Summary
Dopamine orchestrates motor behavior and reward-driven learning. Perturbations of dopamine signaling have been implicated in several neurological and psychiatric disorders, and in drug addiction. The actions of dopamine are mediated in part by the regulation of gene expression in the striatum, through mechanisms that are not fully understood. Here, we show that drugs of abuse, as well as natural reinforcement learning, promote the nuclear accumulation of dopamine- and cAMP-regulated phosphoprotein Mr=32,000 (DARPP-32). This accumulation is mediated through a signaling cascade involving dopamine D1 receptors, cAMP-dependent activation of protein phosphatase-2A, dephosphorylation of DARPP-32 at Ser-97 and inhibition of its nuclear export. The nuclear accumulation of DARPP-32, a potent inhibitor of protein phosphatase-1, increases phosphorylation of histone H3, an important component of nucleosomal response. Mutation of Ser-97 profoundly alters behavioral effects of drugs of abuse, and decreases motivation for food, underlining the functional importance of this signaling cascade.
Midbrain dopamine (DA) neurons, activated following unexpected rewarding stimuli, are essential in reinforcement learning1. Drugs of abuse mimic the physiological action of DA neurons by increasing their firing rate or preventing DA uptake. Thus, they enhance extracellular DA levels in the forebrain, especially in the nucleus accumbens (NAc), a key structure required for the reinforcing effects of addictive drugs2-4. To understand how DA mediates reward-controlled learning, it is necessary to identify the intracellular events that trigger gene transcription alterations supporting long-lasting synaptic changes5-7. DARPP-32 (dopamine- and cAMP-regulated phosphoprotein, Mr=32,000)8 is a prominent mediator of DA signaling in the striatum9. DARPP-32 is highly enriched in striatal GABAergic mediumsize spiny neurons (MSN)10. Following activation of DA D1 receptors (D1R), DARPP-32 is phosphorylated by cAMP-dependent protein kinase (PKA) at Thr-34 and converted into a potent inhibitor of the multifunctional serine/threonine protein phosphatase-1 (PP1)11. DARPP-32-mediated inhibition of PP1 increases the phosphorylation of neurotransmitter receptors and ion channels crucial for synaptic function and plasticity9. DARPP-32 also regulates nuclear events, as demonstrated by alterations of drug-induced gene expression in mice lacking DARPP-32 or bearing a point mutation of Thr-3412,13. Part of the control exerted by DARPP-32 on transcription is mediated by activation of the ERK pathway, dependent on the concomitant stimulation of D1R and glutamate NMDA receptors13,14. However, the precise mechanisms of information transfer from the cytoplasm to the nucleus of striatal neurons are still poorly characterized.
Drugs of abuse and reinforcement learning trigger nuclear accumulation of DARPP-32 in striatal neurons
DARPP-32 has been extensively characterized as a cytoplasmic protein9. On the other hand, electron microscopy revealed DARPP-32-immunoreactivity in the nucleus of some striatal MSN15, the significance of which has not been elucidated. In mice treated with D-amphetamine, Thr-34-phospho-DARPP-32 immunoreactivity was strong in the nuclei of striatal neurons (Fig.1a). D-amphetamine increased total DARPP-32 immunoreactivity in nuclei (Fig.1a,b). Cocaine also induced a rapid nuclear accumulation of DARPP-32 which lasted several hours (Fig.1b, Supp. Fig.1a). D-amphetamine and cocaine triggered DARPP-32 nuclear accumulation in both dorsal striatum and NAc (data not shown). In contrast, morphine increased nuclear DARPP-32 only in the NAc shell (Fig.1b, Supp. Fig.1b), where it potently increases DA release16. A reinforcement learning paradigm, in which mice nose poked for food, also triggered nuclear accumulation of DARPP-32 in the dorsal striatum and NAc shell and core (Fig.1c). In mice receiving no food, or in yoked mice which received food when the “active” animal nose poked, no nuclear accumulation was observed (Fig.1c).
Figure 1. Drugs of abuse and food self-administration induce accumulation of DARPP-32 in nuclei of mouse striatal neurons through a D1R/cAMP-mediated mechanism.
(a) DARPP-32 (green) and phospho-Thr-34-DARPP-32 (P-Thr34-DARPP-32, red) immunofluorescence in dorsal striatum (DStr), 15 min after i.p. injection of saline (Sal) or D-amphetamine (10 mg/kg, Amph), single confocal sections. (b) Effects of D-amphetamine (Student t-test), cocaine (20 mg/kg, time-course, F(6,42)=26.6, p<0.01), and morphine (Mor, 5 mg/kg s.c., 15 min, Student t-test). (c) Effects of reinforcement learning 1-hour session in which mice were placed in the self-administration chamber without food (No food, NF), learned to nose poke for food pellets (Active, A), or received pellets when the active animal nose poked (Yoked, Y). DStr: F(2,14)=15.38, p<0.001; core: F(2,14)=14.45, p<0.001; shell: F(2,14)=17.93, p<0.001). Means±SEM, 4-8 mice per group. One-way ANOVA, Bonferroni test (unless indicated), *p<0.05, **p<0.01, ***p<0.001. Bar: 10 μm.
In drd1a-EGFP mice, which express specifically green fluorescent protein (GFP) in striatonigral neurons17, DARPP-32 nuclear accumulation occurred in D1R-expressing neurons 8 min after cocaine injection (Supp. Fig.2a). Cocaine-induced rapid nuclear accumulation of DARPP-32 was not observed in D1R-null mice (Supp. Fig.2b), indicating the requirement for D1R. In contrast, pretreatment of mice with MK801, an antagonist of NMDA receptors, did not alter the effects of cocaine (Supp. Fig.2c).
In striatal neurons in culture, DARPP-32 immunofluorescence was predominantly cytoplasmic and application of SKF81297, a D1R agonist, induced its nuclear accumulation, which was prevented by a D1R antagonist, SCH23390, or a cAMP antagonist, Rp-cAMPS (Supp Fig.3a,b). Conversely, a cAMP analogue, Sp-5,6-DCl-cBIMPS, increased nuclear DARPP-32 immunoreactivity in a time-dependent manner (Supp. Fig.3c). Similar results were obtained with DARPP-32 fused to green fluorescent protein (D32-GFP), co-expressed with D1R in striatal neurons in culture (Supp. Fig.4a-c). Altogether our results showed that DARPP-32 nuclear accumulation in response to physiological reward-controlled learning or to drugs of abuse was mediated by D1R and that cAMP was necessary and sufficient for this effect.
D1R controls DARPP-32 cyto-nuclear shuttling through regulation of Ser-97 phosphorylation
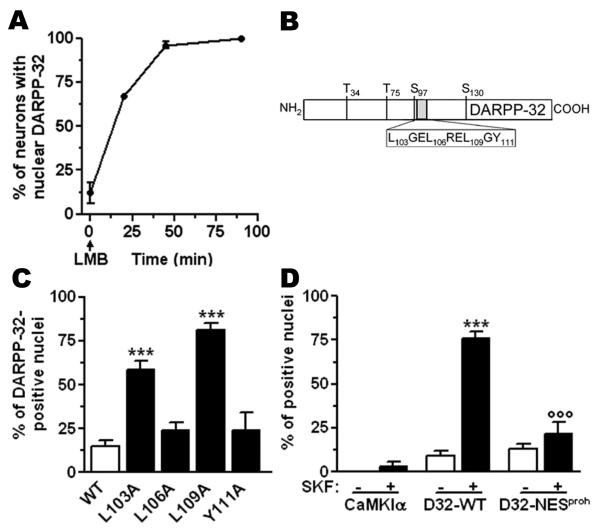
Leptomycin B (LMB), a specific inhibitor of CRM1-mediated nuclear export18 induced a rapid nuclear accumulation of endogenous DARPP-32 (Fig.2a) or transfected D32-GFP (Supp. Fig.5a) in virtually all neurons. We identified at residues 103-111 of DARPP-32 a putative nuclear export signal (NES, Fig.2b), homologous to sequences known to bind CRM119. Mutation of Leu-103 or Leu-109 to alanine induced a dramatic accumulation of DARPP-32-GFP in the nucleus (Fig.2c), confirming the critical role of this sequence in nuclear export. Similar results were observed in CHO cells (Supp. Fig.5b,c). We also found that the N-terminal region of DARPP-32, which encompasses several putative nuclear localization signals, played a critical role in its nuclear import (data not shown).
Figure 2. DARPP-32 undergoes a continuous cyto-nuclear shuttling.
(a) Leptomycin B (LMB, 10 ng/ml) effect on DARPP-32 localization in striatal neurons in culture. (b) DARPP-32 putative NES and major phosphorylation sites. (c) Mutagenesis of hydrophobic residues in the NES increases D32-GFP nuclear accumulation:. F(4,21)=35.0, p<0.001; ***p<0.001 vs. wild type. (d) Stimulation of D1R has no effect on the localization of an unrelated protein with an NES (CaMKIα-GFP) or when DARPP-32 contains an additional NES (D32-NESproh-GFP): Neurons transfected with D1R and GFP-chimeras treated with vehicle or SKF81297 (10 μM, 15 min): F(5,22)=35.2, p<0.001; ***p<0.001 SKF vs. vehicle; ooop<0.001 mutant vs. wild type. Data are means ± SEM, n=3-6, one-way ANOVA, Bonferroni test.
These results suggested that D1R stimulation acted by reducing the export of DARPP-32 out of the nucleus. We ruled out a general inhibitory influence of D1R stimulation on CRM1-dependent nuclear export, by showing that SKF81297 did not alter the localization of another protein with an active NES20, Ca2+/calmodulin-dependent kinase-Iα (CaMKIα-GFP, Fig.2d). Introduction of an ectopic NES sequence derived from prohibitin21 (NESproh) at the C-terminus of DARPP-32 (D32-NESproh-GFP) blocked the translocation induced by SKF81297 (Fig.2d).
To determine how the D1R/cAMP pathway regulated DARPP-32 localization we mutated its major phosphorylation sites (Thr-34, Thr-75, Ser-97, and Ser-130, Fig.2b) to alanine. In striatal neurons in culture, basal and D1R-induced localization of T34A-, T75A- and S130A-DARPP-32-GFP mutants was similar to that of wild type protein (Supp. Fig.6a). In support of these observations, in knock-in mutant mice bearing an alanine point mutation at Thr-34 or Thr-75 the basal localization of DARPP-32 and its cocaine-induced nuclear accumulation was similar to wild type (Supp. Fig.6b,c). In contrast, when Ser-97, located close to the NES of DARPP-32, was mutated to alanine, the protein was nuclear in basal conditions and D1R stimulation did not alter this distribution (S97A-D32-GFP, Fig.3a). Mutation of Ser-97 to phospho-mimic acidic residues, glutamate (S97E) or aspartate (S97D) induced a preferential cytoplasmic localization, which was not modified by SKF81297 (Fig.3a, Supp. Fig.7a).
Figure 3. Phosphorylation of Ser-97 controls intracellular localization of DARPP-32.
(a) S97 mutation alters localization of DARPP-32 (first panel): Striatal neurons transfected with D1R and D32-GFP wild type or Ser-97-Ala (S97A), -Asp (S97D), or -Glu (S97E), treated for 15 min with vehicle (−) or 10 μM SKF81297 (+); F(7,42)=16.6, p<0.001; ***p<0.001 SKF vs. basal; oop<0.01, ooop<0.001, mutant vs. wild type. Inhibition of CK2 by TBB promotes nuclear accumulation of DARPP-32 (second panel): Treatment with vehicle or 10 μM SKF81297 in the absence or presence of TBB (50 μM, 45 min before SKF); F(3,16)=14.9, p<0.001; ***p<0.001 SKF vs. vehicle; ooop<0.001 TBB vs. vehicle. Mutation of Ser-97 to glutamate (S97E) prevents the effects of TBB (third panel): F(3,12)=14.6, p<0.001; ***p<0.001 TBB vs. vehicle. Okadaic acid prevents D1R-induced nuclear accumulation of DARPP-32 (fourth panel): Treatment with vehicle or SKF81297, in the absence or presence of okadaic acid (OA, 500 nM, 45 min before SKF81297); F(3, 21)=38.9, p<0.001; ***p<0.001 SKF vs. vehicle; ooop<0.001 OA vs. vehicle. (b) Stimulation of D1R (10 μM SKF81297) induces phosphorylation of Thr-34 and dephosphorylation of Ser-97 in mouse striatal slices: Phosphorylation of Thr-34 and Ser-97 measured by immunoblotting, normalized to untreated slices (n=5-7); **p<0.01. (c) Forskolin (10 μM) induces phosphorylation of Thr-34 and dephosphorylation of Ser-97 in slices (n=5-7): **p<0.01. (d) Okadaic acid (OA 1 μM) prevents dephosphorylation of Ser-97 induced by forskolin (Forsk) in slices: P-Thr-34-DARPP-32: F(3,24)=16.3, p<0.001, vs. control ***p<0.001; vs. OA ∇∇p<0.01. P-Ser-97-DARPP-32: F(3,26)=24.7, p<0.001, vs. control ***p<0.001. (e) B56δ promotes the dephosphorylation of DARPP-32 Ser-97 in response to forskolin: HEK293 cells transfected with DARPP-32 and vector, B56δ or Bα PP2A subunit, incubated with vehicle or 10 μM forskolin (10 min); DARPP-32-phospho-Ser-97 immunoblotting (n=3, Student t-test *p<0.01). Statistics: means ± SEM, one-way ANOVA and Bonferroni test (unless indicated).
Ser-97 (Ser-102 in rat) is highly phosphorylated in basal conditions by casein kinase-2 (CK2)22. An inhibitor of CK2, 4,5,6,7-tetrabromobenzotriazole (TBB)23, increased markedly nuclear DARPP-32-GFP, and prevented the effects of D1R-agonist treatment (Fig.3a, Supp. Fig.7b). The effects of TBB resulted from prevention of Ser-97 phosphorylation, since they were not observed with S97E-D32-GFP (Fig.3a, Supp. Fig.7c). These results supported the hypothesis that DARPP-32 is mainly nuclear when Ser-97 is dephosphorylated, whereas it is preferentially cytoplasmic when phosphorylated by CK2.
Dopamine and cAMP trigger dephosphorylation of Ser-97 through activation of protein phosphatase-2A
Since our results predicted that Ser-97 dephosphorylation would enhance nuclear DARPP-32, we tested the role of protein phosphatase-2A (PP2A), which dephosphorylates Ser-97 in vitro22. Pretreatment of striatal neurons with okadaic acid, a potent inhibitor of PP2A, did not alter the basal cytoplasmic localization of DARPP-32-GFP, whereas it blocked its D1R-induced nuclear translocation (Fig.3a, Supp. Fig.7d). We next examined the regulation of Ser-97 phosphorylation in striatal slices24. SKF81297 induced a marked increase in Thr-34 phosphorylation, as expected, and a dephosphorylation of Ser-97 (Fig.3b). The precise time-course of the two responses was different, with dephosphorylation of Ser-97 being slower and more persistent than Thr-34 phosphorylation. Forskolin, a potent activator of adenylyl cyclase induced a pronounced and sustained increase in Thr-34 phosphorylation and a slightly delayed, but persistent, decrease in Ser-97 phosphorylation (Fig.3c, Supp. Fig.8). Forskolin-stimulated dephosphorylation of Ser-97 was prevented by okadaic acid (Fig.3d), at a concentration which blocks PP2A activity fully and PP1 activity only partially in striatal slices25.
PP2A activity is controlled by various regulatory subunits, among which the B56δ subunit is highly expressed in the striatum (Supp. Fig.9a), and is activated by cAMP-dependent phosphorylation26,27. When B56δ was co-expressed with DARPP-32 in HEK293 cells, it promoted dephosphorylation of Ser-97 in response to forskolin (Fig.3e, Supp. Fig.9b). In contrast, in cells transfected with the vector alone or another isoform of B subunit (Bα), forskolin slightly increased Ser-97 phosphorylation (Fig.3e, Supp. Fig.9b). Thus, D1R stimulation and increased cAMP induce dephosphorylation of Ser-97 selectively through stimulation of PP2A containing the B56δ subunit.
Ser-97-Ala-DARPP-32 mutant mice display altered behavioral responses to drugs of abuse and decreased motivation for food
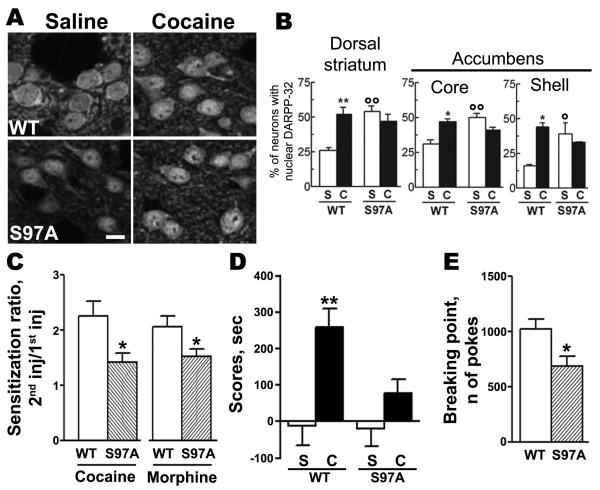
To examine the role of this residue in vivo, we used a knock-in mouse line bearing a Ser-97 to alanine (S97A) point mutation. DARPP-32 was nuclear in approximately half of MSN in the various striatal regions of the mutant mice (Fig.4a). Importantly cocaine injection did not alter the nuclear localization of S97A-DARPP-32 (Fig.4a).
Figure 4. Mutation of Ser-97 alters behavioral responses to drugs of abuse and motivation for food reward.
(a) DARPP-32 immunoreactivity in the dorsal striatum of wild type (WT) or Ser-97-Ala mutant mice (S97A) injected with saline (S) or cocaine (C, 20 mg/kg, 10 min): Single confocal sections; Bar 10 μm; (n=3-8); dorsal striatum: genotype-cocaine interaction F(1,19)=11.5, p<0.01; genotype effect, F(1,19)=5.58, p<0.05; cocaine effect, F(1,19)=3.81, NS; **p<0.01 Sal vs. Coc; oop<0.01 S97A vs. wild type; core: genotype-cocaine interaction, F(1,8)=24.0, p<0.01; genotype effect, F(1,8)=6.5, p<0.05; cocaine effect, F(1,8)=1.9, NS; **p<0.01 Sal vs. Coc; oop<0.01 S97A vs. wild type; shell, genotype-cocaine interaction F(1,10)=12.0, p<0.01; genotype effect, F(1, 10)=1.49, NS; cocaine effect, F(1,10)=5.00, p<0.05; *p<0.05 Sal vs. Coc; op<0.05 S97A vs. wild type. (b) Sensitization of locomotor response to cocaine (20 mg/kg) and morphine (5 mg/kg) is decreased in S97A mutant mice: Mice received two drug injections (on days 1 and 7). Locomotor activity was recorded in a circular maze for 1 h (cocaine) or 3 h (morphine) and sensitization expressed as the ratio of response to the 2nd injection over response to the 1st injection. Student t-test: cocaine: **p<0.01 (n=16); morphine: *p<0.01 (n=8). (c) Conditioned place preference to cocaine was prevented in S97A mice: Scores are differences between times spent in the cocaine-paired compartment after and before conditioning (n=7-8): genotype-treatment interaction, F(1,26)=3.16, NS; genotype effect, F(1,26)=3.76, NS; treatment effect, F(1, 26)=14.21, p<0.01; **p<0.01 vs. saline. (d) Mice trained to nose poke for food reward were subjected to progressive ratio (PR) schedule and the breaking point determined as the number of pokes at which mice stopped working: Student t-test : *p<0.05 (n=7-8). Statistics: means ± SEM, two-way ANOVA and Bonferroni test (unless indicated).
The spontaneous activity, habituation, and acute locomotor response to cocaine was similar in S97A-DARPP-32 and wild type mice (Supp. Fig.10a,b,c), whereas the acute locomotor response to morphine (5 mg/kg) was reduced in the mutant (Supp. Fig.10d). In wild type mice a second injection of cocaine or morphine, 7 days after the first injection, had a much stronger effect on locomotion than the first one, showing a robust sensitization (Supp. Fig.10e,f), as previously reported13. In S97A-DARPP-32 mice, locomotor sensitization to cocaine and morphine, although still present, was less pronounced than in wild type mice (Supp. Fig.10e,f, Fig.4b). Moreover, the rewarding effects of cocaine, evaluated by conditioned place preference, were not observed in S97A-DARPP-32 mice (Fig.4c).
We next examined whether mutation of Ser-97 interfered with responses to physiological rewarding stimuli using a paradigm in which mildly food-deprived mice learned to obtain food pellets by nose poking. Wild type and mutant mice learned equally well, even when the number of nose pokes necessary to obtain a pellet was increased to a fixed ratio of 5 or when the rewarded hole was reversed (Supp. Fig.10g). However, when the required number of nose pokes was progressively increased until the mice interrupted their behavior, the breaking point was much lower in S97A mutant than in wild type mice (Fig.4d). Together these results showed that mutation of Ser-97 altered long-lasting responses to drugs of abuse, and decreased motivation for food reward
In S97A-DARPP-32 mice we found a decreased phosphorylation of DARPP-32 on Thr-34 and of several proteins regulated through DARPP-32 (data not shown). We previously observed using purified bovine DARPP-32 that prior phosphorylation by CK2 facilitated phosphorylation of Thr-34 phosphorylation by PKA22. However, there was no deficit in cAMP-induced Thr-34 phosphorylation of S97A mutant protein in transfected COS7 cells in which overexpressed DARPP-32 was cytoplasmic (Supp. Fig.11). Thus, we conclude that the observed alterations in S97A mice resulted from the blockade of cyto-nuclear shuttling of DARPP-32, rather than from an intrinsic effect of Ser-97 phosphorylation on Thr-34 phosphorylation.
Nuclear translocation of DARPP-32 is essential for D1R-regulated histone H3 phosphorylation in striatal neurons
Since D1R stimulation induces the accumulation of Thr-34-phospho-DARPP-32 (Fig.1a), a potent inhibitor of PP1, we hypothesized that DARPP-32 translocation might regulate the phosphorylation of nuclear proteins. We therefore investigated the phosphorylation of histone H3 on Ser-10, a substrate for several kinases28,29, which is known to be regulated by PP130,31. The phosphorylation of H3 on Ser-10 is a key step in nucleosomal response28,32 and is crucial for memory formation33. It is increased in the striatum after cocaine administration in vivo, and this response is functionally important14,34. Cocaine induced an intense phospho-Ser-10-H3 signal in the nuclei of a number of striatal neurons in wild type mice, whereas this effect was absent in either T34A-DARPP-32 or S97A-DARPP-32 mice (Fig.5a). Similar results were observed using an antibody that detected phospho-acetyl-H3 (phospho-Ser-10-acetylLys-14-H3, data not shown).
Figure 5. Role of nuclear DARPP-32 in the phosphorylation of histone H3 Ser-10 in striatal neurons.
(a) Cocaine-induced phosphorylation of H3 Ser-10 is abolished in Thr-34-Ala and Ser-97-Ala mutant mice: Phospho-Ser-10-H3 immunofluorescence (P-H3) in dorsal striatum of wild type (WT), T34A-DARPPP-32 (T34A) or S97A-DARRP-32 (S97A) mutant mice treated with saline (Sal) or cocaine (20 mg/kg, 30 min). (b) D1R stimulation increases Ser-10 H3 phosphorylation in DARPP-32-transfected neurons: Striatal neurons transfected with D32-GFP and D1R, treated with vehicle or SKF81297 (10 μM, 15 min). Confocal section of GFP fluorescence and phospho-Ser-10-H3 (P-H3), acetyl-Lys-14-H3 (Ac-H3) and phosphoSer-10-acetyl-Lys-14-H3 (P/Ac-H3) immunolabeling. Bars: 5 μm. (c) H3 phosphorylation requires DARPP-32 Thr-34 and nuclear accumulation: Striatal neurons transfected with wild type (WT) or mutant D32-GFP: T34A (unable to inhibit PP1), or S97E and D32-NESproh-GFP, treated with SKF81297. Arrows: median values; Kruskal-Wallis and Dunn's tests. (d) Working model of DARPP-32 regulation of nucleosomal response in striatal neurons.
In striatal neurons transfected with D1R and D32-GFP, the D1R agonist SKF81297 increased dramatically phosphorylation of H3 on Ser-10 (Fig.5b,c, Supp. Fig.12). SKF81297 did not alter H3 Lys-14 acetylation, but increased phospho-Ser-10, acetyl-Lys-14 immunoreactivity (Fig.5b, Supp. Fig.12). D1R-induced phosphorylation of histone H3 was lost when neurons were transfected with T34A-DARPP-32-GFP instead of wild type (Fig.5c), confirming that the ability of DARPP-32 to inhibit PP1 was essential for H3 phosphorylation. We then directly evaluated the role of DARPP-32 translocation to the nucleus, by using mutant forms of DARPP-32-GFP constitutively excluded from the nucleus: S97E-DARPP-32-GFP (see Fig.3a) and DARPP-32-NESproh-GFP (see Fig.2d). In neurons transfected with these “cytoplasmic” variants of DARPP-32-GFP, the D1R agonist SKF81297 did not induce H3 phosphorylation (S97E and NESproh, Fig.5c). These results showed that DARPP-32 phosphorylation on Thr-34, and thus PP1 inhibition, was essential for D1R-induced phosphorylation of Ser-10-H3 and that this effect required the ability of DARPP-32 to accumulate in the nucleus in response to D1R stimulation.
Discussion
Our study identifies nuclear DARPP-32 as a key factor in the phosphorylation of histone H3, a component of the nucleosomal response essential for gene expression28,32,33. Psychostimulant drugs, D-amphetamine and cocaine, and morphine, which act by completely different mechanisms, share the ability to increase extracellular DA in the NAc, and to trigger the nuclear accumulation of DARPP-32. A natural stimulus such as a simple food-reinforced learning paradigm caused a similar effect. DARPP-32 undergoes a very active, continuous cyto-nuclear shuttling regulated by phosphorylation of Ser-97, which is in the vicinity of its NES (summarized in Fig.5d). Phosphorylation of Ser-97 by CK2 appears critical for nuclear export of DARPP-32. CK2 is present in nuclei of striatal neurons (Supp. Fig.13) and can phosphorylate nuclear DARPP-32 and promote its export. Stimulation of D1R triggers rapid phosphorylation of Thr-34 responsible for cytoplasmic effects of DARPP-32, and slower dephosphorylation of Ser-97 by activating PP2A through cAMP/PKA-mediated phosphorylation of its B56δ subunit26,27, thus decreasing the nuclear export of DARPP-32. Although phosphorylation has been reported to regulate cyto-nuclear shuttling of a number of proteins35, this is a rare example of facilitation of CRM1-mediated nuclear export by phosphorylation36,37. Interestingly, cAMP-independent mechanisms also couple DA D2R to a delayed activation of PP2A38. D2R do not seem to be important in the rapid DARPP-32 translocation, but they could have a regulatory role on Ser-97 phosphorylation and DARPP-32 localization in other circumstances.
A large body of evidence demonstrates that long term synaptic plasticity requires the control of gene expression in the striatum as in other brain regions39. We have identified a novel mechanism by which DA controls chromatin through the regulated translocation of a PP1 inhibitor to the nucleus, which promotes histone H3 phosphorylation. DA-controlled inhibition of nuclear PP1, a wide-spectrum protein phosphatase, is likely to have other targets, making it a general means for control of nuclear function. This mechanism is undoubtedly important in the long-term effects of drugs of abuse, which activate gene transcription through D1R stimulation, and in physiological reward-controlled learning. Regulation of DARPP-32 nuclear accumulation may also be important to the functions or dysfunctions of DA that involve transcription-dependent long term plasticity, including neuroleptic- and L-DOPA-induced dyskinesia, and other diseases of the basal ganglia.
METHODS SUMMARY
Knock-in T34A-, T75A-, and S97A-DARPP-3240,41, D1R-deficient42, drd1a-EGFP17 and matched wild-type control mice were habituated to saline injections for 3 days before experiments. Drug-treated mice were anaesthetized with pentobarbital and fixed by intracardiac perfusion with 4% paraformaldehyde. Vibratome-cut brain sections were processed for immunofluorescence13. Primary striatal neurons, from 14-day embryonic Swiss mice, were grown in supplemented Neurobasal medium, and transfected a week later with plasmids expressing D1R and DARPP-32 fused with C-terminal EGFP (D32-GFP). HEK293, CHO-K1, or COS-7 cells were transfected with DARPP-32 constructs associated or not with PP2A subunits, and studied 24-48 hours later. Subcellular localization of DARPP-32 or GFP was classified using confocal single sections into three categories (nuclear staining stronger, equal or weaker than cytoplasmic). For cells in culture, the first two categories were pooled and considered as cells with nuclear staining. Phosphorylated and/or acetylated H3 immunofluorescence was quantified with Image-J-1.34s (NIH). Striatal coronal slices (350 μm) were incubated in oxygenated artificial cerebrospinal fluid and analyzed by western blotting25. Evaluation of locomotor activity, sensitization produced by a single injection of drug, and conditioned place preference were as described13,14. For incentive learning food self-administration, mildly food-deprived mice were placed in an operant chamber with two nose-poke holes. One or several nose pokes (fixed ratios) in the rewarded hole, indicated by a light, triggered the release of a food pellet. Controls were “no food” (mice in the same chamber but no pellet delivered) and “yoked” mice (paired to active mice, in exactly the same conditions, but rewarded when the active mouse nose poked). For the reversal test, the light indicating the rewarded hole was reversed. For analysis of progressive ratio, the number of successive pokes required for food was progressively increased until the mouse stopped poking (breaking point).
METHODS
Animals
Male 8-week old C57BL/6J mice from Elevage Janvier (Le Genest St Isle, France) or Japan SLC (Shizuoka, Japan) were used for in vivo and slice experiments, respectively. Production of knock-in T34A-, T75A- and S97A-DARPP-32 mutant40,41, D1R-deficient42, and drd1a-EGFP17 mice was described previously. Mutant and wild type mice used in experiments, or their parents, were littermates. Mice were kept at least one week in the animal house in stable conditions of temperature (22°C) and humidity (60%) with a constant cycle of 12 h light and 12 h dark and free access to food and water. During the 3 days preceding the experiments, mice were habituated to injections by daily intraperitoneal saline administration. Animal care was in accordance with ethical guidelines (Declaration of Helsinki and NIH, publication no.85-23, revised 1985, European Community Guidelines on the Care and Use of Laboratory Animals, and French Agriculture and Forestry Ministry guidelines for handling animals, decree 87849, license A 75-05-22) and approved by the local ethical committees.
Drugs
(+)-D-methylphenethylamine (D-amphetamine) sulfate salt, cocaine-HCl, SKF81297, SCH23390 (both prepared in 0.1% vol/vol DMSO) and okadaic acid potassium salt Sigma-Aldrich or Tocris; morphine sulfate salt: Francopia, 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole-3′,5′-cyclic-monophosphoro-thioate, Sp-isomer (Sp-5,6-DCl-cBIMPS) and adenosine-3′,5′-cyclic-mono-phosphorothioate, Rp-isomer (Rp-cAMPS): Biolog; leptomycin-B: Amersham; 4,5,6,7-tetrabromobenzotriazole (50 μM in 0.1% DMSO): Calbiochem. For in vivo experiments, drugs were dissolved in 0.9% NaCl and injected intraperitoneally (i.p.) except for morphine which was injected sub-cutaneously (s.c.).
Plasmid constructs and DARPP-32 mutants
To construct D32-GFP, full length cDNA of Rattus norvegicus DARPP-32 (note that for simplicity mouse residue numbering is used throughout) was amplified by PCR using Pfu (Stratagene) and cloned in XhoI/EcoRI sites of EGFP-N2 (Clontech). DARPP-32 mutants were produced by site-directed mutagenesis (Quick-Change, Stratagene). DARPP-32-NESproh was constructed by EcoRI/SalI digestion and ligation of a sequence encoding AAEDIAYQLSRSRNITYLPAGQSVLLQLPQ (NES of prohibitin) to the C-terminus of DARPP-3221. All constructs were confirmed by DNA sequencing. CaMKIα-GFP and pRK5-D1R were gifts of Dr. M.R. Picciotto (Yale University), S. Cottecchia and J.P. Hornung (Lausanne University), respectively.
Cell culture and transfection
Striatal cells (1.8 × 105/well) from 14-day embryonic Swiss mice (Janvier) were cultured14 in B27-supplemented Neurobasal (Invitrogen), 500 nM L-glutamine, 60 μg/ml penicillin G, 25 μM β-mercaptoethanol, on 24-well dishes precoated with 50 μg/ml poly-L-lysine (Sigma) at 37°C in humidified 95% air, 5% CO2. After 7 days in culture, neurons were transfected with D32-GFP (1 μg) and pRK5-D1R (1 μg) DNA, using Lipofectamine 2000 in OptiMEM serum-free medium (Invitrogen). Treatments were done 24 hours later, in fresh Neurobasal medium. CHO-K1 (American Type Culture Collection, Manassas, VA) cells were seeded (25,000 cells/14-mm-diameter coverslips), in Ham's F-12 medium (Invitrogen) with 10% FBS (Invitrogen), 0.5 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin, at 37 °C in 5% CO2. COS-7 cells were cultured in 6-well plates (106 per well) in DMEM (Invitrogen) containing 10% fetal bovine serum. CHO-K1 and COS-7 cells were transfected as neurons with D32-GFP DNA (1 μg). HEK293 cells in 6-well plates (106 per well) to 60-70% confluence in DMEM (Invitrogen) containing 10% fetal bovine serum (FBS) were cotransfected with Myc-tagged DARPP-32 (0.5 μg) and FLAG-tagged B-subunits of PP2A vector, Bα or B56δ (0.5 μg) DNA, using Fugene-6 (Roche). After 48 h, cells were treated with forskolin and lysed in 150 μl of lysis buffer [50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, protease inhibitor cocktail (Roche), and phosphatase inhibitor cocktail I and II (Calbiochem)], sonicated for 5 s, and centrifuged for 10 min at 16,000 × g.
Immunostaining of striatal neurons in culture
Cells were fixed with PBS containing 2% paraformaldehyde (PFA) for 40 min at room temperature (RT) and incubated with methanol/acetone solution (1:1) for 10 min at 4°C. After three rinses in PBS, cells were treated with blocking buffer (3% BSA in PBS except for H3 immunofluorescence: 1% BSA and 1% FCS) for 45 min (2 h for P-Ser-10-H3) at RT. Coverslips were incubated overnight at 4°C in PBS containing 1% BSA with monoclonal antibodies for DARPP-32 (1:4000) and D1R (1:500, gift of Prof. R. Luedtke, University of North Texas Health Science Center at Fort Worth). P-Ser-10-H3, acetyl-Lys-14-H3 and PSer-10-acetyl-Lys-14-H3 antibodies (1:1000, Upstate) were added overnight at 4°C in PBS containing 1% BSA and Tween-20 0.05%. Anti-mouse Alexa488-conjugated antibody or anti-mouse CY3-conjugated antibody (1:400, Molecular Probes) were added for 1 h at RT. Nuclei were counterstained with SYTOX (1:2000, Molecular Probes). Cells were rinsed three times and mounted under coverslips using Vectashield (Vector laboratories). The subcellular localization of D32-GFP and CaMKID-GFP was visualized by direct fluorescence.
Immunohistofluorescence of brain sections and subcellular distribution of DARPP-32
Brains from PFA-perfused mice were post-fixed and 30-μm sections cut with a vibratome (Leica) 13,43. Free-floating sections were incubated with antibodies overnight at 4°C, and after rinses in TBS, for 2 hr at RT with secondary antibody (1:400; Cy3-coupled anti-rabbit-IgG, or Alexa488-conjugated anti-mouse antibodies, Molecular Probes). Nuclei were counterstained with 1 μM of TO-PRO-3 iodide (Molecular Probes). Sections were rinsed three times in TBS and mounted in Vectashield (Vector Laboratories).
Image acquisition and statistical analysis
Image acquisition with sequential laser scanning confocal microscopy (SP2, Leica) was done at the Institut du Fer à Moulin Imaging Facility. For brain immunofluorescence, two sections were analyzed bilaterally and cells were counted in one field for dorsal striatum, NAc shell and core (data points for an animal correspond to 4 acquisitions/structure). For cultures data points correspond to a minimum of 15 transfected cells (minimum of 2 different experiments with 3 independent transfections). Data were analyzed with Prism (GraphPad) software, using parametric or non-parametric statistics, as indicated, depending on the normality of the distribution of variables.
Neostriatal slices
Mice were sacrificed by decapitation, brains were rapidly removed and placed in ice-cold oxygenated Krebs-HCO3− buffer (124 mM NaCl, 4 mM KCl, 26 mM NaHCO3, 1.5 mM CaCl2, 1.25 mM KH2PO4, 1.5 mM MgSO4, and 10 mM D-glucose, pH 7.4)25. Coronal slices (350 μm) were prepared using a vibrating blade microtome (VT1000S; Leica Microsystems, Nussloch, Germany). Striata were dissected from the slices in ice-cold Krebs-HCO3− buffer and placed in a polypropylene incubation tube with 2 ml of fresh Krebs-HCO3− buffer containing adenosine deaminase (10 μg/ml). The slices were preincubated at 30°C under constant oxygenation with 95% O2/5% CO2 for 60 min. The buffer was replaced with fresh Krebs-HCO3− buffer after 30 min of preincubation. After drug treatment, slices were transferred to Eppendorf tubes, frozen on dry ice, and stored at −80°C until assayed.
Behavioral analysis
Experiments were performed after 3 consecutive daily 30-min habituation sessions with saline injections in the experimental box. Locomotor activity was measured in a circular corridor (Imetronic, Pessac, France)13. Mice were placed in the corridor 30 min before injection for the acute experiments. Locomotor sensitization was induced by a single injection of cocaine or morphine 13,44.
Supplementary Material
Acknowledgements
This work was supported by Inserm, and by a grant from Association Nationale de la Recherche to J.A.G. (05-NEUR-020-01), a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to A.N. and grants from NIDA (DA10044), NIMH (MH74866) and DOD (W81XWH-05-1-0146) to P.G. and A.C.N. A.S. was supported by Mission Interministérielle de Lutte contre la Drogue et la Toxicomanie, J.B.G. by Fondation pour la Recherche Médicale. The authors thank Mireille Lambert for her help with time-lapse video, and Peter Ingrassia and Philippe Bernard for their help with mutant mice.
References
- 1.Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- 2.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 3.Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- 4.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 5.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 7.Hyman SE, Malenka RC, Nestler EJ. Neural Mechanisms of Addiction: The Role of Reward-Related Learning and Memory. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 8.Walaas SI, Aswad DW, Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- 9.Svenningsson P, et al. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 10.Ouimet CC, et al. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III Immunocytochemical localization. J.Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmings HC, Jr., Greengard P, Tung HYL, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- 12.Fienberg AA, et al. DARPP-32: Regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–839. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 13.Valjent E, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brami-Cherrier K, et al. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouimet CC, Greengard P. Distribution of DARPP-32 in the basal ganglia: An electron microscopic study. J.Neurocytol. 1990;19:39–52. doi: 10.1007/BF01188438. [DOI] [PubMed] [Google Scholar]
- 16.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 18.Nishi K, et al. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J Biol Chem. 1994;269:6320–6324. [PubMed] [Google Scholar]
- 19.Henderson BR, Eleftheriou A. A comparison of the activity, sequence specificity, and CRM1-dependence of different nuclear export signals. Exp Cell Res. 2000;256:213–224. doi: 10.1006/excr.2000.4825. [DOI] [PubMed] [Google Scholar]
- 20.Stedman DR, et al. Cytoplasmic localization of calcium/calmodulin-dependent protein kinase I-alpha depends on a nuclear export signal in its regulatory domain. FEBS Lett. 2004;566:275–280. doi: 10.1016/j.febslet.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi S, Joshi B, Fusaro G, Chellappan S. Camptothecin induces nuclear export of prohibitin preferentially in transformed cells through a CRM-1-dependent mechanism. J Biol Chem. 2006;281:2951–2959. doi: 10.1074/jbc.M508669200. [DOI] [PubMed] [Google Scholar]
- 22.Girault JA, et al. Phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, by casein kinase II. J.Biol.Chem. 1989;264:21748–21759. [PubMed] [Google Scholar]
- 23.Sarno S, et al. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’) FEBS Lett. 2001;496:44–48. doi: 10.1016/s0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- 24.Nishi A, et al. Amplification of dopaminergic signaling by a positive feedback loop. Proc Natl Acad Sci U S A. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishi A, Snyder GL, Nairn AC, Greengard P. Role of calcineurin and protein phosphatase-2A in the regulation of DARPP-32 dephosphorylation in neostriatal neurons. J Neurochem. 1999;72:2015–2021. doi: 10.1046/j.1471-4159.1999.0722015.x. [DOI] [PubMed] [Google Scholar]
- 26.Usui H, et al. Activation of protein phosphatase 2A by cAMP-dependent protein kinase-catalyzed phosphorylation of the 74-kDa B″ (delta) regulatory subunit in vitro and identification of the phosphorylation sites. FEBS Lett. 1998;430:312–316. doi: 10.1016/s0014-5793(98)00684-x. [DOI] [PubMed] [Google Scholar]
- 27.Ahn JH, et al. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc Natl Acad Sci U S A. 2007;104:2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowak SJ, Corces VG. Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet. 2004;20:214–220. doi: 10.1016/j.tig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Salvador LM, et al. Follicle-stimulating hormone stimulates protein kinase A-mediated histone H3 phosphorylation and acetylation leading to select gene activation in ovarian granulosa cells. J Biol Chem. 2001;276:40146–40155. doi: 10.1074/jbc.M106710200. [DOI] [PubMed] [Google Scholar]
- 30.Murnion ME, et al. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J Biol Chem. 2001;276:26656–26665. doi: 10.1074/jbc.M102288200. [DOI] [PubMed] [Google Scholar]
- 31.Hsu JY, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 32.Bode AM, Dong ZG. Inducible covalent postranslational modification of histone H3. Science's STKE. 2005;281:1–12. doi: 10.1126/stke.2812005re4. [DOI] [PubMed] [Google Scholar]
- 33.Levenson JM, Sweatt JD. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell. Mol. Life Sci. 2006;63:1009–1016. doi: 10.1007/s00018-006-6026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Poon IK, Jans DA. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–186. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 36.Panasyuk G, et al. Nuclear export of S6K1 II is regulated by protein kinase CK2 phosphorylation at Ser-17. J Biol Chem. 2006;281:31188–31201. doi: 10.1074/jbc.M602618200. [DOI] [PubMed] [Google Scholar]
- 37.Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–3114. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 40.Svenningsson P, et al. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, et al. Cocaine self-administration in mice is inversely related to phosphorylation at Thr34 (protein kinase A site) and Ser130 (kinase CK1 site) of DARPP-32. J Neurosci. 2006;26:2645–2651. doi: 10.1523/JNEUROSCI.3923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drago J, et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci U S A. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valjent E, et al. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanderschuren LJ, et al. A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci. 1999;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.