Abstract
Objective
To determine the temporal relationships of clinical, cognitive, Pittsburgh Compound-B (PiB) amyloid imaging, and cerebrospinal fluid (CSF) markers of Alzheimer’s disease (AD).
Design
A case report of a longitudinally assessed participant in a memory and aging study who had serial clinical and psychometric assessments over 6 years, in addition to PiB imaging and CSF biomarker assays, prior to coming to autopsy.
Setting
Alzheimer’s Disease Research Center
Findings
An 85-year old individual was cognitively normal at his initial and next 3 annual assessments. Decline in measures of episodic memory and, to a lesser degree, working memory began at about age 88 years. PiB-PET amyloid imaging was negative at age 88.5 years, but at age 89.5 years there was reduced amyloid-beta 42 (Aβ42) and elevated levels of tau in the CSF. At his 6th assessment, when he was 90 years old, he was diagnosed with very mild dementia of the Alzheimer type. After death at age 91 years, the autopsy revealed foci of frequent neocortical diffuse Aβ plaques, sufficient to fulfill Khachaturian neuropathologic criteria for AD, but neuritic plaques and neurofibrillary tangles were sparse. Postmortem biochemical analysis of the cerebral tissue confirmed that PiB-PET-binding was below the level needed for in vivo detection.
Conclusion
Clinical, cognitive, and CSF markers consistent with AD may precede detection of cerebral Aβ with amyloid imaging agents such as PiB, which primarily label fibrillar Aβ plaques.
The identification of sensitive and specific biomarkers of Alzheimer disease (AD) may improve its early diagnosis and may help to evaluate the efficacy of potential therapeutic interventions. 1–4 In particular, cerebrospinal fluid (CSF) assays of amyloid-beta-42 (Aβ42) and tau and amyloid imaging tracers such as Pittsburgh Compound-B (PiB) 5 may identify the AD pathological process in the brain, regardless of clinical status (ie, whether or not cognitive impairment or dementia is present). 6–12 To our knowledge, there have been no reports of individuals who have been characterized longitudinally with clinical and cognitive measures and who transitioned from cognitive normality to early symptomatic AD during a period when both CSF markers and PiB amyloid imaging were obtained. We now report such a case, with clinicopathological evidence for AD, to provisionally examine the sequence of biomarker and neuropathological abnormalities in AD.
METHODS
PARTICIPANT
The case reported here comes from a sample of community-dwelling volunteers enrolled in a longitudinal study of healthy aging and AD conducted by the Washington University Alzheimer Disease Research Center. Participants in this longitudinal study are 60 years of age or older, in good general health, and have no neurological (other than AD), psychiatric, or systemic medical illness that could contribute importantly to dementia. They also have no medical contraindication to lumbar puncture (LP) or structural or functional neuroimaging. All procedures have been approved by the University’s human subjects committee, and written informed consent is obtained from the participants and their collateral sources.
ASSESSMENTS
At entry and annual followup, experienced clinicians determine the cognitive status of participants based soley on semistructured interviews with the individual and their observant collateral sources (typically the spouse or adult child), followed by a neurological examination of the participant. Impaired cognition is detected when there is decline from previously attained cognitive abilities that interfere to at least some degree with the individual’s performance in everyday activities. 13 The clinical battery includes the administration of the MiniMental State Examination (MMSE). 14 Based on all information, the clinician determines the Clinical Dementia Rating (CDR,) 15 where 0 indicates no dementia and excludes even minimal cognitive impairment, whereas CDR 0.5 indicates very mild dementia. In demented individuals, a diagnosis of AD is made in accordance with standard definitions and criteria. 16
The CDR and dementia diagnosis is completed without reference to psychometric performance, which is obtained 2 weeks after the clinical assessment. The 1.5 hour psychometric test battery is administered annually. The tests include three measures of episodic memory: Logical Memory and Associate Learning from the Wechsler Memory Scale 17 and the Free and Selective Reminding Test (sum of three free recall trials). 18 There were two measures of semantic memory, Information19 and the Boston Naming Test.20 Working memory measures included Mental Control and Digit Span (forward and backward) 17 and word fluency for S and P. 21 Visuospatial ability was assessed with Block Design 19 and Digit Symbol 19and the Trailmaking Test A. 22 Scores can be converted to z scores using means and standard deviations from the first time of assessment of a reference group of 310 individuals who were enrolled as CDR 0 and remained that way as long as followed (for at least two assessments). 23
At entry, a blood sample is obtained for determination of apolipoprotein E (APOE) genotype as previously described. 24
CSF COLLECTION, PROCESSING, AND ASSESSMENT
Cerebrospinal fluid (20–30 mL) is collected at 8 AM after overnight fasting. Samples are gently inverted to avoid possible gradient effects, briefly centrifuged at low speed, and aliquoted into polypropylene tubes prior to freezing at −84°C. The samples are analyzed for total tau, phosphorylated tau181 (ptau181), and Aβ42 by enzyme-linked immunosorbant assay (Innotest; Innogenetics, Ghent, Belgium) as previously described. 7
IN VIVO AMYLOID IMAGING
Positron emission tomography (PET) with PiB is obtained as previously described. 10 A “positive” PET-PiB image, denoting the presence of cerebral Aβ deposits, is defined by a mean cortical binding potential (MCBP) for PiB of 0.2 or more (averaging the binding potentials in prefrontal cortex, precuneus, lateral temporal cortex, and gyrus rectus). 10
NEUROPATHOLOGICAL ASSESSMENT
Formalin-fixed tissue from fifteen standard cortical and subcortical regions are embedded in paraffin wax and sections cut at 7 µm as previously described.25 Hematoxylin and eosin (H&E) and a modified Bielschowsky silver impregnation is used on representative brain areas. Immunohistochemistry is performed using anti-ubiquitin (1:1,000, rabbit polyclonal antibody (PAB; Dako, Glostrup, Denmark), anti-TDP-43 (1:4,000, rabbit PAB, ProteinTech Inc., Chicago, IL) anti-tau (PHF-1; a gift from Dr P Davies, Albert Einstein School of Medicine, NY, NY), anti-α-synuclein (1:500, mouse monoclonal antibody (MAB) LB-509; Zymed, San Francisco, CA), and anti-Aβ (1:100,000, mouse MAB, 10D5; gift of Eli Lilley, Indianapolis, IN) antibodies. Additional immunohistochemical and histological analyses utilize the 6E10 antibody, targeting amino acids 1-16 (N-terminus) of Aβ (1:3,000, mouse MAB, Signet, Emeryville, CA) and β-sheet markers 6-CN-PiB and X-34 (the highly fluorescent derivatives of PiB26 and Congo red,27 respectively.
NOTE: The neuropathologist is aware of the clinical diagnoses of autopsied participants. However, the clinical, psychometric, CSF, and PET-PiB imaging assessments are completed by investigators who are unaware of the results from the other assessments.
CASE REPORT
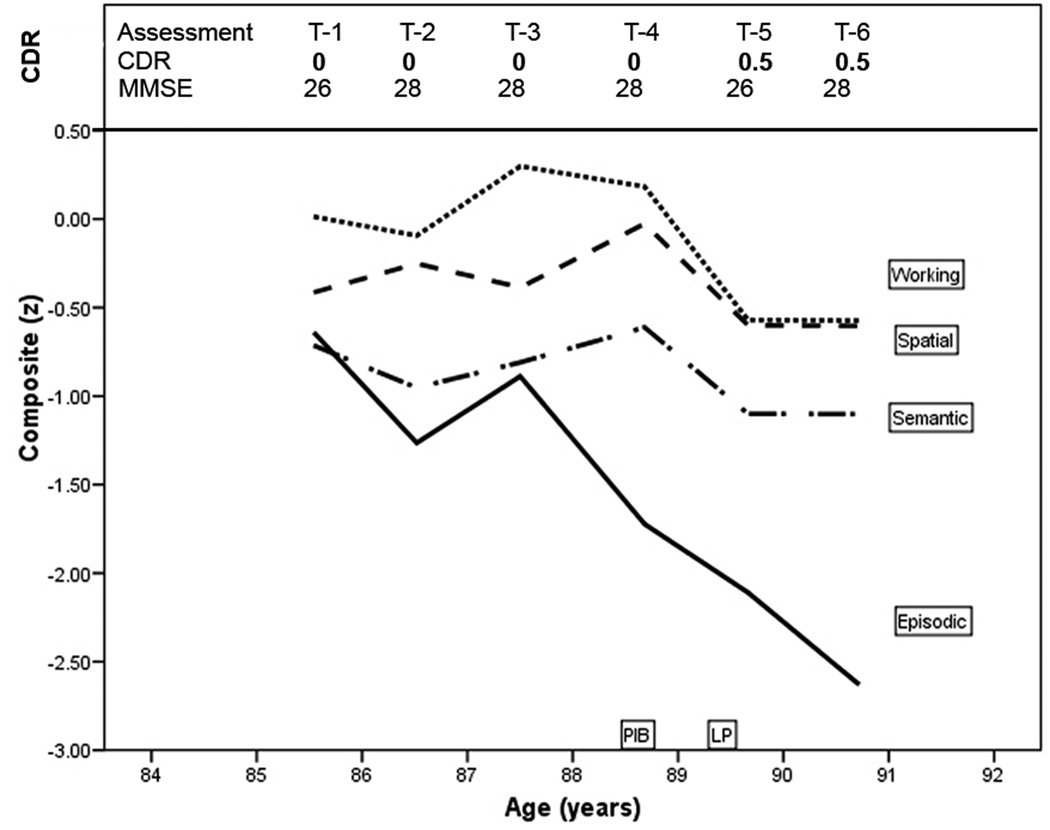
An 85-year-old male civil servant with 12 years of education and without a family history of dementia was CDR 0 (cognitively normal) at entry (T-1) and at the next 3 annual assessments through age 89 years (Figure 1). At age 90 years, his collateral source reported declining cognitive abilities with forgetfulness, poor decisional capacity, and mild interference with daily function (e.g., impaired driving abilities causing a motor vehicle accident). The participant could not recall reliably recent events in which he had participated. The MMSE score at age 90 was 26. He was diagnosed with very mild (CDR 0.5) dementia of the Alzheimer type (DAT) and died of congestive heart failure 6 months later, shortly after his 91st birthday. His APOE genotype was homozygous for ε3.
Figure 1.
Clinical and cognitive course of PiB-PET-negative case. T-1, first clinical assessment; CDR, Clinical Dementia Rating; z score, the means of four neuropsychological test composites: episodic memory, semantic memory, working memory, and visuospatial ability.
The longitudinal performance of this participant on measures of episodic memory, semantic memory, working memory, and visuospatial ability is shown in Figure 1. The most striking feature of Figure 1 is the two standard deviation drop on the episodic memory composite accompanied by a less precipitous decline in working memory (half a standard deviation), with maintained semantic memory and visuospatial ability.
At age 88.5 years a PET-PiB scan was unremarkable (PiB mean cortical binding potential = −0.006). At age 89.5 years CSF assays showed elevated total tau = 575 pg/mL (>500 pg/mL is abnormal) and ptau181 = 83 pg/mL (>80 pg/mL is abnormal) and lowered Aβ42 = 303 pg/mL (<500 pg/mL is abnormal), and Aβ40 = 12,943 pg/mL, which was in the normal range.7, 28
The autopsy was performed 2.5 years after the PiB-PET scan. The unfixed brain weighed 1,310 g (normal range: 1,250 – 1,400 g). External examination showed no cerebral atrophy. Coronal slicing revealed mild to moderate dilatation of the lateral ventricle with rounding of the angle and only modest increase in space in the inferior horn; the hippocampus appeared only slightly smaller than normal. The substantia nigra and locus coeruleus were well-pigmented.
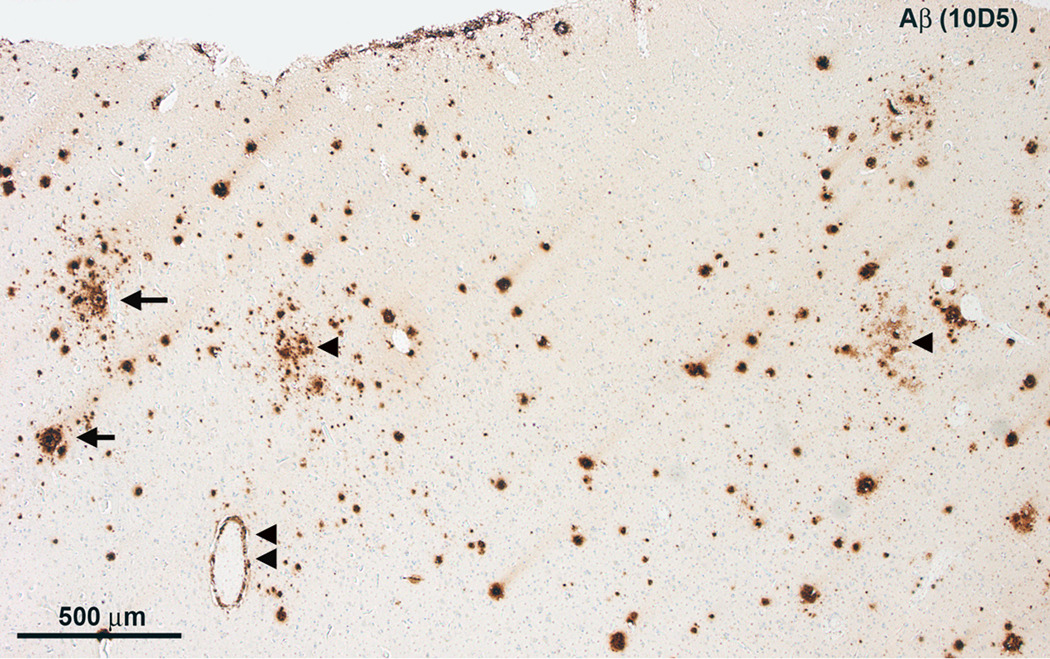
Microscopy of H&E stained tissue sections revealed some neuronal loss and gliosis throughout the frontal neocortex (not shown). There were sparse to focally numerous Aβ plaques (Figure 2) but only infrequent neuritic plaques. Isolated neurofibrillary tangles were seen using Bielschowsky silver staining and PHF-1 (not shown). There was no α-synucleinopathy and no TDP-43 proteinopathy[c1]. There was mild amyloid angiopathy and modest arteriolosclerosis. Moderate neuronal loss and gliosis in the CA1 subfield of the hippocampus were accompanied by modest granulovacuolar degeneration and several neurofibrillary tangles. There was also neuronal loss, gliosis, and a few neurofibrillary tangles, but no neuritic plaques, in the parahippocampal gyrus.
Figure 2.
Microscopy of the left frontal lobe. A, There are numerous diffuse Aβ plaques (arrowheads), but only few ring-with-core plaques (arrows) and modest cerebral amyloid angiopathy (double arrowhead).
The AD lesions met neurofibrillary stage III for tangles and amyloid stage C for plaques in the Braak and Braak staging system. 29, 30 The densities of diffuse plaques were sufficient to meet the age-adjusted neuropathological criteria for AD according to Khachaturian, 31 but the low densities of neuritic plaques and tangles were consistent only with “possible AD” according to the Consortium to Establish a Registry for Alzheimer’s Disease 32 criteria, and there was only a “low” probability that the very mild dementia was caused by AD according to the National Institute on Aging-Reagan Institute (NIA-Reagan) criteria. 33 There was no evidence of any other neurodegenerative or clinically meaningful vascular disease.
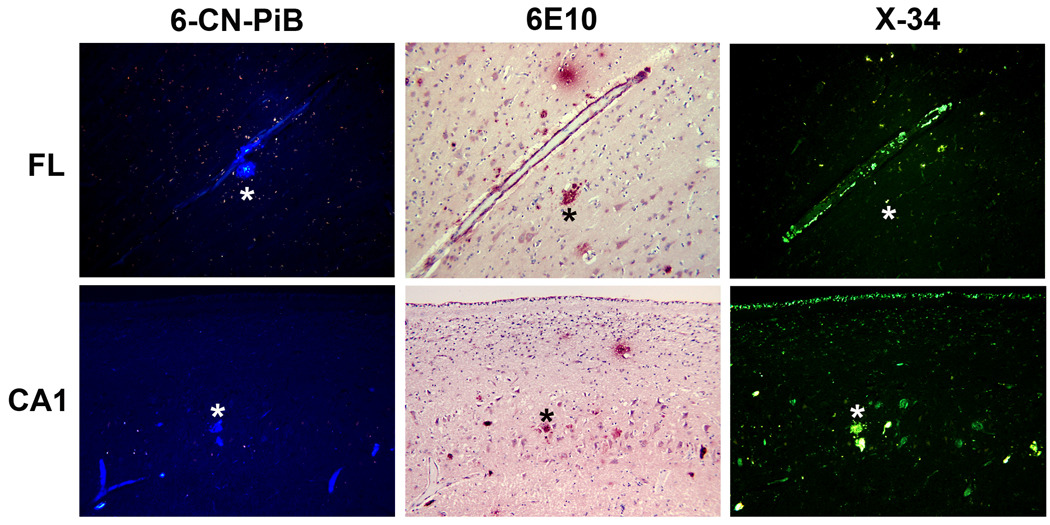
Biochemical analysis of frozen brain tissue included [3H]-PiB binding to brain tissue homogenates (Table 1). The binding levels were below those needed for in vivo PiB detection of fibrillar Aβ plaques, and X-34 and 6-CN-PiB histofluorescence staining revealed only scarce fibrillar Aβ plaques (Figure 3). 26, 27
Table 1.
PiB cortical binding potential in PiB-negative participant and Aβ load at autopsy
| Aβ | FL | AC | TL | PL | PC | OL | Mean |
|---|---|---|---|---|---|---|---|
|
PiB Mean BP |
−0.04 | −0.33 | 0.07 | 0.43 | 0.15 | 0.01 | 0.05* |
|
Aβ load (area fraction of 10D5 (%)) |
4.4 | 1.6 | 2.0 | 2.9 | 5.4 | 0.9 | 2.9 |
|
Aβ load [3H]PiB bound(pmol/g) |
249 | 124 | 161 | 116 | 225 | 295 | 195 |
|
Aβ load (Aβ42pmol/g) |
1582 | 837 | 722 | 687 | 1785 | 1525 | 1190 |
Abbreviations: FL, superior frontal gyrus of frontal lobe; TL, superior temporal gyrus; PL, inferior parietal lobe; OL, calcarine sulcus of occipital lobe; AC, anterior cingulate gyrus; PiB BP = binding potential as calculated by the Distribution Volume ratio minus 1.0;
indicates that cortical BP is less than that of white matter (0.34).
Figure 3.
Fluorescent β-pleated sheet stains label a spectrum of Aβ structures in the frontal lobe (upper panels) and CA1 subfield of hippocampus (lower panels) of postmortem brain of PiBPET-amyloid-negative participant. Amyloid is visible using 6-CN-PiB and X-34, highly fluorescent derivatives of PiB17 and Congo red18, respectively; the monoclonal antibody 6E10, targeting amino acids 1-16 (N-terminus) of Aβ identifies similar structures as denoted by the asterixes.
COMMENT
This case met conventional criteria for mild cognitive impairment (MCI).34 Our clinicians, however, are trained to use the observations of a collateral source to detect an individual’s impaired ability to conduct accustomed activities (e.g., driving a motor vehicle) due to decline in that individual’s previously attained cognitive function. The clinician determines the CDR based on these observations and the clinician’s assessment of the individual, but without knowledge of the individual’s psychometric performance or imaging or CSF results. When an individual is determined to have cognitive impairment, even at the CDR 0.5 level, the clinician then judges the likely cause or causes of that impairment. If that cause is judged on clinical grounds to be AD, the individual is diagnosed with DAT. Although this diagnosis may occur at an earlier stage of symptomatic AD than is common elsewhere, and even at a stage where psychometric performance is insufficiently impaired to meet criteria for MCI, it is supported by subsequent progressive cognitive and functional decline and by the histopathologic confirmation of AD in 92% of those individuals who come to autopsy.13, 35
The clinical diagnosis of very mild DAT (CDR 0.5) in this case was independently supported by cognitive decline in measures of episodic and working memory and by a CSF biomarker phenotype for AD, namely elevated tau and ptau181 levels and reduced Aβ42 levels. Neuropathological examination showed increased densities of diffuse plaques but more mature neuritic plaques were scarce and thus not likely to be identified in vivo by PiB-PET amyloid imaging.. These findings suggest that substantial densities of diffuse plaques, which themselves may be downstream of more toxic species of Aβ,36 are not benign as they can be associated with very early symptomatic stages of AD. Hence, neuropathological criteria such as the NIA-Reagan recommendations32 that do not incorporate diffuse Aβ plaques into their algorithms may overlook an important early pathogenic feature of AD.
Amyloid tracers such as PiB bind strongly to fibrillar Aβ in compact/cored plaques and cerebral amyloid angiopathy but only weakly to amorphous cortical Aβ plaques. 26, 37 These tracers thus may be unable to detect AD variants that are characterized predominantly by diffuse Aβ plaques. We reported previously that elevated mean cortical binding potential for PiB is almost always associated with low CSF Aβ42 but that low levels of CSF Aβ42 can occur in the absence of elevated mean cortical binding potentials for PiB, 6, 8 possibly because diffuse plaques can first appear in the absence of significant amount of fibrillar Aβ, as suggested by the current case. For individuals with substantial amounts of diffuse non-fibrillar Aβ deposits, reduced levels of CSF Aβ42 may reflect AD pathology prior to the presence of sufficient fibrillar plaques to allow detection by PiB. Although this is but one case, it implies that a CSF profile of reduced Aβ42 and elevated tau in nondemented individuals can serve as an antecedent biomarker for AD as it may predict future DAT.
A limitation of this single case study is the temporal dissociation between the various assessments. The scarcity of fibrillar Aβ plaques at autopsy, however, suggests that it is unlikely that the PiB-PET would have been positive even if imaging occurred closer to the time of lumbar puncture (one year later) or time of death (2.5 years later). Another limitation is that the battery of psychometric tests was chosen many years ago for the longitudinal study in which this man participated; it did not include the more sensitive measures of working memory that are available today. Newer instruments may have detected earlier and more profound decline in this domain. The battery also did not include measures of attention, which may also be affected very early in the course of the disease. 38
Acknowledgments
Funding/Support: This work was supported by grants P50-AG05681, P01-AG03991, and P01-AG26276 from the National Institute on Aging and P30-NS048056 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland, and by the Charles and Joanne Knight Alzheimer's Research Initiative of the Washington University Alzheimer's Disease Research Center. Sumi Chakraverty, MS, of the Center’s Genetics Core performed the APOE genotyping.
Footnotes
Author Contributions:
Study concept and design: Cairns, Benzinger, Storandt, Ikonomovic, Klunk, Holtzman, Mintun, Morris.
Acquisition of data: Cairns, Benzinger, Storandt, Fagan, Shah, Taylor Reinwald, Ikonomovic, Mintun, Morris
Drafting of the manuscript: Cairns, Storandt, Ikonomovic, Mintun, Morris
Critical revision of the manuscript for important intellectual content: Cairns, Benzinger, Storandt, Fagan, Shah, Taylor-Reinwald, Ikonomovic, Klunk, Holtzman, Mintun, Morris
Statistical analysis: Storandt.
Obtained funding: Morris and Klunk.
Administrative, technical, and material support: Cairns, Fagan, Klunk, and Morris.
Study supervision: Cairns and Morris.
Financial Disclosure: None reported.
Reference List
- 1.Frank RA, Galasko D, Hampel H, et al. Biological markers for therapeutic trials in Alzheimer's disease. Proceedings of the biological markers working group; NIA initiative on neuroimaging in Alzheimer's disease. Neurobiol Aging. 2003;24:521–536. doi: 10.1016/s0197-4580(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 2.Morris JC, Quaid KA, Holtzman DM, et al. The role of biomarkers in studies of presymptomatic Alzheimer's disease. Alzheimer's & Dementia. 2005;1:145–151. doi: 10.1016/j.jalz.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Mathis CA, Lopresti BJ, Klunk WE. Impact of amyloid imaging on drug development in Alzheimer's disease. Nucl Med Biol. 2007;34:809–822. doi: 10.1016/j.nucmedbio.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonnen JA, Montine KS, Quinn JF, Kaye JA, Breitner JCS, Montine TJ. Biomarkers for cognitive impairment and dementia in elderly people. Lancet Neurol. 2008;7:704–714. doi: 10.1016/S1474-4422(08)70162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 6.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 7.Fagan AM, Roe CM, Xiong C, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 8.Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid AB42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mintun MA, LaRossa GN, Sheline YI, et al. [11C] PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 11.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007 Nov;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 12.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathological outcomes in original versus revised MCI and in PreMCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. Mini-mental State: A practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alz Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D, Stone CP. Manual: Wechsler Memory Scale. New York: Psychological Corporation; 1973. [Google Scholar]
- 18.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Manual: Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1955. [Google Scholar]
- 20.Goodglass H, Kaplan E. Boston Naming Test scoring booklet. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 21.Thurstone LL, Thurstone LG. Examiner Manual for the SRA Primary Mental Abilities Test. Chicago: Science Research Associates; 1949. [Google Scholar]
- 22.Armitage SG. An analysis of certain psychological tests used in the evaluation of brain injury. Psych Mono. 1946;60:1–48. [Google Scholar]
- 23.Johnson DK, Storandt M, Morris J, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009 doi: 10.1001/archneurol.2009.158. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastor P, Roe CM, Villegas A, et al. APOE ε4 modifies Alzheimer's disease onset in an E280A PS1 kindred. Ann Neurol. 2003;54:163–169. doi: 10.1002/ana.10636. [DOI] [PubMed] [Google Scholar]
- 25.Behrens MI, Mukherjee O, Tu P-H, et al. Neuropathologic heterogeneity in HDDD1: A familial frontotemporal lobar degeneration with ubiquitin-positive inclusions and progranulin mutation. Alz Dis Assoc Disord. 2007;21:1–7. doi: 10.1097/WAD.0b013e31803083f2. [DOI] [PubMed] [Google Scholar]
- 26.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in-vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikonomovic MD, Abrahamson EE, Isanski BA, et al. X-34 labeling of abnormal protein aggregates during the progression of Alzheimer's disease. Methods Enzymol. 2006;412:123–144. doi: 10.1016/S0076-6879(06)12009-1. [DOI] [PubMed] [Google Scholar]
- 28.Fagan AM, Younkin LH, Morris JC, et al. Differences in Aβ40/Aβ42 associated with CSF lipoproteins as a function of apolipoprotein E genotype. Ann Neurol. 2000;48:201–210. [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol (Berl) 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 32.Mirra SS, Heyman A, McKeel DW, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 33.National Institute on Aging, Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 34.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 35.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer's disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 36.Shankar GM, Li S, Mehta TH, et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–434. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 38.Storandt M. Cognitive deficits in the early stages of Alzheimer's disease. Current Directions in Psychological Science. 2008;17:198–202. [Google Scholar]