3′-Diindolylmethane stimulates murine immune function in vitro and in vivo. J Nutr Biochem 19:336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HH, Aronchik I, Brar GA, Nguyen DH, Bjeldanes LF, Firestone GL 2008 The dietary phytochemical indole-3-carbinol is a natural elastase enzymatic inhibitor that disrupts cyclin E protein processing. Proc Natl Acad Sci USA 105:19750–19755 [DOI] [PMC free article] [PubMed] [Google Scholar]