Abstract
Here, we review the clinical and translational implications of the caveolin gene family for understanding the pathogenesis of human diseases, including breast and prostate cancers, pulmonary hypertension, cardiomyopathy, diabetes, and muscular dystrophy. Detailed phenotypic analysis of caveolin knock-out mice has served to highlight the crucial role of a caveolin-deficiency in the pathogenesis of many human disease processes. Mutations in the human caveolin genes are associated with a number of established genetic disorders (such as breast cancer, lipodystrophy, muscular dystrophy, and cardiomyopathy), making the caveolins important and novel targets for drug development. The implementation of new strategies for caveolin-replacement therapy—including caveolin-mimetic peptides—is ongoing.
Keywords: Caveolae, Caveolins, Human Disease Pathogenesis, Mouse Animal Models
Overview
Caveolins are the signature proteins of specialized invaginations of the plasma membrane, named caveolae, that function to regulate signal transduction within the cell. Three members of the caveolin family protein have been identified, caveolin-1, -2 and -3. Caveolin-1 and -2 are co-expressed in a wide range of tissues, whereas caveolin-3 is muscle specific. The generation of caveolin null mice has demonstrated clear roles for the caveolin proteins in mammalian physiology. Caveolin-1 null mice present several pathological phenotypes, suggesting that caveolin-1 has pleiotropic functions in various organs. Caveolin-1 null mice show increased susceptibility towards the development of epidermal and mammary tumors, but exhibit decreased tumor formation in the prostate, demonstrating that caveolin-1 behaves as a tumor suppressor or a tumor promoter depending on the cellular context. Moreover, several other phenotypes of caveolin-1 null mice indicate that caveolin-1 plays an important role in the regulation of insulin signaling, and in pulmonary and cardiac function.
Caveolin-2 null mice reveal unique functions of caveolin-2 in the lung and in the skeletal muscle. Indeed, caveolin-2 null mice display lung defects with increased alveolar septa and fibrosis, and exhibit a peculiar skeletal muscle phenotype, with tubular aggregates formation and mitochondrial proliferation. Caveolin-3 null mice exhibit pathological changes in the skeletal muscle and the heart, and show an unexpected metabolic phenotype, with increased adiposity and insulin resistance.
Importantly, data from human samples confirm that caveolins are involved in human pathology. Mutations in the caveolin-1 gene are found in breast cancer as well as in oral squamous cell carcinomas. Caveolin-1 mutations are associated exclusively with estrogen receptor positive breast tumors, suggesting that caveolin-1 normally regulates estrogen receptor expression and signaling. Mutations in the caveolin-3 gene are detected in a wide spectrum of skeletal muscle disorders, including limb girdle muscular dystrophy, distal myopathy, idiopathic hyperCKemia, rippling muscle disease, as well as in cardiac pathologies such as familial hypertrophic cardiomyopathy, Long-QT Congenital Syndrome, and sudden infant death syndrome.
Thus, modulation of caveolin expression may represent a novel therapeutic approach for the cure of a number of pathological conditions. Preliminary studies have shown that a caveolin-1 mimetic peptide may be successfully used for the treatment of cancer and of pulmonary hypertension. More studies will be required to evaluate the possibility of using caveolin-1 mimetic peptides in other pathological contexts.
Caveolae and the Caveolin Gene Family
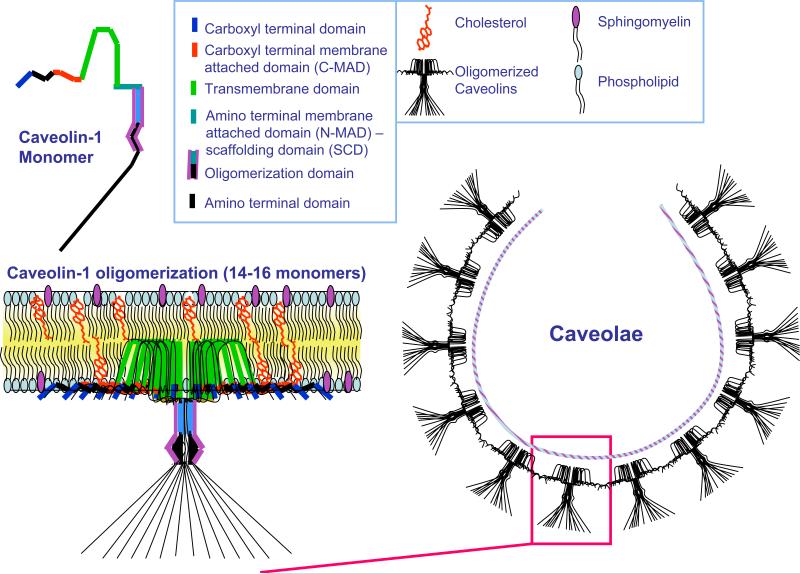
Caveolae are 50-100-nm omega-shaped invaginations of the plasma membrane. Due to their high content of cholesterol, glycosphingolipids and sphingomyelin, caveolae are considered a specialized subset of detergent-insoluble plasma membrane microdomains, named lipid rafts (1). The signature proteins of caveolae are a family of proteins called caveolins (2). Structurally, the formation of caveolae requires the capability of caveolin to bind cholesterol and to oligomerize (Figure 1) (3-5). Three members of the caveolin family have been discovered so far. Caveolin-1 (Cav-1) is widely expressed and is most abundant in adipocytes, fibroblasts, endothelial cells as well as epithelial cells such as mammary epithelial cells and type I pneumocytes (6). Cav-1 is essential for caveolae formation, and Cav-1 expression levels correlate with caveolae numbers (7, 8). Caveolin-2 (Cav-2) is expressed in the same cell types as Cav-1 (9, 10). Caveolin-3 (Cav-3) is the muscle specific family member and is mainly found in heart, skeletal muscle, as well as smooth muscle cells (11). Caveolins are more than just caveolae-associated proteins. They help the formation of a microenvironment to compartmentalize several signaling molecules, thereby facilitating the cross-talk between different signaling pathways (12). The caveolin-scaffolding domain (CSD) serves as a docking site for the binding and tonic inhibition of several classes of molecules belonging to pro-proliferative and pro-survival pathways. Thus, deregulation of caveolin expression and function is involved in many pathological conditions. The physiological implications of caveolins became evident with the generation of null (−/−) mice for each caveolin.
Figure 1. Caveolin Domain Organization, Membrane Topology, Oligomerization, and Caveolar Assembly.
This diagram summarizes the domain organization of a caveolin-1 monomer, its self-oligomerization into caveolar assembly units, and the intergration of these assembly units into a caveolar membrane domain. The cytoplasmic surface of the caveolar coat provides an “integrated scaffold” onto which various classes of signaling molecules can assemble at the plasma membrane, such as G-proteins, Src-family tyrosine kinases, endothelial nitric oxide synthase (eNOS), and components of the Ras-p42/44 MAP kinase cascade. Caveolae organelles also function as docking sites for many different classes of cell surface receptors, including G-protein coupled receptors, cytokine receptors, and receptor-tyrosine kinases.
Cav-1 in Tumor Biology: Is Cav-1 a Tumor Suppressor, an Oncongene or Both?
Several lines of evidences indicate that Cav-1 may act as a tumor suppressor or a tumor promoter depending on the organ. Cav-1 expression is downregulated in NIH-3T3 cells transformed with various oncogenes such as H-Ras (G12V), Bcr-Abl and v-Abl (13). In soft agar, the anchorage-independent growth of transformed cells can be reversed by Cav-1 re-expression (14, 15). In NIH-3T3 cells, antisense-mediated down-regulation of endogenous Cav-1 induces anchorage-independent growth, promotes tumor formation in nude mice with hyperactivation of the MAP kinase pathway (16). Importantly, loss of Cav-1 in mice was shown to promote cellular growth in several contexts, including the mammary gland and the skin. For example, although the ablation of Cav-1 is not sufficient to induce spontaneous tumor formation, Cav-1 null mammary glands display cellular hyperplasia, and accelerated mammary gland development during pregnancy (17, 18). In the MMTV-PyMT (mouse mammary tumor virus-polyoma middle T antigen) mouse model background, loss of Cav-1 accelerates dysplastic lesion formation, promotes premature development of mammary tumors, and increases metastatic potential (19, 20). Additionally, the combined loss of INK4a, a tumor suppressor, and Cav-1 lead to severe mammary hyperplasia with increased side branching and fibrosis (21). In the skin, Cav-1 (−/−) mice demonstrate hypersensitivity to carcinogen-induced epidermal tumors. Following 16 weeks of treatment with the carcinogen DMBA, Cav-1 (−/−) null mice show very significant increases in tumor incidence, tumor area and tumor number per mouse when compared to wild type counterparts (22). Mechanistically, in the mammary gland and in the skin, Cav-1 is thought to inhibit proliferation and cellular growth. Indeed, in the context of Cav-1 genetic ablation, carcinogen-induced epidermal tumors and oncogene-induced mammary tumors display hyperactivation of the mitogenic p42/44 MAP kinase pathway, with increased levels of Cyclin D1 (20, 22).
However, Cav-1 does not behave as a tumor suppressor in all cellular contexts. A growing body of evidence derived from cellular, mouse, and human studies clearly indicates that Cav-1 acts as a tumor promoter in other organs, such as the prostate. In the TRAMP (TRansgenic Adenocarcinoma of Mouse Prostate) mouse model of prostate cancer, Cav-1 expression is increased when compared to the normal prostate epithelium (23). Genetic ablation of Cav-1 in TRAMP mice decreases incidence of prostate tumors at 28 weeks of age and reduces metastasis to regional lymph node and to distant organs, such as the lungs (23). In patients with prostate cancer, Cav-1 expression is increased in 13% of well-differentiated tumors, 24% of moderately differentiated tumors and 39% of poorly differentiated tumors (24). These results suggest that Cav-1 over-expression in prostate cancer cells correlates with tumor progression (24). Other studies have shown that high levels of Cav-1 are associated with high Gleason's score, reduced survival and poor prognosis in prostate cancer (25, 26). Finally, a higher incidence of Cav-1 positivity is observed in metastatic lesions and metastasis-derived cell lines, as compared to primary tumors and primary tumor-derived cell lines, respectively (27).
In the mammary gland and skin, Cav-1 negatively regulates proliferation. However, the anti-apoptotic properties of Cav-1 seem to contribute to the development of prostate cancer. Prostate tumors derived from TRAMP/Cav-1 null mice exhibit increased apoptosis as assessed by TUNEL staining, with increased levels of prostate apoptosis response factor-4 and PTEN (23). However, the role of Cav-1 in apoptosis in different cell types still remains largely unexplored and highly controversial. In prostate cancer cells, it is believed that Cav-1 can shift from its conventional membrane-bound position to a secreted form outside the cell. Indeed, Cav-1 has been detected in the serum of patients with prostate cancer (28). It was also found that the Cav-1 secreted form is bioactive since it could promote cell survival (28). The phosphorylation of Cav-1 on serine 80 seems to be involved in the conversion of Cav-1 from an intracellular protein to a secreted form (29).
Table 1 summarizes the main phenotypes of Cav-1 (−/−) mice and the implications for different human pathologies.
Table 1.
Phenotypes of Cav-1 null mice and their implications in human pathologies
| Gene | Expression | Knockout mouse phenotypes | Relevance to human pathologies |
|---|---|---|---|
| Cav-1 | Epithelial cells Fibroblasts Endothelial cells Adipocytes Type I pneumocytes |
Mammary epithelial cell hyperplasia and premature lactation during pregnancy | Breast Cancer |
| Enhanced tumor formation and increased metastasis in a mammary tumor model background (MMTV-PyMT) | |||
| Increased mammary epithelial hyperplasia with fibrosis in INK4a (−/−) background | |||
| Increased mammary stem cell population | |||
| Increased DMBA-induced epidermal tumors | Skin Cancer | ||
| Decreased incidence of prostate tumors and metastasis in a prostate cancer model background (TRAMP) | Prostate Cancer | ||
| Reduced pulmonary alveolar space, increased wall thickening, hypercellularity, and fibrosis | Pulmonary Disease | ||
| Concentric left ventricular hypertrophy Right ventricular dilation | Cardiovascular Diseases | ||
| Increased neointima formation | |||
| Decreased atheromatous lesions in Western type diet fed ApoE (−/−)/Cav-1(−/−) | |||
| Resistance to diet-induced obesity | Insulin Resistance & Diabetes | ||
| Adipose tissue atrophy | |||
| Impaired insulin signaling | |||
| Increased ischemic injury | Cerebral Ischemia |
Cav-1 and Adult Stem Cells
Studies have shown that Cav-1 may normally regulate the proper differentiation of stem/progenitor cell populations in organs, such as the skin, the mammary gland, and the intestine, that are physiologically subjected to constant self-renewal (Figure 2). For example, Cav-1 deficiency leads to an amplification of an adult mammary stem cell population, both in vivo and in vitro. The expression of stem cell markers, such as Sca-1 and Keratin 6, is greatly increased in the hyperplastic mammary ducts of Cav-1 deficient mice, as well as in 3D cultures of Cav-1 (−/−) primary mammary epithelial cells. Such an amplification of progenitor cells is functionally associated with the abnormal presence of myoepithelial cells in the hyperplastic lesions of Cav-1 deficient mammary glands (30).
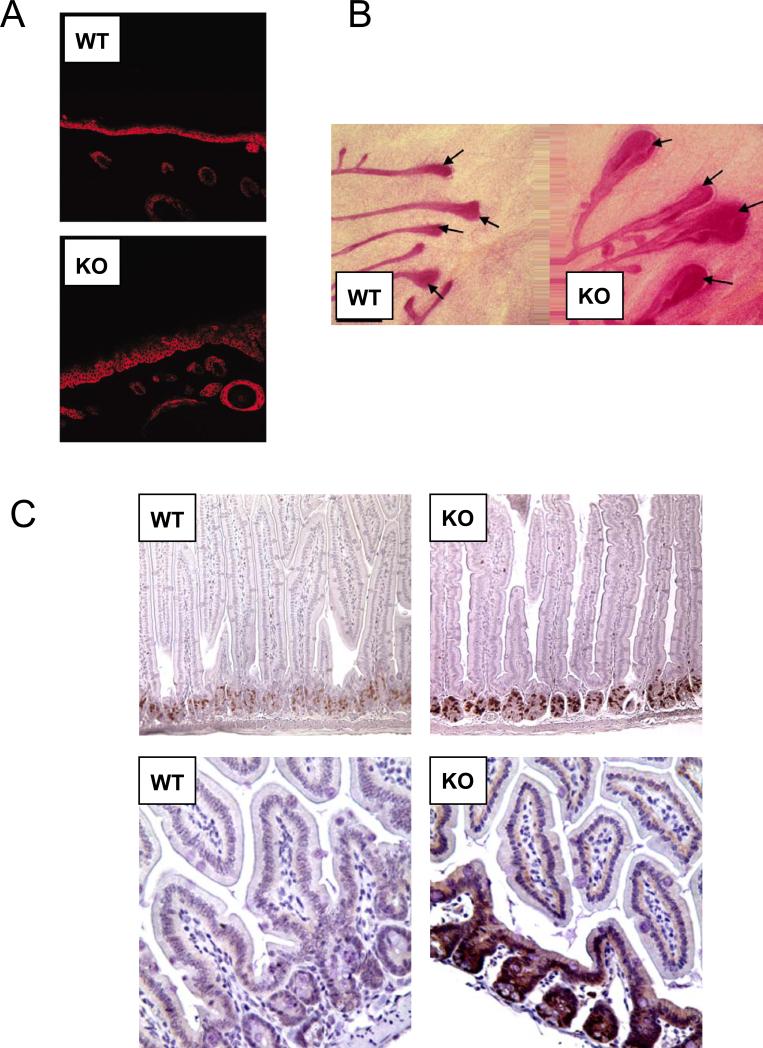
Figure 2. Loss of Cav-1 Increases the Adult Epithelial Stem Cell Compartment.
A) Skin. After DMBA-treatment, Cav-1 (−/−) mice show an expansion of the basal keratinocyte cell layer in the skin, as marked by immuno-staining with anti-keratin-14 IgG.
B) Mammary Gland. Cav-1 (−/−) mice show an increase in the size of terminal end buds (TEBs), the site of mammary stem/progenitor cells during adult mammary gland development. Ducts and TEBs are stained red with Carmine dye.
C) GI tract. The intesinal crypt stem cells from Cav-1 (−/−) mice show increases in both proliferation (BrdU incorporation) and expression of β-catenin, a stem cell marker.
Reproduced with permission from Capozza et al, 2003; Williams et al, 2006; and Li et al, 2005 (22, 31, 78).
In addition, genetic ablation of Cav-1 induces an abnormal amplification of small intestine crypt stem cells, resulting in increased susceptibility to gamma-radiation. Cav-1 null small intestine crypt stem cells display higher proliferation rates, as compared to wild-type controls. Because of its fast renewing nature, the small intestine constitutes one of the main targets of radiation. After gamma-radiation exposure, Cav-1 deficient mice display a decreased survival rate, as compared to wild-type mice (31). Mechanistically, Wnt/beta-catenin signaling, which normally controls stem cell self-renewal, is up-regulated in Cav-1 null mammary and crypt stem cells. The longevity and slow-dividing properties of stem cells facilitates the accumulation of genetic alterations, and renders progenitor cells the likely precursors of malignant derivatives. As such, loss of Cav-1 may induce the accumulation of stem cells, and that this event may be an initiating factor during tumorigenesis.
Cav-1: Insulin Signaling, Pulmonary and Cardiac Function, & Ischemia
The analysis of Cav-1 (−/−) mice has highlighted how loss of Cav-1 function may lead to a number of important pathological conditions. In addition to its involvement in breast, skin and prostate cancer, Cav-1 also plays important roles in diabetes, lung and heart disease, and ischemia (Table 1).
Cav-1, Insulin Signaling and Diabetes
Cav-1 (−/−) mice show resistance to diet-induced obesity, and display adipose tissue atrophy (32). Metabolically, plasma levels of insulin and glucose are normal in Cav-1 null mice (32). However, an insulin tolerance test revealed abnormally low glucose uptake in young Cav-1 (−/−) mice, suggestive of impaired insulin signaling (33). This defect in glucose uptake is due to a severe deficiency of insulin receptor protein expression in Cav-1 (−/−) adipose tissue (33). In direct support of these findings, Cav-1 null adipose tissue displays decreased insulin signaling, as assessed by phosphorylation of insulin receptor and its downstream targets (33). Although loss of Cav-1 is not sufficient to induce diabetes, it may function as a predisposing factor for the development of insulin resistance in humans.
Cav-1 and Pulmonary Function
Cav-1 is highly expressed in the lung, and is found in several pulmonary cell types, including endothelial cells and pneumocytes. Histological analysis of Cav-1 null mice reveals a deeply modified lung morphology with reduced alveolar spaces, increased wall thickening, fibrosis, and hypercellularity (34, 35). In addition, Cav-1 null mice develop pulmonary hypertension (36, 37). Reduced Cav-1 levels in the lung have been documented in several animal models of pulmonary hypertension and in patients with severe pulmonary hypertension. These findings may have important implications for understanding human respiratory pathologies, such as pulmonary hypertension, fibrosis, as well as acute respiratory syndrome.
Cav-1 and Cardiovascular Function
Cav-1 is implicated in several cardiovascular pathologies, including cardiac hypertrophy, neointima formation and atherosclerosis. Cardiac hypertrophy is a critical pathology leading to heart failure. Cav-1 (−/−) mice show progressive concentric left ventricular hypertrophy, as well as right ventricular dilation (36, 38). Cav-1 expression is restricted to the supporting cells of the heart, such as fibroblasts and endothelial cells. Excessive activation of the Ras-p42/44 MAP kinase cascade in Cav-1 (−/−) cardiac fibroblasts is considered one of the upstream key factors promoting hypertrophy and fibrosis in the adjacent myocytes (39). Notably, Cav-1 (−/−) cardiac fibroblasts exhibit p42/44 MAP kinase hyperactivation when compared to wild type fibroblasts (38), suggesting that the hypertrophy of Cav-1 null hearts occurs via a paracrine mechanism.
Neointimal hyperplasia is the principal cause for clinical failures in angioplasty and is a critical component of re-stenosis. During the development of neointimal hyperplasia, the arterial wall thickens and the lumen narrows as a consequence of smooth muscle cells (SMC) accumulation and proliferation in the intima (40, 41). Genetic ablation of Cav-1 in mice facilitates SMC proliferation and neointima formation. Four weeks after ligation, Cav-1 (−/−) carotid arteries showed significantly more neointimal hyperplasia with subtotal luminal occlusion, as compared to wild-type mice. Mechanistically, the development of neointimal hyperplasia in Cav-1 (−/−) mice is mediated by elevated levels of phospho-p42/44 MAP kinase and cyclin D1 (42).
Atherosclerosis is characterized by the accumulation of modified lipoproteins in the subendothelial space followed by the recruitment and proliferation of monocytes/macrophages and SMC. Genetic ablation of Cav-1 in mice confers protection against atherosclerosis. In the apolipoprotein E-deficient (ApoE−/−) atherogenic mouse model background, loss of Cav-1 prevents the development of aortic atheromas, with a ~70% reduction in atherosclerotic lesion area. Mechanistically, loss of Cav-1 is associated with the down-regulation of proatherogenic molecules, namely CD36 and vascular cell adhesion molecule-1 (VCAM-1) (43).
Thus, Cav-1 (−/−) mice are more sensitive to the development of neointimal hyperplasia (luminal narrowing), but are strongly resistant to the development of atheromas, clearly demonstrating the different patho-physiology of sub-endothelial versus mural narrowing of the arterial lumina.
Cav-1 and Ischemia
Recent studies have directly addressed the functional role of Cav-1 in ischemic injury. First, Cav-1 ablation in mice was shown to promote ischemic injury in a model of hindlimb ischemia (44). Similarly, in a model of cerebral artery occlusion, Cav-1 null mice display a significant increase in the volume of cerebral infarcts, as compared with wild-type mice. Mechanistically, Cav-1 null ischemic brains exhibited decreased proliferation of endothelial cells and an elevated apoptotic index, as compared with wild-type counterparts (45). Ischemic pre-conditioning has previously been shown to increase the cardiac phosphorylation of Cav-1 and SRC in mice subjected to myocardial ischemia-reperfusion. These interesting observations suggest that phosphorylation of Cav-1 and SRC might play a cardio-protective role in ischemic injuries. Hence, genetic ablation of the Cav-1 gene in mice was shown to attenuate the protective effect of myocardial ischemic pre-conditioning (46).
Caveolin-2: Pulmonary and Skeletal Muscle Functions
Cav-2 requires the presence of Cav-1 for stabilization and membrane targeting, such that in the absence of Cav-1, Cav-2 is retained in the endoplasmic reticulum/Golgi complex and undergoes degradation through a proteaosomal pathway (47, 48). As a consequence, Cav-2 expression is nearly abrogated in Cav-1 null mice (34, 35). Thus, Cav-2 was considered an “accessory” protein that just functions in conjunction with Cav-1. However, the generation and analysis of Cav-2 null mice revealed unique roles for Cav-2 in the physiology of the lung and of the skeletal muscle. Cav-2 null mice show normal caveolae formation and nearly normal levels of Cav-1 expression (49). Cav-2 null lungs show thickened alveolar septa with increased number of endothelial cells. Consequently, Cav-2 null mice show exercise intolerance, suggestive of an impaired gas exchange that is often associated with human pulmonary diseases (49). Interestingly, the Cav-2 null lung phenotypes are identical to the ones observed in Cav-1 null mice (34, 35). Because Cav-2 expression is severely reduced in Cav-1 null mice, a Cav-2 deficiency is considered to be the root cause of this lung disorder.
Cav-2 null mice also exhibit an unexpected skeletal muscle phenotype. Cav-2 null skeletal muscles show peculiar abnormalities, such as tubular aggregate formation, and mitochondrial proliferation/aggregation. In addition, Cav-2 deficiency induces increased numbers of satellite cells, which are skeletal muscle specific stem/precursor cells (50). For a summary of Cav-2 null mice phenotypes and their implications in human pathologies, please refer to Table 2.
Table 2.
Phenotypes of Cav-2 and Cav-3 knockout mice and their implications in human pathologies
| Genes | Expression | Knockout mouse phenotypes | Relevance to human pathologies |
|---|---|---|---|
|
Cav-2
|
Same as Cav-1 | Reduced pulmonary alveolar space and increased wall thickening with hypercellularity and fibrosis | Pulmonary Disease |
| Epithelial cells Fibroblasts Endothelial cells Adipocytes Type I pneumocytes |
Tubular aggregate formation, mitochondrial proliferation, increased numbers of satellite cells |
Tubular Aggregates |
|
| Cav-3 | Cardiac and skeletal muscle | Skeletal muscle myopathic changes, mononuclear cell infiltration, variable fibers size and presence of necrosis | Limb-Girdle Muscular Dystrophy |
| Cardiac hypertrophy, dilatation, reduced fractional shortening | Cardiomyopathy | ||
| Increased adiposity, Insulin resistance | Diabetes |
Caveolin-3: Skeletal Muscle Disorders, Heart Disease and Diabetes
Caveolin-3 is the muscle specific family member, and is necessary for caveolae formation in muscle tissues. Indeed, electron microscopy revealed a lack of caveolae in all striated and cardiac muscle cells of Cav-3 null mice (51, 52). Cav-3 null skeletal muscle shows signs of mild myopathic changes, such as mononuclear cell infiltration, variable fibers size, and presence of necrosis. These phenotypes are similar to the ones found in a human disease, termed limb-girdle muscular dystrophy (LGMD-1C), which is due to mutations in the Cav-3 gene.
A Cav-3 deficiency also affects cardiac function, with the development of cardiac hypertrophy, dilatation, and reduced fractional shortening at 4 months of age (53). Cav-3 null cardiac myocytes show hypertrophy, with cellular infiltrates and progressive interstitial and perivascular fibrosis. Hyperactivation of the Ras-p42/44-MAP kinase pathway is associated with these cardiac phenotypes (53).
Interestingly, transgenic over-expression of either wild-type or dominant-negative mutant Cav-3 was shown to induce cardiomyopathy, with cardiac tissue degeneration, and reductions in cardiac functions (54, 55). These results suggest that maintenance of normal Cav-3 expression levels is essential for proper cardiac function.
Surprisingly, Cav-3 null mice also show an interesting metabolic phenotype. Cav-3 null mice demonstrate increased adiposity and develop insulin resistance, as shown by decreased glucose uptake and reduced glucose metabolic flux in their skeletal muscles. During fasting, Cav-3 null skeletal muscles exhibit normal insulin receptor protein levels. However, insulin stimulation induces a severe reduction in insulin receptor levels in Cav-3 null mice, suggesting that Cav-3 may function to stabilize insulin receptor. These results show that Cav-3 contributes to the regulation of whole body glucose metabolism in vivo and indicates that Cav-3 may play a role in the development of insulin resistance (56, 57).
For a summary of Cav-3 null mouse phenotypes and their implications in human pathologies, refer to Table 2.
Caveolin Mutations in Human Disease
Over the last few years, mutations in the caveolin genes have been detected in several types of human diseases, clearly indicating the importance of these proteins for normal human physiology, and demonstrating that dys-regulation of caveolin function is a critical factor for the development of human disease.
Cav-3 mutations
The first mutations in a caveolin gene were identified a decade ago, when mutations in the Cav-3 gene were found in patients with limb-girdle muscular dystrophy (LGMD-1C) (58). Since then, several other mutations have been found in patients with skeletal muscle disorders. The spectrum of disease phenotypes associated with Cav-3 mutations is quite variable, ranging from LGMD-1C, to idiopathic hyperckemia, rippling muscle disease, and distal myopathy. For a comprehensive review of Cav-3 and muscle disorders, please consult the GeneReview database available at http://www.genetests.org (search CAV3). Most of the Cav-3 mutations are heterozygous and cause a dramatic decrease in Cav-3 expression in skeletal muscle, thus acting in a dominant-negative fashion (59). Finally, mutations in the Cav-3 gene have been found in familial hypertrophic cardiomyopathy (60), in the arrhythmogenic syndrome Long-QT Congenital Syndrome (LQTS) (61), as well as in sudden infant death syndrome (SIDS) (62).
Cav-1 mutations
Somatic mutations in the Cav-1 gene are involved in human cancer. We and others have shown that a proline to leucine substitution at position 132 (P132L) in the Cav-1 gene is found in 16-20% of breast cancers (63, 64). This heterozygous mutation acts in a dominant-negative fashion and induces the intracellular retention of the normal Cav-1 protein at the level of the ER/Golgi complex (17). Other mutations were also found in human breast cancer samples, such as W128stop, Y118H, S136R, I141T, Y148H and Y148S (64). Most interestingly, Cav-1 mutations only correlate with estrogen receptor alpha (ER-alpha) positive breast tumors, and are found in up to 35% ER-alpha positive tumors (64). These results suggest that Cav-1 may regulate ER-alpha expression. In fact, we have shown that ER-alpha is dramatically upregulated in the luminal mammary epithelial cells of Cav-1 knockout mice, as compared to their wild type counterparts (64). Because ER-alpha expression increases as mammary cell transformation progresses, these results suggest that Cav-1 mutations may be an initiating event in the development of human breast cancers. Thus, screening of patients biopsies for Cav-1 mutations could become a routine analysis as a preventive measure to detect estrogen-dependent breast cancer at an early stage. These findings provide new insights into the regulation of ER-alpha in breast cancer. Finally, mutations and abnormal expression of Cav-1 have been also found in human oral squamous cell carcinomas (65).
Recently, a homozygous mutation in the Cav-1 gene has been detected in Berardinelli-Seip Congenital Lipodystrophy, a rare genetic disorder characterized by near absence of adipose tissue, severe dyslipidemia, and insulin resistance. This mutation induces a complete loss of Cav-1 expression in skin fibroblasts (66). These findings provide human genetic evidence to directly support a critical role for Cav-1 in regulation of adipocyte function and insulin signaling. Additional heterozygous Cav-1 mutations have now been observed in patients with atypical partial lipodystrophy and hypertriglyceridemia (67). See Garg et al, 2008, for a recent review on this subject (68).
Caveolin Mimetic Peptides: A Novel Therapeutic Strategy for Human Pathologies
Cav-1 functions as a powerful tumor suppressor in certain types of cancer and its re-expression in tumor cells could potentially become a new therapeutic avenue for the treatment of human cancers. Tumor progression in mice can be blocked by the administration of a cell-permeable peptide, containing the homeodomain of the antennapedia (penetratrin) coupled to the Cav-1 scaffolding domain (CSD) (69). After subcutaneous implantation of Lewis lung carcinoma cells (LLC), the injection of the CSD peptide reduced tumor size and tumor vascular permeability, and increased tumor necrosis, suggesting that Cav-1 prevents tumorigenesis by affecting tumor blood supply (69). Similar experiments in WT and Cav-1 (−/−) mice further demonstrated the role of Cav-1 in vascular permeability and tumor development. In Cav-1 KO mice, LLC-derived tumors showed significantly higher growth rates, with increased angiogenesis and decreased apoptosis than tumors implanted in WT hosts. Most interestingly, administration of CSD peptide was able to reverse tumor hyperpermeability as well as to attenuate the increased tumor growth (70).
Therapeutic administration of the Cav-1 scaffolding domain peptide has been employed in other experimental models. Indeed, administration of CSD peptide has been successfully used to prevent the development of pulmonary hypertension (PH), right ventricular hypertrophy, and pulmonary artery medial hypertrophy in a monocrotaline-induced PH rat model (71). Administration of CSD peptide to monocrotaline-treated rats significantly prevented PH and normalized pulmonary artery medial hypertrophy and right ventricular hypertrophy (71). Finally, in an experimental mouse model of inflammation, the administration of the CSD peptide has also been shown to decrease inflammation, reduce edema formation and vascular permeability (72). Thus, the administration of Cav-1 peptide mimetics might become a novel alternative treatment for cancer as well as pulmonary hypertension patients.
Future Directions: Role of Caveolins in the Cancer Stroma
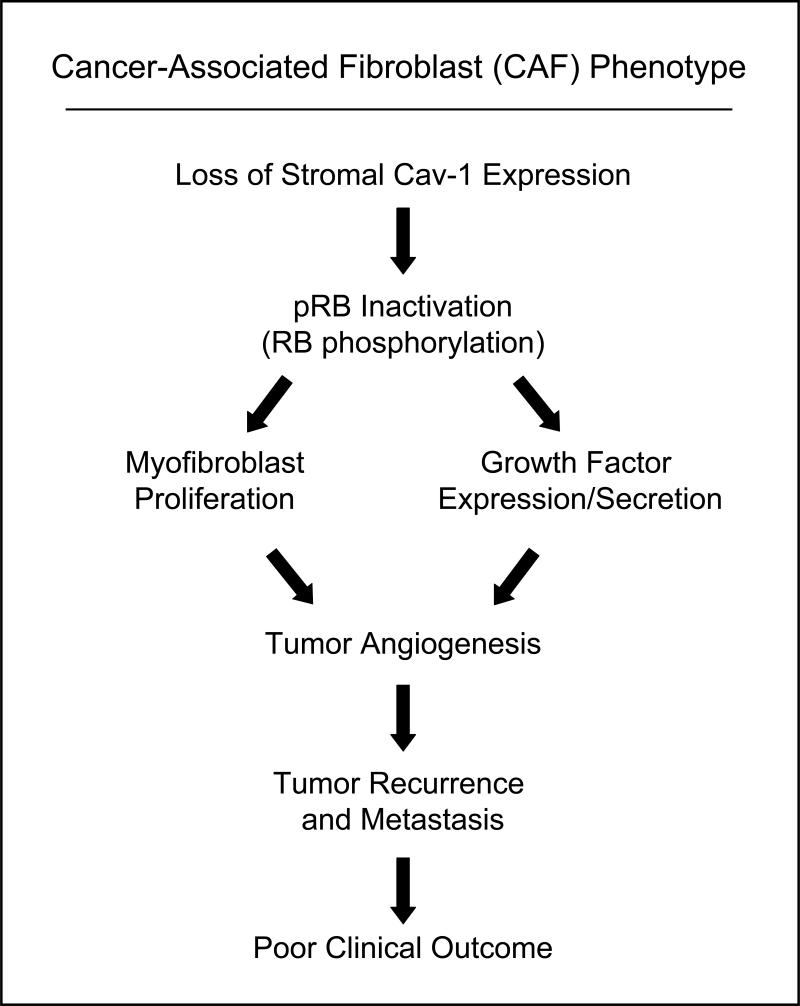
Normal epithelial cells are often surrounded by fibroblasts which serve mainly as support to protect the tissue from damage. Fibroblasts can proliferate and secrete extracellular matrix proteins as a response to injury. Once the wound healing process is complete, they undergo apoptosis and the ECM production lessens. Several lines of evidence now suggest that stromal fibroblasts become “activated” and play a very dynamic role in tumor initiation and progression. These cancer-associated fibroblasts (CAFs) express smooth muscle actin, have contractile functions and secrete ECM proteins. Unlike normal fibroblasts involved in wound repair, these CAFs do not undergo spontaneous apoptosis, rather they remain activated and hyper-proliferate. Studies suggest the recruitment of CAFs is not only a secondary event to tumor initiation, but is very dynamic as CAFs can increase the growth of adjacent epithelial cells through paracrine mechanisms. For a complete review on stromal fibroblasts and cancer please see Kalluri et al (73). However, very little is known about the mechanism(s) controlling the proliferation of CAFs and their clinical implications in cancer progression and outcome. Recently, it was shown that Cav-1 protein expresssion is decreased in primary cultures of CAFs isolated from patients with invasive breast cancer and that loss of Cav-1 function might play a role in their hyper-proliferation (74). Accordingly, treatment of these cells with a cell-permeable Cav-1 mimetic peptide decreased the phosphorylation of RB (retinoblastoma protein) as well as PCNA (Proliferating Cell Nuclear Antigen) and MCM7 (MiniChromosome Maintenance) proteins levels, two pRB target proteins. Therefore, caveolin proteins might play an important role in cancer through their novel “tumor suppressor” role in the neoplastic stroma (Figure 3).
Figure 3. Loss of Stromal Cav-1 Expression Predicts Poor Clinical Outcome in Human Breast Cancer Patients.
Mechanistically, loss of stromal Cav-1 expression in the tumor micro-environment leads to RB-inactivation, increased myofibroblast proliferation, and the secretion of angiogenic growth factors. This, in turn, greatly facilitates tumor recurrence and metastasis, leading to poor clinical outcome.
In accordance with this idea, we have recently shown that loss of stromal Cav-1 is a novel breast cancer biomarker that predicts early disease recurrence, metastasis, survival, and tamoxifen-resistance (75). For example, lymph-node-positive breast cancer patients showed an ~11.5-fold reduction in 5-year progression-free survival in the absence of stromal Cav-1 (80% versus 7% survival) (75). Mechanistically, this appears to be related to the idea that the loss of Cav-1 expression in mammary fibroblasts leads to pRB-inactivation, increased growth factor secretion, and stromal angiogenesis (76, 77) (Figure 3). As such, breast cancer patients lacking stromal Cav-1 might greatly benefit from anti-angiogenic therapy, in addition to standard treatment regimens.
Thus, future studies are warranted to explore this novel role for caveolins in the stromal pathogenesis of human cancers.
Acknowledgements
M.P.L. and his laboratory were supported by grants from the NIH/NCI (R01-CA-80250; R01-CA-098779; R01-CA-120876), the American Heart Association (AHA), the Muscular Dystrophy Association (MDA), the American Association for Cancer Research (AACR), and the Department of Defense-Breast Cancer Research Program (Synergistic Idea Award). I.M. was supported by a Post-doctoral Fellowship from the Susan G. Komen Breast Cancer Foundation. P.G.F. was supported by a grant from the W.W. Smith Charitable Trust, and a Career Catalyst Award from the Susan G. Komen Breast Cancer Foundation. F.S. was supported by grants from the Elsa U. Pardee Foundation, the W.W. Smith Charitable Trust, and a Research Scholar Grant from the American Cancer Society (ACS). J.F.J. was supported by a Career Catalyst Award from the Susan G. Komen Breast Cancer Foundation. R.G.P. was supported by grants from the NIH/NCI (R01-CA-70896, R01-CA-75503, R01-CA-86072, and R01-CA-107382) and the Dr. Ralph and Marian C. Falk Medical Research Trust. The Kimmel Cancer Center was supported by the NIH/NCI Cancer Center Core grant P30-CA-56036 (to R.G.P.).
This work was funded, in part, under a grant with the Pennsylvania Department of Health (to M.P.L.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Abbreviations
- Cav
Caveolin
- CSD
caveolin scaffolding domain
- MMTV-PyMT
mouse mammary tumor virus-polyoma middle T antigen
- TRAMP
TRansgenic Adenocarcinoma of Mouse Prostate
- SMC
smooth muscle cells
- LGMD
limb-girdle muscular dystrophy
- ER
estrogen receptor
- LLC
Lewis lung carcinoma cells
- PH
pulmonary hypertension
References
- 1.Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106(4):403–11. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 2.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68(4):673–82. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 3.Murata M, Peranen J, Schreiner R, Weiland F, Kurzchalia T, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci, USA. 1995;92:10339–43. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sargiacomo M, Scherer PE, Tang Z-L, Kubler E, Song KS, Sanders MC, et al. Oligomeric structure of caveolin: Implications for caveolae membrane organization. Proc Natl Acad Sci, USA. 1995;92:9407–11. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia T. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–27. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glenney JR, Jr., Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci U S A. 1992;89(21):10517–21. doi: 10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S, Song KS, Koh SS, Kikuchi A, Lisanti MP. Baculovirus-based expression of mammalian caveolin in Sf21 insect cells. A model system for the biochemical and morphological study of caveolar biogenesis. J Biol Chem. 1996;271:28647–54. doi: 10.1074/jbc.271.45.28647. [DOI] [PubMed] [Google Scholar]
- 8.Vogel U, Sandvig K, van Deurs B. Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells. J Cell Sci. 1998 Mar;111(Pt 6):825–32. doi: 10.1242/jcs.111.6.825. [DOI] [PubMed] [Google Scholar]
- 9.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci, USA. 1996;93:131–5. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, et al. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–46. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 11.Tang Z-L, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, et al. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–61. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- 12.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002 Sep;54(3):431–67. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 13.Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1381–5. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem. 1997 Jun 27;272(26):16374–81. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- 15.Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998 Mar;16(11):1391–7. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- 16.Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, et al. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. Embo J. 1998 Nov 16;17(22):6633–48. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Park DS, Razani B, Russell RG, Pestell RG, Lisanti MP. Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: caveolin-1 (P132L) behaves in a dominant-negative manner and caveolin-1 (-/-) null mice show mammary epithelial cell hyperplasia. Am J Pathol. 2002 Oct;161(4):1357–69. doi: 10.1016/S0002-9440(10)64412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park DS, Lee H, Frank PG, Razani B, Nguyen AV, Parlow AF, et al. Caveolin-1-deficient mice show accelerated mammary gland development during pregnancy, premature lactation, and hyperactivation of the Jak-2/STAT5a signaling cascade. Mol Biol Cell. 2002 Oct;13(10):3416–30. doi: 10.1091/mbc.02-05-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams TM, Cheung MW, Park DS, Razani B, Cohen AW, Muller WJ, et al. Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol Biol Cell. 2003 Mar;14(3):1027–42. doi: 10.1091/mbc.E02-08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in Vivo role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004 Sep 7; doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 21.Williams TM, Lee H, Cheung MW, Cohen AW, Razani B, Iyengar P, et al. Combined loss of INK4a and caveolin-1 synergistically enhances cell proliferation and oncogene-induced tumorigenesis: role of INK4a/CAV-1 in mammary epithelial cell hyperplasia. J Biol Chem. 2004 Jun 4;279(23):24745–56. doi: 10.1074/jbc.M402064200. [DOI] [PubMed] [Google Scholar]
- 22.Capozza F, Williams TM, Schubert W, McClain S, Bouzahzah B, Sotgia F, et al. Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation. Am J Pathol. 2003 Jun;162(6):2029–39. doi: 10.1016/S0002-9440(10)64335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams TM, Hassan GS, Li J, Cohen AW, Medina F, Frank PG, et al. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in tramp mice. J Biol Chem. 2005 Jul 1;280(26):25134–45. doi: 10.1074/jbc.M501186200. [DOI] [PubMed] [Google Scholar]
- 24.Yang G, Truong LD, Wheeler TM, Thompson TC. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 1999 Nov 15;59(22):5719–23. [PubMed] [Google Scholar]
- 25.Rajjayabun PH, Garg S, Durkan GC, Charlton R, Robinson MC, Mellon JK. Caveolin-1 expression is associated with high-grade bladder cancer. Urology. 2001 Nov;58(5):811–4. doi: 10.1016/s0090-4295(01)01337-1. [DOI] [PubMed] [Google Scholar]
- 26.Fong A, Garcia E, Gwynn L, Lisanti MP, Fazzari MJ, Li M. Expression of caveolin-1 and caveolin-2 in urothelial carcinoma of the urinary bladder correlates with tumor grade and squamous differentiation. Am J Clin Pathol. 2003 Jul;120(1):93–100. doi: 10.1309/292N-HAYN-WAVR-EJ37. [DOI] [PubMed] [Google Scholar]
- 27.Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, et al. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4:1873–80. [PubMed] [Google Scholar]
- 28.Tahir SA, Yang G, Ebara S, Timme TL, Satoh T, Li L, et al. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001 May 15;61(10):3882–5. [PubMed] [Google Scholar]
- 29.Schlegel A, Arvan P, Lisanti MP. Caveolin-1 binding to endoplasmic reticulum membranes and entry into the regulated secretory pathway are regulated by serine phosphorylation. Protein sorting at the level of the endoplasmic reticulum. J Biol Chem. 2001;276(6):4398–408. doi: 10.1074/jbc.M005448200. [DOI] [PubMed] [Google Scholar]
- 30.Sotgia F, Williams TM, Cohen AW, Minetti C, Pestell RG, Lisanti MP. Caveolin-1-deficient mice have an increased mammary stem cell population with upregulation of Wnt/beta-catenin signaling. Cell Cycle. 2005 Dec;4(12):1808–16. doi: 10.4161/cc.4.12.2198. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Hassan GS, Williams TM, Minetti C, Pestell RG, Tanowitz HB, et al. Loss of caveolin-1 causes the hyper-proliferation of intestinal crypt stem cells, with increased sensitivity to whole body gamma-radiation. Cell Cycle. 2005 Dec;4(12):1817–25. doi: 10.4161/cc.4.12.2199. [DOI] [PubMed] [Google Scholar]
- 32.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002 Mar 8;277(10):8635–47. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 33.Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, et al. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol. 2003 Jul;285(1):C222–35. doi: 10.1152/ajpcell.00006.2003. [DOI] [PubMed] [Google Scholar]
- 34.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of Caveolae, Vascular Dysfunction, and Pulmonary Defects in Caveolin-1 Gene-Disrupted Mice. Science. 2001;293(5539):2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 35.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001 Oct 12;276(41):38121–38. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 36.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11375–80. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jasmin JF, Mercier I, Hnasko R, Cheung MW, Tanowitz HB, Dupuis J, et al. Lung remodeling and pulmonary hypertension after myocardial infarction: pathogenic role of reduced caveolin expression. Cardiovasc Res. 2004 Sep 1;63(4):747–55. doi: 10.1016/j.cardiores.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, et al. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol. 2003 Feb;284(2):C457–74. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 39.Booz GW, Dostal DE, Baker KM. Paracrine actions of cardiac fibroblasts on cardiomyocytes: implications for the cardiac renin-angiotensin system. Am J Cardiol. 1999 Jun 17;83(12A):44H–7H. doi: 10.1016/s0002-9149(99)00257-x. [DOI] [PubMed] [Google Scholar]
- 40.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 41.Kohler TR, Kirkman TR, Kraiss LW, Zierler BK, Clowes AW. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ Res. 1991 Dec;69(6):1557–65. doi: 10.1161/01.res.69.6.1557. [DOI] [PubMed] [Google Scholar]
- 42.Hassan GS, Jasmin JF, Schubert W, Frank PG, Lisanti MP. Caveolin-1 deficiency stimulates neointima formation during vascular injury. Biochemistry. 2004 Jul 6;43(26):8312–21. doi: 10.1021/bi049609t. [DOI] [PubMed] [Google Scholar]
- 43.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004 Jan;24(1):98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 44.Sonveaux P, Martinive P, DeWever J, Batova Z, Daneau G, Pelat M, et al. Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis. Circ Res. 2004 Jul 23;95(2):154–61. doi: 10.1161/01.RES.0000136344.27825.72. [DOI] [PubMed] [Google Scholar]
- 45.Jasmin JF, Malhotra S, Singh Dhallu M, Mercier I, Rosenbaum DM, Lisanti MP. Caveolin-1 deficiency increases cerebral ischemic injury. Circ Res. 2007 Mar 16;100(5):721–9. doi: 10.1161/01.RES.0000260180.42709.29. [DOI] [PubMed] [Google Scholar]
- 46.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. Faseb J. 2007 May;21(7):1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 47.Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, et al. Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane. Retention of caveolin-2 at the level of the golgi complex. J Biol Chem. 1999;274(36):25718–25. doi: 10.1074/jbc.274.36.25718. [DOI] [PubMed] [Google Scholar]
- 48.Mora R, Bonilha VL, Marmorstein A, Scherer PE, Brown D, Lisanti MP, et al. Caveolin-2 localizes to the golgi complex but redistributes to plasma membrane, caveolae, and rafts when co-expressed with caveolin-1. J Biol Chem. 1999;274(36):25708–17. doi: 10.1074/jbc.274.36.25708. [DOI] [PubMed] [Google Scholar]
- 49.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, et al. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002 Apr;22(7):2329–44. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schubert W, Sotgia F, Cohen AW, Capozza F, Bonuccelli G, Bruno C, et al. Caveolin-1(-/-)- and caveolin-2(-/-)-deficient mice both display numerous skeletal muscle abnormalities, with tubular aggregate formation. Am J Pathol. 2007 Jan;170(1):316–33. doi: 10.2353/ajpath.2007.060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, et al. Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet. 2000;9(20):3047–54. doi: 10.1093/hmg/9.20.3047. [DOI] [PubMed] [Google Scholar]
- 52.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, et al. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and T- tubule abnormalities. J Biol Chem. 2001;19:19. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- 53.Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, et al. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002 Oct 11;277(41):38988–97. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 54.Aravamudan B, Volonte D, Ramani R, Gursoy E, Lisanti MP, London B, et al. Transgenic overexpression of caveolin-3 in the heart induces a cardiomyopathic phenotype. Hum Mol Genet. 2003 Nov 1;12(21):2777–88. doi: 10.1093/hmg/ddg313. [DOI] [PubMed] [Google Scholar]
- 55.Ohsawa Y, Toko H, Katsura M, Morimoto K, Yamada H, Ichikawa Y, et al. Overexpression of P104L mutant caveolin-3 in mice develops hypertrophic cardiomyopathy with enhanced contractility in association with increased endothelial nitric oxide synthase activity. Hum Mol Genet. 2004 Jan 15;13(2):151–7. doi: 10.1093/hmg/ddh014. [DOI] [PubMed] [Google Scholar]
- 56.Oshikawa J, Otsu K, Toya Y, Tsunematsu T, Hankins R, Kawabe J, et al. Insulin resistance in skeletal muscles of caveolin-3-null mice. Proc Natl Acad Sci U S A. 2004 Aug 24;101(34):12670–5. doi: 10.1073/pnas.0402053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Capozza F, Combs TP, Cohen AW, Cho YR, Park SY, Schubert W, et al. Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol. 2005 Jun;288(6):C1317–31. doi: 10.1152/ajpcell.00489.2004. [DOI] [PubMed] [Google Scholar]
- 58.Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M, et al. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nature Genetics. 1998;18:365–8. doi: 10.1038/ng0498-365. [DOI] [PubMed] [Google Scholar]
- 59.Galbiati F, Volonte D, Minetti C, Chu JB, Lisanti MP. Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C). Retention of LGMD-1C caveolin-3 mutants within the golgi complex. J Biol Chem. 1999;274(36):25632–41. doi: 10.1074/jbc.274.36.25632. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi T, Arimura T, Ueda K, Shibata H, Hohda S, Takahashi M, et al. Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2004 Jan 2;313(1):178–84. doi: 10.1016/j.bbrc.2003.11.101. [DOI] [PubMed] [Google Scholar]
- 61.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006 Nov 14;114(20):2104–12. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 62.Cronk LB, Ye B, Kaku T, Tester DJ, Vatta M, Makielski JC, et al. Novel mechanism for sudden infant death syndrome: persistent late sodium current secondary to mutations in caveolin-3. Heart Rhythm. 2007 Feb;4(2):161–6. doi: 10.1016/j.hrthm.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayashi K, Matsuda S, Machida K, Yamamoto T, Fukuda Y, Nimura Y, et al. Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res. 2001;61(6):2361–4. [PubMed] [Google Scholar]
- 64.Li T, Sotgia F, Vuolo MA, Li M, Yang WC, Pestell RG, et al. Caveolin-1 mutations in human breast cancer: functional association with estrogen receptor alpha-positive status. Am J Pathol. 2006 Jun;168(6):1998–2013. doi: 10.2353/ajpath.2006.051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han SE, Park KH, Lee G, Huh YJ, Min BM. Mutation and aberrant expression of Caveolin-1 in human oral squamous cell carcinomas and oral cancer cell lines. Int J Oncol. 2004 Feb;24(2):435–40. [PubMed] [Google Scholar]
- 66.Ae Kim C, Delepine M, Boutet E, El Mourabit H, Le Lay S, Meier M, et al. Association of a homozygous nonsense Caveolin-1 mutation with Berardinelli-Seip Congenital Lipodystrophy. J Clin Endocrinol Metab. 2008 Jan 22; doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 67.Cao H, Alston L, Ruschman J, Hegele RA. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis. 2008;7:3. doi: 10.1186/1476-511X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garg A, Agarwal AK. Caveolin-1: a new locus for human lipodystrophy. J Clin Endocrinol Metab. 2008 Apr;93(4):1183–5. doi: 10.1210/jc.2008-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gratton JP, Lin MI, Yu J, Weiss ED, Jiang ZL, Fairchild TA, et al. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003 Jul;4(1):31–9. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 70.Lin MI, Yu J, Murata T, Sessa WC. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 2007 Mar 15;67(6):2849–56. doi: 10.1158/0008-5472.CAN-06-4082. [DOI] [PubMed] [Google Scholar]
- 71.Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation. 2006 Aug 29;114(9):912–20. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]
- 72.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6(12):1362–7. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 73.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006 May;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 74.Mercier I, Casimiro MC, Wang C, Rosenberg AL, Quong J, Minkeu A, et al. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: Implications for the response to hormonal therapy. Cancer Biol Ther. 2008 Aug;7(8):1212–25. doi: 10.4161/cbt.7.8.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, et al. An Absence of Stromal Caveolin-1 Expression Predicts Early Tumor Recurrence and Poor Clinical Outcome in Human Breast Cancers. Am J Pathol. 2009 doi: 10.2353/ajpath.2009.080873. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sotgia F, Del Galdo F, Casimiro MC, Bonuccelli G, Mercier I, Whitaker-Menezes D, et al. Caveolin-1 (-/-) Null Mammary Stromal Fibroblasts Share Characteristics with Human Breast Cancer-Associated Fibroblasts. Am J Pathol. 2009 doi: 10.2353/ajpath.2009.080658. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mercier I, Casimiro MC, Zhou J, Wang C, Plymire C, Bryant KG, et al. Genetic Ablation of Caveolin-1 Drives Estrogen-Hypersensitivity and the Development of DCIS-like Mammary Lesions. Am J Pathol. 2009 doi: 10.2353/ajpath.2009.080882. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams TM, Sotgia F, Lee H, Hassan G, Di Vizio D, Bonuccelli G, et al. Stromal and epithelial caveolin-1 both confer a protective effect against mammary hyperplasia and tumorigenesis: Caveolin-1 antagonizes cyclin D1 function in mammary epithelial cells. Am J Pathol. 2006 Nov;169(5):1784–801. doi: 10.2353/ajpath.2006.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]