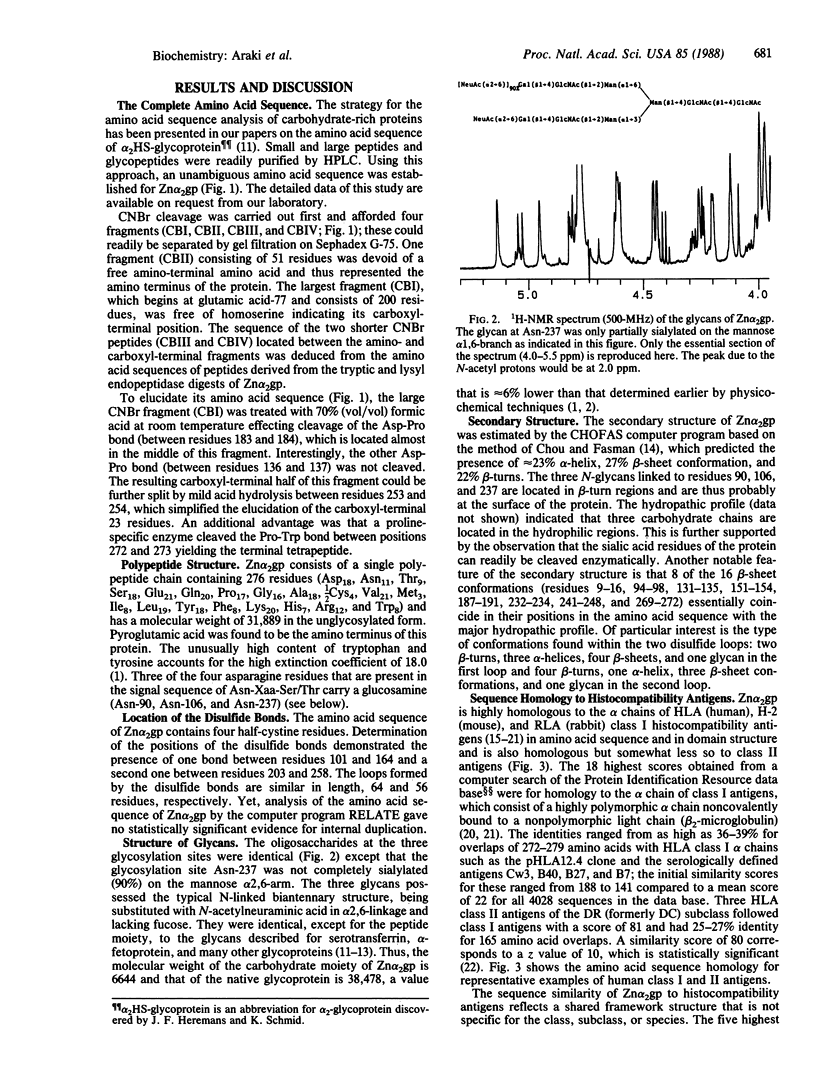
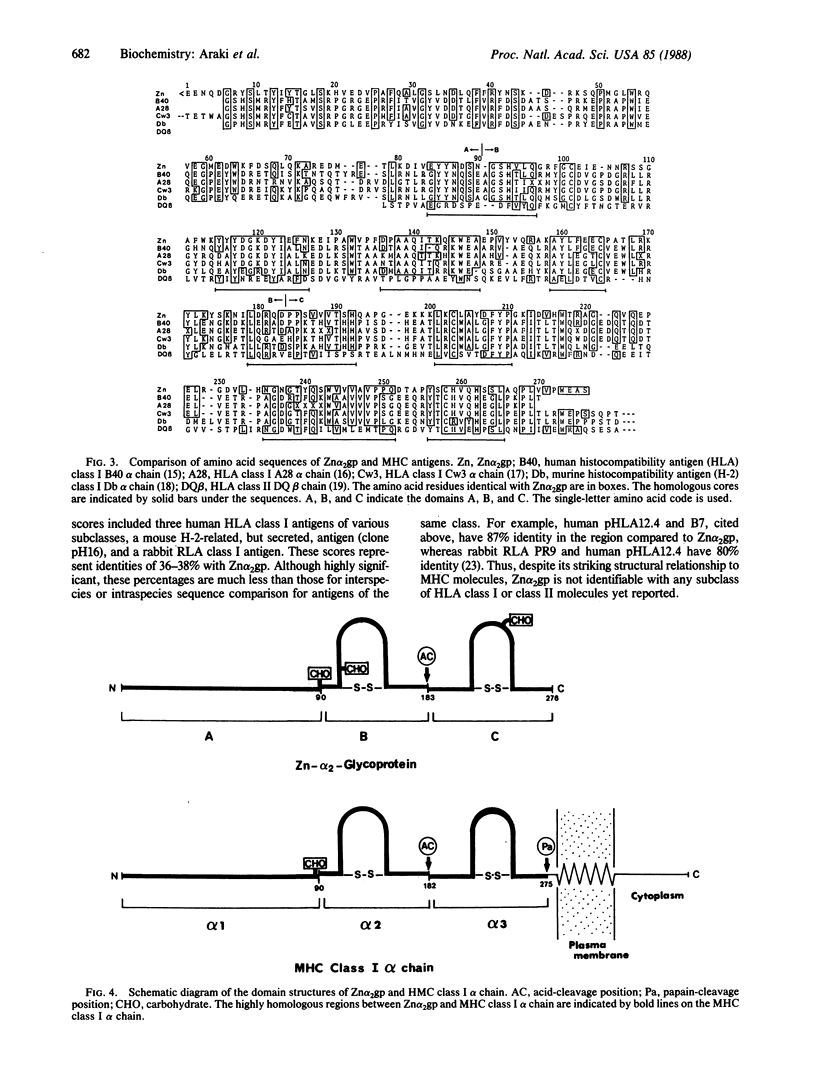
Abstract
In the present study the complete amino acid sequence of human plasma Zn-alpha 2-glycoprotein was determined. This protein whose biological function is unknown consists of a single polypeptide chain of 276 amino acid residues including 8 tryptophan residues and has a pyroglutamyl residue at the amino terminus. The location of the two disulfide bonds in the polypeptide chain was also established. The three glycans, whose structure was elucidated with the aid of 500 MHz 1H NMR spectroscopy, were sialylated N-biantennas. The molecular weight calculated from the polypeptide and carbohydrate structure is 38,478, which is close to the reported value of approximately equal to 41,000 based on physicochemical measurements. The predicted secondary structure appeared to be comprised of 23% alpha-helix, 27% beta-sheet, and 22% beta-turns. The three N-glycans were found to be located in beta-turn regions. An unexpected finding was made by computer analysis of the sequence data; this revealed that Zn-alpha 2-glycoprotein is closely related to antigens of the major histocompatibility complex in amino acid sequence and in domain structure. There was an unusually high degree of sequence homology with the alpha chains of class I histocompatibility antigens. Moreover, this plasma protein was shown to be a member of the immunoglobulin gene superfamily. Zn-alpha 2-glycoprotein appears to be a truncated secretory major histocompatibility complex-related molecule, and it may have a role in the expression of the immune response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. R., Atkinson P. H., Grimes W. J. Major carbohydrate structures at five glycosylation sites on murine IgM determined by high resolution 1H-NMR spectroscopy. Arch Biochem Biophys. 1985 Dec;243(2):605–618. doi: 10.1016/0003-9861(85)90538-7. [DOI] [PubMed] [Google Scholar]
- Auffray C., Strominger J. L. Molecular genetics of the human major histocompatibility complex. Adv Hum Genet. 1986;15:197–247. doi: 10.1007/978-1-4615-8356-1_4. [DOI] [PubMed] [Google Scholar]
- BURGI W., SCHMID K. Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem. 1961 Apr;236:1066–1074. [PubMed] [Google Scholar]
- Bayard B., Kerckaert J. P., Strecker G., Dorland L., van Halbeek H., Vliegenthart J. F. Structure determination of the carbohydrate chains of rat alpha-fetoprotein. Eur J Biochem. 1983 Dec 1;137(1-2):319–323. doi: 10.1111/j.1432-1033.1983.tb07831.x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Götz H., Kratzin H., Thinnes F. P., Yang C., Kruse T., Pauly E., Kölbel S., Egert G., Wernet P., Hilschmann N. Primary structure of human class II histocompatibility antigens 3rd communication. Amino acid sequence comparison between DR and DC subclass antigens derived from a lymphoblastoid B cell line homozygous at the HLA loci (HLA-A3,3; B7,7; Dw2,2; DR2,2: MT1,1; Dc1,1: MB1,1). Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):749–755. doi: 10.1515/bchm2.1983.364.1.749. [DOI] [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Hortin G., Green E. D., Baenziger J. U., Strauss A. W. Sulphation of proteins secreted by a human hepatoma-derived cell line. Sulphation of N-linked oligosaccharides on alpha 2HS-glycoprotein. Biochem J. 1986 Apr 15;235(2):407–414. doi: 10.1042/bj2350407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboh M. I., Ferrell R. E. Genetic studies of low-abundance human plasma proteins. I. Microheterogeneity of zinc-alpha 2-glycoprotein in biological fluids. Biochem Genet. 1986 Dec;24(11-12):849–857. doi: 10.1007/BF00554524. [DOI] [PubMed] [Google Scholar]
- Kress M., Cosman D., Khoury G., Jay G. Secretion of a transplantation-related antigen. Cell. 1983 Aug;34(1):189–196. doi: 10.1016/0092-8674(83)90149-6. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Castro J., Bragado R., Strong D. M., Strominger J. L. Primary structure of papain-solubilized human histocompatibility antigen HLA-B40 (-Bw60). An outline of alloantigenic determinants. Biochemistry. 1983 Aug 2;22(16):3961–3969. doi: 10.1021/bi00285a036. [DOI] [PubMed] [Google Scholar]
- Maloy W. L., Coligan J. E. Primary structure of the H-2Db alloantigen. II. Additional amino acid sequence information, localization of a third site of glycosylation and evidence for K and D region specific sequences. Immunogenetics. 1982;16(1):11–22. doi: 10.1007/BF00364438. [DOI] [PubMed] [Google Scholar]
- SCHMID K., TAKAHASHI S. POLYMORPHISM OF ZINC-ALPHA-2-HUMAN GLYCOPROTEIN. Nature. 1964 Jul 25;203:407–408. doi: 10.1038/203407a0. [DOI] [PubMed] [Google Scholar]
- Shibata S., Miura K. Nephritogenic glycoprotein. IX. Plasma Zn-alpha2-glycoprotein as a second source of nephritogenic glycoprotein in urine. Nephron. 1982;31(2):170–176. doi: 10.1159/000182638. [DOI] [PubMed] [Google Scholar]
- Sodoyer R., Damotte M., Delovitch T. L., Trucy J., Jordan B. R., Strachan T. Complete nucleotide sequence of a gene encoding a functional human class I histocompatibility antigen (HLA-CW3). EMBO J. 1984 Apr;3(4):879–885. doi: 10.1002/j.1460-2075.1984.tb01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Vivona G., Atkinson P. H. 1H NMR spectroscopy of carbohydrates from the G glycoprotein of vesicular stomatitis virus grown in parental and Lec4 Chinese hamster ovary cells. Arch Biochem Biophys. 1984 Apr;230(1):363–374. doi: 10.1016/0003-9861(84)90119-x. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T., Miyata T., Iwanaga S., Toyo'oka T., Imai K. Application of a fluorogenic reagent, ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate for detection of cystine-containing peptides. J Biochem. 1985 Jun;97(6):1811–1813. doi: 10.1093/oxfordjournals.jbchem.a135241. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Atkinson P. H. Structure of yeast external invertase Man8-14GlcNAc processing intermediates by 500-megahertz 1H NMR spectroscopy. J Biol Chem. 1986 Jul 25;261(21):9815–9824. [PubMed] [Google Scholar]
- Tykocinski M. L., Marche P. N., Max E. E., Kindt T. J. Rabbit class I MHC genes: cDNA clones define full-length transcripts of an expressed gene and a putative pseudogene. J Immunol. 1984 Oct;133(4):2261–2269. [PubMed] [Google Scholar]
- Yoshioka Y., Gejyo F., Marti T., Rickli E. E., Bürgi W., Offner G. D., Troxler R. F., Schmid K. The complete amino acid sequence of the A-chain of human plasma alpha 2HS-glycoprotein. J Biol Chem. 1986 Feb 5;261(4):1665–1676. [PubMed] [Google Scholar]


