Abstract
Recent studies on the neural bases of sensorimotor adaptation demonstrate that the cerebellar and striatal thalamocortical pathways contribute to early learning. Transfer of learning involves a reduction in the contribution of early learning networks, and increased reliance on the cerebellum. The neural correlates of learning to learn remain to be determined, but likely involve enhanced functioning of general aspects of early learning.
Keywords: sensorimotor adaptation, cerebellum, basal ganglia, skill learning, rehabilitation
INTRODUCTION
Adaptive and flexible modification of motor behavior is fundamental to the variety of skilled actions that characterize human behavior. As such, it is not surprising that motor learning is a heavily studied topic. In addition to examining the learning process itself, many studies make use of transfer tests to determine the generalizability and flexibility of acquired motor representations. Generalization of learning refers to the degree to which newly acquired skills can be produced with a new effector (23, 8), in a new workspace (28), or under new modes of movement (i.e., transfer from continuous tracking to discrete pointing movements (1)). The patterns of transfer are then used to infer whether acquired representations are spatial, motor (i.e., joint-based or tied to the effector used during practice), or more abstract.
In addition to studying motor acquisition and the nature of acquired representations, recent studies have also focused on transfer of learning, and learning to learn. The savings associated with transfer of learning (cf. 30) can be tested by having participants adapt movements to a perturbation, wash out the effects of learning, and then re-adapt to the same or a similar perturbation. This allows the determination of whether individuals can make use of the previously acquired motor memory to learn something new. There is also evidence that people can learn to learn new motor skills (4, 18). In this case, participants acquire multiple unrelated motor tasks (adapt to a change in the gain of display of movements, visual feedback display rotation, sequence learning, etc.) successively, with the end result that they show faster learning than naive participants do. These data provide evidence that people can acquire something very general and transferable about the learning process itself.
The purpose of this review is to provide an overview of our current understanding of these multiple forms of motor learning. The underlying neural and behavioral mechanisms of each will be outlined, with a particular focus on whether motor learning, transfer of learning, and learning to learn are dissociable or overlapping processes. Here, I advance the hypothesis that transfer of learning and learning to learn differ from motor learning in unique ways. I propose that transfer of learning is similar, both in terms of the neural substrates and behavioral processes that are engaged, to the late phase of motor learning. In contrast, I propose that learning to learn reflects an enhancement of early phase motor learning processes.
MOTOR LEARNING
Skill acquisition (used interchangeably with the term “motor learning” in this review) has been defined as “…a set of processes associated with practice or experience leading to relatively permanent changes in the capability for responding” (17). Motor learning can be divided into (at least) two major categories: sequence learning and sensorimotor adaptation. When learning new sequences, individuals combine isolated movements into one smooth, coherent action, such as when practicing the components of a tennis serve. For sensorimotor adaptation, participants modify movements in response to changes in sensory inputs or motor outputs, for example when adapting the motor commands for arm movements in response to the altered limb dynamics associated with holding a tennis racquet. In the current review, I will focus predominantly on the sensorimotor adaptation literature, as a comprehensive review of both types of learning is beyond the scope of this article. Multiple theories of motor learning that encompass both adaptation and sequence learning have been previously proposed (cf. 9, 33), and the reader is referred to these articles for further discussion of the topic.
The motor neuroscientist faces many challenges when aiming to identify the processes underlying motor learning. Because individuals learn at different rates and often adopt different strategies, it is difficult to characterize the dynamics of evolving motor behaviors. Moreover, motor learning is largely implicit, so verbal reports about the learning process are imprecise and unreliable. The greatest challenge occurs, however, because many of the same brain regions contribute to both skill learning and to motor execution, making it particularly difficult to determine their respective roles in each. Parametric manipulation of variables such as movement rate, force, or motor error have revealed corresponding changes in activity in the sensory and motor cortical and subcortical regions of the brain (cf. 2, 26). Since these same variables change with skill acquisition, it is difficult to disentangle whether changes in brain activation are reflecting the differing performance levels that occur with practice, or rather represent true contributions to the learning process. To address this issue in the context of our motor learning experiments outlined in this review, we conducted a control study in which we induced performance changes in the absence of learning (26). Participants made manual aiming movements using a joystick to hit targets of differing sizes on a computer display. Movements to smaller targets were slower and characterized by larger errors. We found that “better performance” (movements to larger targets, which participants made faster and with fewer errors) was associated with greater activation in the contralateral primary motor cortex, premotor cortex, and the basal ganglia (see Fig. 1, panels A and B). In contrast, we found that “poorer performance” (movements to smaller targets, which participants made more slowly and with larger errors) was associated with greater activation in the ipsilateral motor cortex, insular cortex, cingulate motor area, and multiple cerebellar regions (see Fig.. 2, panels A and B). We are able to use these results to aid in the interpretation of our motor learning studies by determining whether a brain region that changes activation across the time course of learning is also associated with performance change in our control study. Such a finding would imply that a brain region may not be actively involved in the learning process per se, but rather is reflecting the changes in performance that occur with practice.
Figure 1.
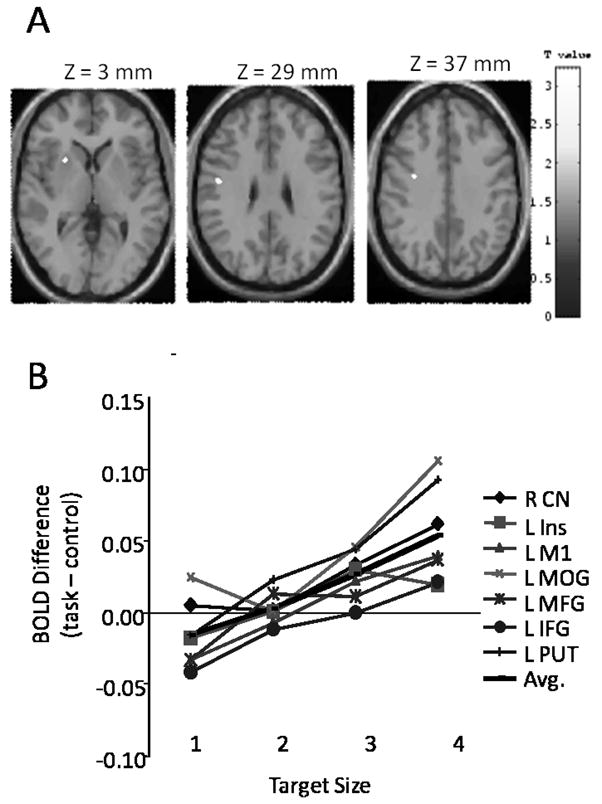
Panel A highlights brain regions that increase their activity with increasing target size, including the left putamen (left slice), left primary motor cortex (middle slice), and left premotor cortex (right slice). Panel B presents signal change for all areas that increased activation with increasing target size: CN (caudate nucleus), Ins (insula), M1 (primary motor cortex), MOG (middle occipital gyrus), MFG (middle frontal gyrus), IFG (inferior frontal gyrus), PUT (putamen). [Adapted from Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. NeuroImage. 2004:22(4):1775–1783. Copyright © 2004 Elsevier. Used with permission.]
Figure 2.
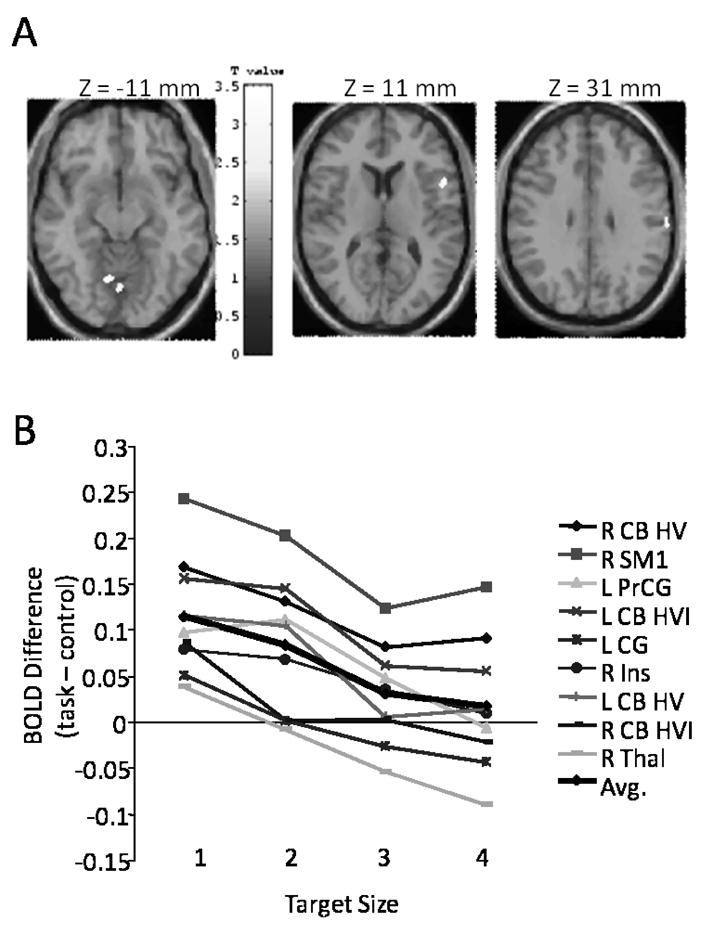
Panel A highlights brain regions that decrease their activity with increasing target size, including the medial cerebellum (left slice), right ventral premotor cortex (middle slice), and right sensorimotor cortex (right slice). Panel B presents signal change for all areas that decreased activation with increasing target size: CB HV (cerebellum hemisphere V), SM1 (primary sensorimotor cortex), PrCG (precentral gyrus), CB HVI (cerebellum hemisphere VI), CG (cingulate gyrus), Ins (insula), Thal (thalamus). [Adapted from Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. NeuroImage. 2004:22(4):1775–1783. Copyright © 2004 Elsevier. Used with permission.]
Sensorimotor adaptation tasks are used to gain insight into how humans represent their environment, the mechanics of the body, and interactions between the two during movement planning and production. When individuals adapt movements to altered visual input, produced for example by prismatic lenses (32), or altered visual feedback on a computer screen (4, 5) they perform kinematic adaptations. When they adapt movements to force field perturbations (28), they perform kinetic adaptations. There is evidence that the cerebellum plays a central role in sensorimotor adaptation, predicting the sensory consequences of actions based on the motor commands and an internal model of the body dynamics (15). These representations are updated via error feedback during sensorimotor adaptation (28). Work by Imamizu and colleagues (12) has consistently shown brain activation in the cerebellar regions surrounding the posterior superior fissure during adaptation of movements to differing visual distortions, even after correction for performance differences occurring across the time course of learning. These findings support the notion that the cerebellum plays a role in sensorimotor adaptation.
Recent neuroimaging studies have shown involvement of the basal ganglia networks during kinetic adaptations (27), but not during kinematic adaptations (with the exception of adaptation to an altered gain of display, 13). In contrast, patients with Parkinson’s or Huntington’s disease, which affect basal ganglia function, exhibit deficits in kinematic adaptation (14). In an effort to resolve this discrepancy, we conducted an fMRI investigation of kinematic adaptation, focusing specifically on the early stage of learning (24). Participants adapted manual aiming movements to either a 30° or 45° clockwise rotation of the visual feedback display (see Fig.. 3), while we acquired functional magnetic resonance images. A novel finding of this study was bilateral basal ganglia activation. We observed activation in the right globus pallidus and putamen, along with the right prefrontal, premotor and parietal cortex during early adaptation, which we proposed may support spatial cognitive processes of adaptation. We also observed activation in the left globus pallidus and caudate nucleus, along with the left premotor and supplementary motor cortex, which we proposed may support sensorimotor processes of adaptation. These results demonstrate a clear involvement of the basal ganglia networks in this type of kinematic motor adaptation task (24). These findings are consistent with a recent theory of motor learning (9) and a computational model of adaptive processes (10).
Figure 3.
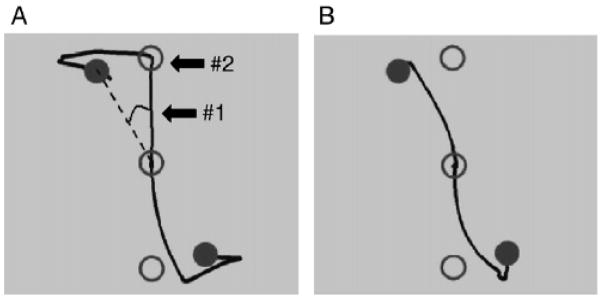
Adaptation data from a typical participant. Panel A depicts single trial spatial trajectories for two trials under the 30° feedback rotation condition early in adaptation. The open circles represent target location in visual space, while the filled circles represent the target locations in joystick space. The spatial trajectory is presented in joystick coordinates as well (participants would view the cursor moving along this path in real time, rotated clockwise by 30°). Panel B depicts single trial spatial trajectories from the same participant performing under the 30° rotation late in adaptation. Learning is evidenced by the straighter trajectories compared to panel A. The arrow labeled #1 in panel A indicates where direction error (DE) is calculated, and refers to the point along the spatial trajectory at which peak velocity was achieved. DE is the angle between the dashed line from the start to the target position, and a straight line from the start to the position at peak velocity. The arrow labeled #2 indicates where initial endpoint error (IEE) is calculated, which is at the endpoint of the initial ballistic movement towards the target. IEE is the distance from this spatial location to the target. [Adapted from Anguera JA, Russell CA, Noll DC, Seidler RD. Neural correlates associated with intermanual transfer of adaptation. Brain Research 2007;1185: 136–151. Copyright © 2007 Elsevier. Used with permission.]
It is possible that the cortico-striatal circuitry may be playing a role in on-line error corrections (movement adjustments that are made within a trial) as opposed to playing a direct role in the learning process (adaptive adjustments made from one trial to the next). For example, patients with Huntington’s disease, a basal ganglia pathology, are unable to perform within-trial adjustments for motor errors, but they do adapt their performance across trials (29). In contrast, patients with cerebellar damage show the complementary deficit; that is, they can make on-line motor adjustments, but they do not show adaptive performance across trials (16, 29). Both types of corrections are made early in the learning process, so the basal ganglia activation that we observed early in the adaptation process (24) may be reflective of on-line error corrections. However, the studies showing basal ganglia contributions to corrective actions have all involved responses to force (kinetic) perturbations as opposed to kinematic perturbations, such as the one that we employed. Patients with basal ganglia pathology may have exhibited deficits for these tasks due to the known role that the basal ganglia play in force control (cf. 31), rather than due to a generalized role for this system in making error corrections. This hypothesis is supported by our control study (26), in which we did not see increased activation in the basal ganglia nuclei for conditions in which participants made larger corrective submovements (i.e., movements to smaller targets). We believe that a more plausible reason for engagement of the basal ganglia circuitry during the early phases of adaptation is to support cognitive processes such as attention and working memory. This seems like a reasonable interpretation of our findings, given that the activation we observed was bilateral (as opposed to contralateral to the moving hand) and encompassed the caudate nucleus (24).
In summary, our work and that of others shows clear involvement of the cerebellar thalamocortical networks to sensorimotor adaptation. These structures may contribute to adaptation through detection and correction of motor errors, as well as storage of newly acquired representations. Our work also demonstrates involvement of the cortico-striatal system during adaptation. It remains to be determined whether engagement of this system is related to performance (i.e., on-line error corrections) or instead actively supports cognitive components of the learning process.
TRANSFER OF LEARNING
Skill learning consists not only of the learning process itself, but also the ability to transfer what has been learned to new conditions and task variants (savings of learning). For example, let’s say that you have mastered the game of tennis, and now you want to learn to play racquetball. There are similarities between the two sports, such as using a racquet to hit a ball and the need to predict an opponent’s actions, and differences as well, including the dynamics of the ball and racquet, the court surface, and the rules. These similarities and differences can lead to both positive and negative (due to interference) transfer of what you’ve already learned while practicing tennis. In the rehabilitation domain, an example of transfer of learning would be transitioning from making a movement under physical guidance to performing the task unconstrained. Recent work has focused on whether the mechanisms underlying motor acquisition (i.e., learning to play tennis) overlap with those of motor transfer (i.e., learning to play racquetball after already having learned tennis). I propose that transfer of learning is neurally and behaviorally similar to the late phase of motor learning.
We began to investigate the mechanisms of motor transfer in a study in which participants learned three related kinematic adaptation tasks in a sequential fashion (adaptation of manual aiming movements to three different magnitudes of clockwise feedback display rotation), allowing us to evaluate their ability to transfer what they were learning across the three tasks. We found that adaptation on one task occurred at a faster rate when that task was preceded by other adaptive experiences. Moreover, we found that the amount of savings that participants exhibited depended on the ordering of conditions and the variables used to assess transfer (19). These results suggest that multiple fundamentally distinct processes are at play during motor transfer. We have also shown that motor acquisition and transfer are differentially affected by the aging process. That is, both young and older adults exhibit better adaptation performance following other adaptive experiences (22), despite the fact that older adults exhibit impairments in initial adaptation (20). This finding is important, as it indicates that some aspects of sensorimotor plasticity are not detrimentally affected by the aging process. Effective rehabilitation paradigms should exploit this preserved plasticity in older adults. The findings also suggest that the underlying mechanisms of learning and transfer may differ, with those supporting learning, but not transfer, affected by the aging process.
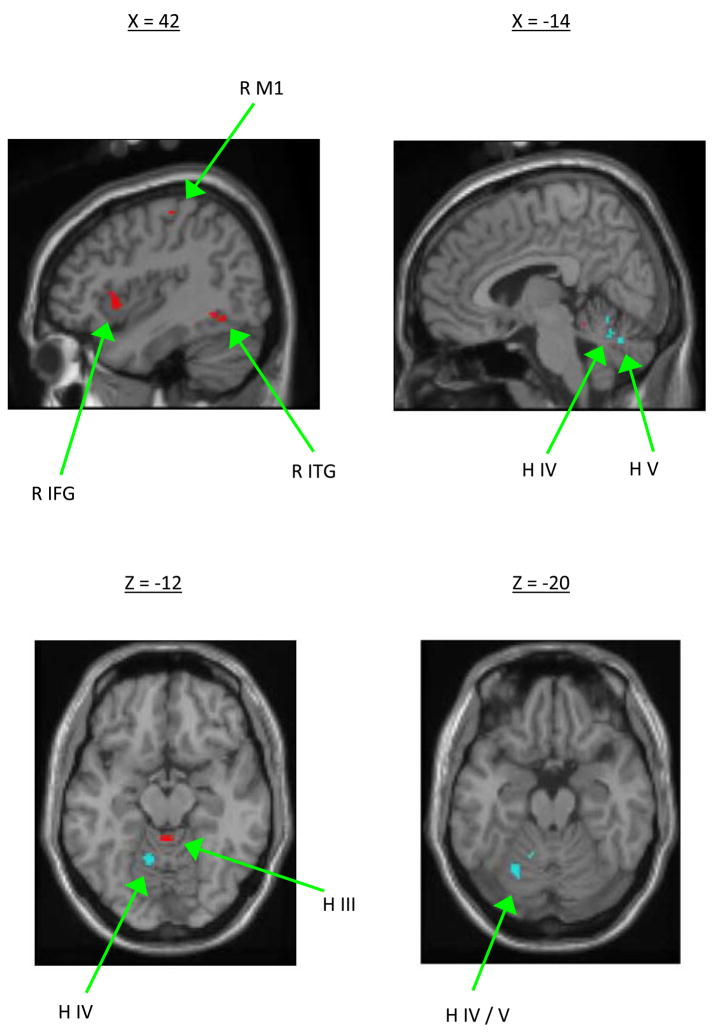
Previous investigations of motor savings demonstrate that a memory of prior learning remains, which can be relied upon for faster subsequent learning. Smith et al. (30) developed a computer model of short-term motor adaptation consisting of two processes: a fast learning module that was also fast to forget, and a slow learning module that was slow to forget. The model successfully recreated several aspects of adaptation, including savings. Based on this model, we hypothesized that brain regions which are more active during the fast, early stages of learning would not be re-engaged at transfer, because their memory for the learning decays quickly. In support of this idea, we found that motor transfer is associated with a reduction in activity of brain regions that play a role early in the adaptation process, including the right inferior frontal gyrus, primary motor cortex, inferior temporal gyrus, and the cerebellum (medial HIII and lateral HIV/V, see Fig.. 4) (25). Moreover, while these regions exhibit activation that is correlated across participants with the rate of acquisition, the degree of savings at transfer was correlated with activity in the right cingulate gyrus, left superior parietal lobule, right inferior parietal lobule, left middle occipital gyrus, and bilaterally in the cerebellum (HV/VI). The cerebellar activation was in the regions surrounding the posterior superior fissure (see Fig. 4), which is thought to be the site of storage associated with sensorimotor adaptation learning. Thus, we found that motor transfer is associated with brain activation that typically characterizes late learning and storage. Transfer seems to involve retrieval of a previously formed motor memory, allowing the learner to move more quickly through the early stage of learning (25).
Figure 4.
The top left slice shows brain regions that reduced their activation at transfer of learning in comparison to motor learning, including the right primary motor cortex, inferior frontal gyrus, and inferior temporal gyrus. The top right slice shows cerebellar activity that was correlated with the magnitude of transfer shown by individual participants, including activation in HIV and HV. The bottom two slices depict the location of this activation (shown in blue) in comparison to the cerebellar region showing a reduction in activation at transfer of learning (shown in red). (Reprinted from Seidler, R. D. & Noll, D. C. Neuroanatomical correlates of motor acquisition and motor transfer. J Neurophys. 2008:99:1836–1845. Copyright © 2008 The American Physiological Society. Used with permission.)
In summary, our work on motor transfer provides evidence that motor acquisition and transfer are unique processes, dissociated by age and their neural substrates. Transfer of learning involves retrieval and modification of previously acquired representations, and is neurally similar to the late phase of learning. Future work should be aimed at elucidating the underlying component processes contributing to these adaptive motor behaviors.
LEARNING TO LEARN – A NOVEL HYPOTHESIS
Traditionally, it has been thought that motor learning follows a specificity of learning principle, which suggests that learning is specific to the learning context and the task performed (3) and that generalized motor abilities do not exist (11). In contrast with this idea, recent evidence demonstrates that a facilitation of learning can be observed when participants transfer to a new skill that is independent from those recently experienced, if they first participate in multiple bouts of learning (4, 18). This would be akin to your prior experiences learning tennis and racquetball benefitting your ability to learn to ski, for example. The popular rehabilitation paradigm constraint-induced movement therapy includes components of learning to learn as well, since patients typically perform multiple functional behaviors during a single session.
To test the specificity of the “learning to learn” phenomenon, we asked participants to learn five different motor tasks, three that were similar to each other (three magnitudes of visuomotor rotation) and two that were not related (gain adaptation and sequence learning). Participants showed transfer of learning across the three visuomotor rotations, and, surprisingly, also exhibited transfer of learning to the gain change and the movement sequence, showing faster learning than that seen in the control group. However, the experimental group had less stable movements when faced with transient perturbations. These data demonstrate that humans can acquire a general enhancement in motor skill learning capacity through experience, but it comes with a cost. Although movement becomes more adaptable following multiple learning experiences, it also becomes less resistant to external perturbation (18).
The mechanisms underlying the learning to learn effect are unclear. One possibility is that participants modulate limb stiffness, which can be done in a task-dependent manner across the time course of learning (cf. 6). Decreasing limb stiffness could be a useful method for the system to gain more information about environmental and task changes, because it would make the limb more sensitive to perturbations. This would result in larger errors, providing the system more information to learn from. Another potential mechanism is that participants become better at some generalized aspect of skill learning, such as pattern detection or error detection and correction. These are functions that have been attributed to the anterior cingulate cortex (cf. 7), which is typically engaged in the early phase of the motor learning process, regardless of the type of learning that participants are engaged in (24). These and other potential mechanisms of the learning to learn effect remain to be tested.
Interestingly, we (21) and others (5) have shown that older adults exhibit intact “learning to learn” for motor skills. These findings have implications for the design of rehabilitation interventions. In order to maximize rate of learning and degree of transfer, training should not be specifically focused; rather it should encompass a varied program of motor learning experiences.
To summarize, we have demonstrated that individuals have an ability to learn how to improve their rate of motor learning. This ability is not negatively impacted by the aging process. The neural bases of this effect remain to be determined; similar to transfer of learning, though, the dissociation between learning deficits and intact learning to learn in older adults (21) suggests that the underlying mechanisms differ between these components of sensorimotor adaptability. Our hypothesis that learning to learn involves an enhancement of early motor learning processes remains to be tested in future experiments.
CONCLUSIONS
Our ongoing work has been aimed at determining the neural and behavioral bases of different forms of skill acquisition including motor learning, transfer of learning, and learning to learn. For sensorimotor adaptation tasks, early learning engages the basal ganglia thalamocortical loops, medial cerebellum, the anterior cingulate cortex, the inferior frontal gyrus, and visual and parietal cortical areas (see Fig. 5). This activation pattern likely supports cognitive aspects of the task, such as error detection and correction, working memory, and attention. As stated in the introduction, I hypothesize that learning to learn involves enhanced functioning of these component processes and their underlying neural systems. This seems plausible given that the learning to learn benefit is evident primarily in the early phase of skill learning, but concrete evidence is lacking. This remains a fruitful area for future investigation.
Figure 5.
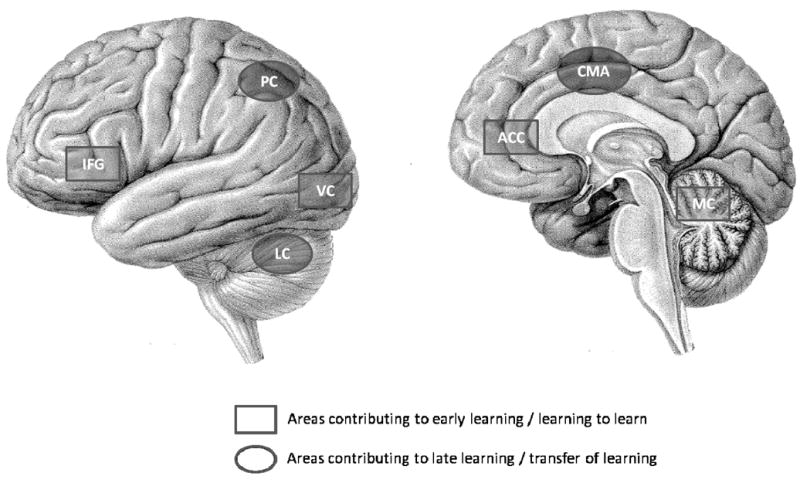
The left image shows a lateral view of the brain while the right depicts a midsaggital image. We and others have shown that the early phase of learning sensorimotor adaptation tasks involves the basal ganglia thalamocortical loops (not shown), the medial cerebellum (MC), the anterior cingulate cortex (ACC), the inferior frontal gyrus (IFG), and visual (VC) and parietal (PC) cortical areas. We hypothesize that learning to learn involves enhanced contributions by these regions. Late learning involves the lateral cerebellum (LC), parietal (PC) and cingulate motor areas (CMA). Transfer of learning is associated with reduced contributions of the early learning network, and overlapping activation with late learning regions. [Adapted from Martin J. Neuroanatomy: Text and Atlas (3rd edition), 2003. McGraw-Hill Medical. New York. Copyright © 2003 McGraw Hill. Used with Permission.)
In contrast, the late phase of motor learning engages the lateral cerebellum, parietal and cingulate motor cortical areas (see Fig. 5). This brain activation pattern likely supports storage and refinement of newly acquired sensorimotor representations. Transfer of learning, which involves retrieval and modification of previously acquired internal models, shows brain activation patterns that are similar to those of the late phase of motor learning, suggesting the engagement of overlapping processes for the two types of learning. Early learning processes may also be engaged at transfer, albeit at a reduced amplitude and over a much shorter timescale.
The encompassing scheme of motor learning processes outlined in this review extends existing models (cf. 9, 33) to include data and hypotheses regarding the neural and behavioral bases of transfer of learning and learning to learn. Understanding the neural bases and underlying cognitive components of motor learning, transfer of learning, and learning to learn, helps to identify why certain movement disorder populations, such as patients with Parkinson’s disease, experience difficulty with aspects of skill acquisition. Moreover, the use of neuroimaging techniques can inform our knowledge of relationships between brain structure and function in the healthy brain, leading to a greater understanding of changes in motor performance associated with neurological disease and age. This information provides a necessary prerequisite to the design of effective interventions and treatments for these populations. Future rehabilitation paradigms should be based on newly emerging principles of motor learning, such as those outlined in the current review.
Acknowledgments
Disclosure of Funding: NIH AG 24106, Gustavus and Louise Pfeiffer Research Foundation
Footnotes
Summary: This article compares and contrasts the neural bases of sensorimotor adaptation learning, transfer of learning, and learning to learn.
References
- 1.Abeele S, Bock O. Transfer of sensorimotor adaptation between different movement categories. Exp Brain Res. 1993;148:128–32. doi: 10.1007/s00221-002-1317-0. [DOI] [PubMed] [Google Scholar]
- 2.Ashe J. Force and the motor cortex. Behavioural Brain Research. 1997;87:255–69. doi: 10.1016/s0166-4328(97)00752-3. [DOI] [PubMed] [Google Scholar]
- 3.Bachman JC. Specificity vs. generality in learning and performing two large muscle motor tasks. Research Quarterly. 1961;32:3–11. [Google Scholar]
- 4.Bock O, Schneider S, Bloomberg J. Conditions for interference versus facilitation during sequential sensorimotor adaptation. Exp Brain Res. 2001;138:359–365. doi: 10.1007/s002210100704. [DOI] [PubMed] [Google Scholar]
- 5.Bock O, Schneider S. Sensorimotor adaptation in young and elderly humans. Neurosci Biobehav Rev. 2002;26(7):761–7. doi: 10.1016/s0149-7634(02)00063-5. [DOI] [PubMed] [Google Scholar]
- 6.Burdet E, Osu R, Franklin DW, Milner TE, Kawato M. The central nervous system stabilizes unstable dynamics by learning optimal impedance. Nature. 2001;414:446–449. doi: 10.1038/35106566. [DOI] [PubMed] [Google Scholar]
- 7.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 8.Chase C, Seidler R. Degree of handedness affects intermanual transfer of skill learning. Exp Brain Res. 2008;190:317–328. doi: 10.1007/s00221-008-1472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobio. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Grosse-Wentrup M, Contreras-Vidal JL. The role of the striatum in adaptation learning: a computational model. Biological Cybernetics. 2007;96:377–388. doi: 10.1007/s00422-007-0142-8. [DOI] [PubMed] [Google Scholar]
- 11.Henry FM. Factorial structure of speed and static strength in a lateral arm movement. Research Quarterly. 1960;31:440–447. [Google Scholar]
- 12.Imamizu H, Miyauchi S, Tamada T, et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- 13.Krakauer JW, Ghilardi MF, Mentis M, et al. Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J Neurophys. 2004;91:924–933. doi: 10.1152/jn.00675.2003. [DOI] [PubMed] [Google Scholar]
- 14.Laforce R, Jr, Doyon J. Differential role for the striatum and cerebellum in response to novel movements using a motor learning paradigm. Neuropsychologia. 2002;40(5):512–7. doi: 10.1016/s0028-3932(01)00128-2. [DOI] [PubMed] [Google Scholar]
- 15.Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Networks. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 16.Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt RA. Motor Control and Learning: A Behavioral Emphasis. 2. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 18.Seidler RD. Multiple motor learning experiences enhance motor adaptability. J Cog Neuro. 2004;16:65–73. doi: 10.1162/089892904322755566. [DOI] [PubMed] [Google Scholar]
- 19.Seidler RD. Differential transfer processes in incremental visuomotor adaptation. Motor Control. 2005;9:40–58. doi: 10.1123/mcj.9.1.40. [DOI] [PubMed] [Google Scholar]
- 20.Seidler RD. Differential effects of age on sequence learning and sensorimotor adaptation. Brain Res Bull. 2006;70:337–346. doi: 10.1016/j.brainresbull.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Seidler RD. Older adults can learn to learn new motor skills. Behav Brain Res. 2007;183:118–122. doi: 10.1016/j.bbr.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidler RD. Aging affects motor learning but not savings at transfer of learning. Learn Mem. 2007;14:17–21. doi: 10.1101/lm.394707. [DOI] [PubMed] [Google Scholar]
- 23.Seidler RD, Bloomberg JJ, Stelmach GE. Patterns of transfer of adaptation among body segments. Behav Brain Res. 2001;122:145–157. doi: 10.1016/s0166-4328(01)00183-8. [DOI] [PubMed] [Google Scholar]
- 24.Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res. 2006;175:544–555. doi: 10.1007/s00221-006-0571-y. [DOI] [PubMed] [Google Scholar]
- 25.Seidler RD, Noll DC. Neuroanatomical correlates of motor acquisition and motor transfer. J Neurophys. 2008;99:1836–1845. doi: 10.1152/jn.01187.2007. [DOI] [PubMed] [Google Scholar]
- 26.Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. NeuroImage. 2004;22(4):1775–1783. doi: 10.1016/j.neuroimage.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277(5327):821–5. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- 28.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophys. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- 30.Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biology. 2006;4:1035–1043. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophys. 2007;98(2):821–834. doi: 10.1152/jn.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Helmholtz H. In: A treatis on physiological optics. Southall TJPC, translator. New York: Dover; 1909 1962. [Google Scholar]
- 33.Willingham DB. A neuropsychological theory of motor skill learning. Psych Rev. 1998;105:558–584. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]