Abstract
Outer hair cell electromotility is a rapid, force generating, length change in response to electrical stimulation. DC electrical pulses either elongate or shorten the cell and sinusoidal electrical stimulation results in mechanical oscillations at acoustic frequencies. The mechanism underlying outer hair cell electromotility is thought to be the origin of spontaneous otoacoustic emissions. The ability of the cell to change its length requires that it be mechanically flexible. At the same time the structural integrity of the organ of Corti requires that the cell possess considerable compressive rigidity along its major axis. Evolution appears to have arrived at novel solutions to the mechanical requirements imposed on the outer hair cell. Segregation of cytoskeletal elements in specific intracellular domains facilitates the rapid movements. Compressive strength is provided by a unique hydraulic skeleton in which a positive hydrostatic pressure in the cytoplasm stabilizes a flexible elastic cortex with circumferential tensile strength. Cell turgor is required in order that the pressure gradients associated with the electromotile response can be communicated to the ends of the cell. A loss in turgor leads to loss of outer hair cell electromotility. Concentrations of salicylate equivalent to those that abolish spontaneous otoacoustic emissions in patients weaken the outer hair cell’s hydraulic skeleton. There is a significant diminution in the electromotile response associated with the loss in cell turgor. Aspirin’s effect on outer hair cell electromotility attests to the role of the outer hair cell in generating otoacoustic emissions and demonstrates how their physiology can influence the propagation of otoacoustic emissions.
THERE HAS BEEN a dramatic change in our understanding of the biophysics of the mammalian inner ear over the past decade. The concept of the cochlea as a passive organ that converts the mechanical vibrations of sound into neural energy has been altered by the startling finding that outer hair cells possess a unique electromotile capacity (Brownell, 1983; 1984; Brownell, Bader, Bertrand, & de Ribaupierre, 1985). The electromotility appears to be responsible for the cochlea’s surprising ability to generate sound (Kemp, 1978; see also other articles in this issue). The hearing science community now accepts the presence of otoacoustic emissions after nearly a decade of experimental confirmation. Skepticism from the more general sensory science community is understandable. The fact that the ear can make sound is, at one level, equivalent to the eye producing light or the nose expelling odors. Energy conversion in both directions (bidirectional transduction) appears unique to hearing and is thought to contribute to the remarkable sensitivity and exquisite frequency selectivity of mammalian hearing. This contribution is discussed below in a brief review of the evidence implicating outer hair cells in generating otoacoustic emissions. The physiological characteristics of outer hair cell electromotility is then presented followed by an analysis of the structure-function relationships within the organ of Corti. This analysis introduces the concept of the hydraulic skeleton as a solution for the mechanical requirements imposed on the outer hair cell. Evidence, including the effect of aspirin on outer hair cell turgor and its impact on otoacoustic emissions, is reviewed. The evidence supports the presence of a hydraulic skeleton.
EVIDENCE FOR A SOURCE OF MECHANICAL ENERGY IN THE COCHLEA
Investigations of the inner ear have, for years, been dominated by paradigms originally proposed by Helmholtz and implemented by Békésy. Békésy (1960) provided strong experimental evidence that the basilar membrane is mechanically tuned by demonstrating that it is most sensitive to low frequencies at one end of the cochlear spiral and high frequencies at the other. The accuracy and the functional importance of Békésy’s findings stimulated inner ear research for decades.
The mechanical tuning Békésy measured was considerably broader than the neural tuning recorded from the VIIIth nerve (Kiang, Watanabe, Thomas, & Clark, 1965) and the inner hair cell (Russell & Sellick, 1978). As new techniques were applied to the measurement of cochlear partition movement, each improvement resulted in narrower mechanical tuning curves and better agreement between mechanical and neural data (Khanna & Leonard, 1982; Rhode, 1971; Rhode, 1978; Robles, Ruggero, & Rich, 1986; Sellick, Patuzzi, & Johnstone, 1982). Anoxia or other insults to the cochlea lead to a degradation of the narrow mechanical tuning of a healthy ear till the movements resemble those measured by Békésy (Rhode, 1971; Sellick et al, 1982). These observations lead to the conclusion that narrow mechanical tuning of the living cochlea is based on a physiologically vulnerable mechanism. The change that follows anoxia and death suggests that an energy consuming or active process is required for normal tuning.
Békésy’s description of the traveling wave motivated a number of mechanical engineering treatments. Analytic and numerical models were developed based on fundamental hydrodynamic and mechanical principles using passive mechanical elements. These models explain the general features of Békésy’s traveling wave but they are unable to account for the narrow mechanical tuning measured in undamaged, living cochlea. Gold (1948) had made an early suggestion that mechanical energy from an active process in the cochlea could produce narrower mechanical tuning. Recent models (Geisler, 1986; Jen & Steele, 1987; Mountain, 1986; Neely & Kim, 1983), incorporating a source of mechanical energy and specifically electromechanical transduction in the cochlear partition, describe mechanical behavior that resembles the narrow tuning of the intact cochlea. The discovery (Kemp, 1978) and subsequent characterization of otoacoustic emissions (more fully described in other articles in this issue) supported the existence of the postulated source of mechanical energy in the cochlea. The evidence for sound production by the inner ear has been around for centuries. The annals of medicine provide numerous reports of sound coming from patients ears. While some of these sounds are noncochlear in origin, the possibility of a cochlear origin for the remainder was not entertained until Kemp’s demonstration of otoacoustic emissions in 1978.
EVIDENCE FOR AN OUTER HAIR CELL INVOLVEMENT IN OTOACOUSTIC EMISSIONS
The crossed olivo-cochlear bundle is a collection of neural fibers that originates in the brain stem and travels to the cochlea, terminating predominately on outer hair cells. Crossed olivo-cochlear bundle stimulation modulates inner hair cell receptor potentials without significantly changing their membrane resistance (Brown & Nuttal, 1984; Brown, Nuttal, & Masta, 1983). Such stimulation also changes the strength of acoustical distortion products in the ear canal (Mountain, 1980; Siegel & Kim, 1982). Both effects suggest an outer hair cell involvement in cochlear mechanics and specifically in the generation of otoacoustic emissions.
An outer hair cell involvement is also suggested by comparing the otoacoustic emissions produced by mammals and nonmammals. Otoacoustic emissions have been measured in vertebrates other than mammals (Klinke & Smolder, 1984; Manley & Schulze, 1987; Palmer & Wilson, 1981; Strack, Klinke, & Wilson, 1981; Whitehead, Wilson, & Baker, 1986; Wit, van Dijk, & Segenhaut, 1988) and are generally of lower magnitude and frequency than can be found in mammals. The hearing organs of most nonmammals bear a strong resemblance to the sensory epithelium of vertebrate vestibular organs. The bird’s acoustic papilla has a dual hair cell organization (tall and short hair cells) with all the hair cells surrounded by supporting cells. Stimulated otoacoustic emissions, but not spontaneous otoacoustic emissions have been reported for the avian ear. The generation of otoacoustic emissions in nonmammalian species may result from electrically evoked movements of the stereociliar bundle (Assad, Hacohen, & Corey, 1989; Crawford & Fettiplace, 1985). Stereociliar bundle movements are low frequency events (<1 kHz) that match the otoacoustic emission frequencies measured in nonmammals. Mammalian otoacoustic emissions can occur at frequencies nearly an order of magnitude higher compelling us to look for structural features unique to the mammalian inner ear. The organ of Corti is a mammalian specialization and it was the discovery of the outer hair cell’s unique electromotile response (Brownell, 1983; Brownell, 1984; Brownell et al, 1985) that greatly strengthened the possible role of the outer hair cell in generating otoacoustic emissions.
OUTER HAIR CELL ELECTROMOTILITY
Static Mechanical Features
The electromechanical abilities of the outer hair cell have been examined in isolated cells that have been dissociated from the organ of Corti and maintained with primary tissue culture techniques. Outer hair cells have proven tolerant of the procedures and have been maintained for up to 4 days (Brownell, 1983; Brownell, 1984; Brownell et al, 1985). The static mechanical features of the isolated cell provide the first hint of the cell’s unique physiological characteristics. The healthy cell behaves as if it is a turgid rod. It resists bending and its cytoplasmic membranes resist deformation by probes. Evidence that the turgidity is related to a positive hydrostatic pressure comes from the fact that insults to the cytoplasmic membrane cause the cytoplasm to spew from the cell, often with sufficient force to eject tbe nucleus (Brownell, 1983; Brownell, 1984).
Shape Changes—Cell Length Increases or Decreases
Electrical stimulation of isolated outer hair cells generates reversible shape changes (Ashmore & Brownell, 1986; Brownell, 1983, 1984, 1986; Brownell et al, 1985; Brownell & Kachar, 1986; Evans, Dallos, & Hallworth, 1989; Holley & Ashmore, 1988a; Kachar, Brownell, Altschuler, & Fex, 1986; Santos-Sacchi, 1989; Santos-Sacchi & Dilger, 1988; Zenner, Zimmerman, & Schmitt, 1985). Length changes are the most conspicuous feature of the shape change because of the cell’s cylindrical form. The cells elongate or shorten about a “resting length.” The mechanism responsible for deforming the cell generates mechanical forces in opposite directions depending on the polarity of the electrical stimulus. While the force that is generated has not been directly measured, a single cell is able to move groups of attached cells that are many times its own mass. The ability of the outer hair cell to produce forces in opposite directions is the first of several physiological characteristics that distinguish outer hair cell electromotility from muscle cell motility. Skeletal muscle cells can generate force only when contracting. The relaxation phase of a skeletal muscle cell does not produce a force. Elongation of muscle cells is passive and generally occurs when forces produced by antagonistic muscles stretch the cell. The outer hair cell must either possess two carefully balanced mechanisms (of opposite sign) for producing its movements, or the movements are due to a single mechanism capable of producing force in either direction in response to electrical signals of opposite sign.
Voltage Dependence, Calcium Independence
The source of energy for outer hair cell electromotility appears to be the potential gradient that drives it. Partial evidence for this comes from experiments that demonstrate the movements are proportional to the applied voltage and not the current (Santos-Sacchi & Dilger, 1988). The movements occur even when most of the membrane ion channels have been blocked or if no calcium is present in the cell. The dependence on stimulus voltage and not stimulus current highlights yet another major difference between outer hair cell electromotility and conventional muscle motility.
Movements do not Require Cellular Stores of ATP
Further evidence that the movements result from a direct conversion of electrical potential energy to mechanical energy comes from experiments that demonstrate movements even after cellular stores of adenosine triphosphate (ATP) are depleted (Brownell & Kachar, 1986; Holley & Ashmore, 1988a; Kachar, Brownell, Altschuler & Fex, 1986). Muscle cells must convert cellular chemical energy stores in order to produce movements. They utilize a series of enzymatically driven biochemical steps that are triggered by an influx of calcium into the cell. The ATP independence of the outer hair cell movements is consistent with an absence of Na+, K+-ATPase in the outer hair cells (Drescher & Kerr, 1985; Kerr, Ross, & Ernst, 1982; Schulte & Adams, 1989). The absence of this enzyme is another unique feature of the outer hair cell Na+, K+-ATPase is ubiquitous in animal cells and is generally essential for life as it helps to maintain transmembrane ionic gradients in the presence of leakage currents.
The outer hair cells have a number of different potassium channels (Ashmore & Meech, 1986; Gitter, Zenner, & Fromter, 1986; Santos-Sacchi & Dilger, 1988) and a portion of these channels are open at any one time. Outer hair cells will therefore begin to lose, and not be able to replenish intracellular potassium from the moment they are removed from the organ of Corti. The transmembrane concentration gradient for potassium with decline with the passage of time. The resting membrane potential will decline with the transmembrane concentration gradient. It is not surprising that their resting membrane potentials are low when measured in vitro with micropipettes (Brownell, 1983; Brownell, 1984; Brownell et al, 1985). When “patch” electrodes are used, the potassium inside the recording pipette quickly restores a “normal” potassium level to the cell and the resting membrane potential will generally stabilize near the high values (Gitter et al, 1986; Santos-Sacchi & Dilger, 1988) that have been measured in vivo (Dallos, Santos-Sacchi & Flock, 1982; Russell & Sellick, 1983). Outer hair cells maintain high “resting” membrane potentials in the intact cochlea even though they lack Na+,K+-ATPase because their intracellular potassium ion concentration is maintained by a standing current driven by the stria vascularis. A standing concentration gradient for potassium has been measured in scala tympani (Dulguerov, Zidanic, & Brownell, 1985; Johnstone, Patuzzi, Syka, & Sykova, 1989) which indicates that a significant portion of the standing current (silent current) is carried by potassium ions. The presence of the standing current was predicted by the high potassium ion concentration and the endocochlear potential found in scala media (Brownell, 1982; Brownell et al, 1986).
The Silent Current
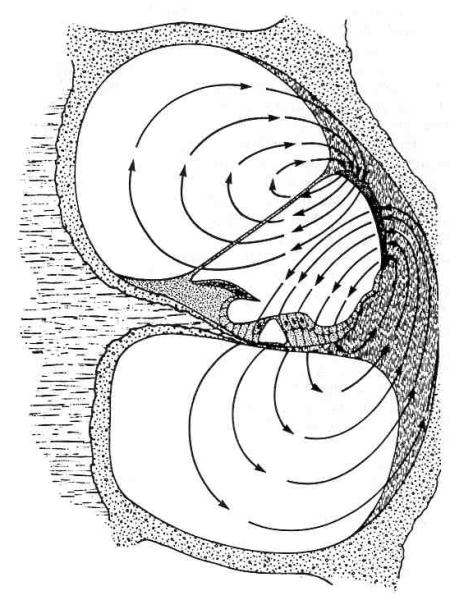
The energy for muscle cell motility comes from cellular stores of adenosine triphosphate (ATP) produced in the cell via the Krebs cycle. Outer hair cell electromotility is also fueled by the Krebs cycle but the oxidative phosphorylation occurs outside the cell in a separate organ. ATP produced in the stria vascularis is used to drive an electrogenic pump in the marginal cells of that organ. In contrast to the outer hair cells, Na+,K+-ATPase is found throughout the stria vascularis and spiral ligament. These pumps give rise to the silent current illustrated in Figure 1 (Brownell, 1982; Brownell, Manis, Zidanic, & Spirou, 1983; Brownell, Zidanic, & Spirou, 1986; Zidanic & Brownell, 1989; Zidanic & Brownell, 1990). The receptor potential in the outer hair cell results from the modulation of the silent current. Outer hair cell electromotility is thought to be driven by the receptor potential, so that ultimately, the energy for the movements comes from the stria vascularis. The energy for movements observed in vitro derives from the applied electrical signal.
Figure 1.
Schematic of the electrical environment that powers outer hair cell electromotility (from Zidanic & Brownell, 1990). The standing (or silent) current is represented in terms of current density field lines. The energy from oxidative phosphorylation in the lateral wall of scala media is used to establish an electrochemical gradient across the organ of Corti. The modulation of the silent current results in outer hair cell receptor potentials which are thought to drive rapid electromotility in vivo.
Further evidence for a division between energy utilization and energy production in the cochlea comes from deoxyglucose uptake experiments that reveal significant glucose utilization in the same structures containing the Na+,K+-ATPase, but considerably less glucose utilization in the organ of Corti (Pujol, Sans, & Calas, 1981; Ryan, Goodwin, Nigel, & Sharp, 1982; Ryan & Sharp, 1982). The division of labor between the stria vascularis and the organ of Corti permits the organ of Corti to be avascular. This provides two benefits for hearing. The first is to reduce the mass of the organ of Corti which helps to improve its sensitivity to acoustic stimulation. The second benefit is that the possibility of detecting cardiovascular sounds is reduced.
The force generating mechanism for outer hair cell electromotility is thought to be driven by the receptor potential. The receptor potential results from the modulation of the standing current that occurs when the stereocilia are bent as a result of cochlear partition displacement. The cochlear partition describes the basilar membrane together with the organ of Corti. The electromotile response is thought to provide a positive mechanical feedback that increases the movement of the cochlear partition near threshold (Geisler, 1986; Gold, 1948; Neely & Kim, 1983). The forces associated with this mechanical nonlinearity may be transmitted back to the ear canal in the form of synchronous otoacoustic emissions. High gain, positive feedback systems are inherently unstable and must be kept under control to prevent oscillations. Failure to keep the energy under control could lead to spontaneous otoacoustic emissions. Multiple rows of outer hair cells may help to stabilize the cochlear partition as random mechanical movements that might trigger oscillations with one row would have to be considerably larger to produce the same result with multiple rows. The three rows typically found in the organ of Corti may represent a balance between increasing the number of rows for stability and the deleterious effect of increasing the mass of the cochlear partition.
Frequency Response
Outer hair cells can move at rates no muscle cell is capable of. They have been monitored following electrical stimulation well into the audio frequency range (Ashmore & Brownell, 1986; Evans, 1988; Evans et al, 1989; Zenner, Zimmerman, & Gitter, 1987). The magnitude of the movements are greatest for low frequencies and decrease with frequency. Some concern has been expressed over the fact that the magnitude of the electrically evoked OHC length changes measured in vitro does not approach the magnitude of threshold cochlear partition movements until the stimulus voltage reaches a value in excess of receptor potentials measured in vivo (Santos-Sacchi, 1989). The same concern calls into question the role of the outer hair cells in the generation of otoacoustic emissions particularly when some stimulated otoacoustic emissions have been measured in response to acoustic stimulation well below the threshold for hearing (Wilson, 1979; Zwicker & Manley, 1981). The movements of isolated outer hair cells are governed by the mechanical properties of the outer hair cell and the electromotile force-generating mechanism (Steele & Jen, 1988). The transfer af force and the resulting movements of the cochlear partition will be determined by its mechanical properties. The mechanical properties of the cochlear partition are different than, and encompass the mechanical properties of, the isolated outer hair cell. It is possible that the outer hair cell undergoes no length change in vivo and that isometric changes in hair cell force are transmitted directly to the cochlear partition. The important feature of the rapid outer hair cell electromotility in isolated cells is that the force generating mechanism can operate at high frequencies.
Asymmetries in Movement
Hyperpolarizing pulses injected into the cell cause the free end (or ends) of the cell to elongate while depolarization results in a shortening (Brownell, 1983; Brownell, 1984; Brownell et al, 1985; Santos-Sacchi, 1989; Santos-Sacchi & Dilger, 1988). Extracellular stimulation follows the same basic rules in that the free ends of the cell move in the same direction as the transcellularly applied potential gradient (Brownell, 1984; Brownell et al, 1985; Brownell & Kachar, 1986; Evans, 1990; Evans et al, 1989; Kachar et al, 1986). Transcellularly applied alternating potential gradients have produced symmetric displacements at low frequencies (Brownell & Kachar, 1986; Kachar, Brownell, Altschuler, & Fex, 1986) and nonlinear “DC” responses at high frequencies (Brownell, 1983; Brownell, 1984, Brownell et al, 1985). Recent investigations of response asymmetries (Evans, 1988; Evans, 1990; Evans et al, 1989; Santos-Sacchi, 1989) confirm that the outer hair cell can produce nonlinear asymmetric displacements. The amount of mechanical rectification seems to be governed by the holding potential (Santos-Sacchi, 1989). There is a range of membrane potentials around which the mechanical response is linear, but when held near −70 mV elongation saturates and shortening dominates. If the membrane potential of transcellularly stimulated cells is considerably less than −70 mV, the mechanical response is likely to be in the linear range. Since the in vivo “resting” potential of outer hair cells appears to be around −70 mV (Dallos et al, 1982; Russell & Sellick, 1983), it is likely that the nonlinear response asymmetries are presented in the living cochlea. Response nonlinearities are necessary in order to account for certain types of evoked otoacoustic emission such as distortion products.
Rapid “DC” displacements of the basilar membrane have been measured (LePage, 1987) in response to tone bursts between 55 and 75 dB SPL. The “DC” displacements may reflect an interaction between the nonlinear nature of outer hair cell electromotility and the inherent rectification present in the receptor potential generator mechanism (Dallos et al, 1982; Russell & Sellick, 1983). Cumulative displacement shifts of the basilar membrane in response to repeated tone bursts have also been measured (LePage, 1987). These may reflect longer time constant mechanical events that could represent an interaction between volume control mechanisms in the outer hair cell and the architectonics of the organ of Corti.
THE ARCHITECTONICS OF THE ORGAN OF CORTI
Outer hair cells are found in the precisely organized cellular matrix of the organ of Corti. The cells in most organs (e.g., liver, heart, skin, retina, maculae, and cristae of the vestibular system) are tightly packed with only 20 to 40 nm separating one cell from the next (Traynelis & Dingledine, 1989). The organ of Corti, in contrast, contains large fluid-filled spaces (see Fig. 2). The spaces of Nuel around the outer hair cells provide up to 1000 nm separation between cells and impart the striking colonnade appearance seen in scanning electron micrographs. This organization allows outer hair cell length changes to occur without having to work against the mechanical restrictions imposed by closely packed neighboring cells. Loose attachments with adjacent cells would impede the movement of the lateral cytoplasmic membrane during length changes. The free space can also accommodate diameter changes associated with outer hair cell length changes.
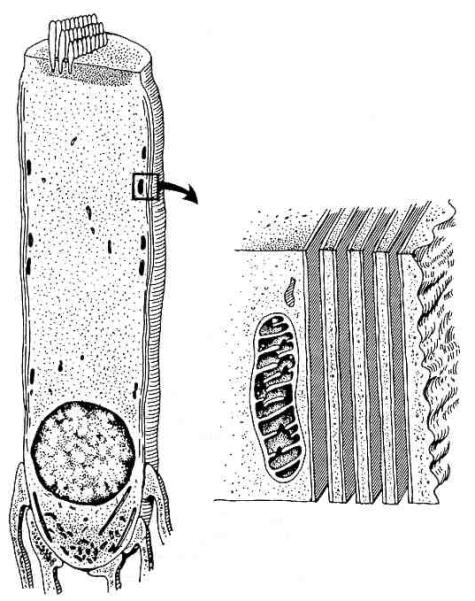
Figure 2.
Drawing of a portion of a single row of outer hair cells and associated Deiter’s cells as viewed from the otic capsule looking toward the modiolus. The apical or high frequency end of the cochlea is to the right, lower frequencies are to the left. The three longitudinal domains of the organ of Corti are from top to bottom: (1) the reticular lamina, (2) the fluid space between the reticular lamina, and (3) the Deiter’s cell body layer. The reticular lamina is composed of the apical ends of the hair cells together with the ends of the Deiter’s cells phalangeal processes that interdigitate between them. The Deiter’s cells sit on the basilar membrane (represented by the row of small circles as its constituent fibers project from the plane of the figure).
The fluid space around outer hair cells is bordered by the reticular lamina apically and the cell bodies of Deiter’s cells basally. The reticular lamina is formed by the apical ends of the hair cells and mechanically rigid extensions of the Deiter’s cells that interdigitate between the hair cells (Fig. 2). Extremely well developed, tight junctional complexes join the cells where they make contact. The tight junctions contribute toward making the reticular lamina rigid and prevent the mixing of fluids on either side of the reticular lamina. The outer hair cell’s apical end is firmly anchored in the reticular lamina while its basal end makes contact with afferent and efferent nerve terminals. The basal end with its attached neural processes rests in a cuplike indentation of a Deiter’s cell. The structural integrity of the layer of Deiter’s cell bodies is provided by their cytoskeletal elements, the close packing of the cells, and the basilar membrane on which they sit.
The outer hair cells and the thin processes of the Deiter’s cells bridge the distance across the fluid space in the organ of Corti. The Deiter’s cell process contains a cytoskeletal core that extends from the reticular lamina through the cell body to the basal end of the cell where it rests on basilar membrane. Mechanical probing of the Deiter’s cell process reveals that it has little if any compressive strength (WE Brownell, personal observations). Even if it possessed compressive rigidity, the fact that it angles toward the basal end of the cochlear spiral and bends freely about the point of attachment with the Deiter’s cell body means that it alone could not prevent the reticular lamina coming closer to the basilar membrane. The separation between the reticular lamina and the Deiter’s cell bodies must, therefore, be maintained by outer hair cells. The need for outer hair cells to maintain the separation can be appreciated by imagining the vibrations of the cochlear partition during acoustic stimulation. Since it is crucial that the movements be transmitted to the stereocilia anchored in the reticular lamina, it is understandable as to why the outer hair cells must have sufficient rigidity to withstand the longitudinal compressive forces associated with such movements. The compressive forces of the outer hair cell acting against the tensile properties of the Deiter’s cell processes will contribute to the overall compliance of that portion of the cochlear partition.
THE INTRACELLULAR DOMAINS OF THE OUTER HAIR CELL
The shape and static mechanical properties of most animal cells are determined by a network of long chain molecules that are collectively called a cytoskeleton (Alberts et al, 1983). It can be argued on theoretical grounds that the presence of a cytoskeleton would be an impediment to rapid movement. This argument gains support from the fact that there is often an absence or depolymerization of the cytoskeleton in cells or portions of the cell undergoing rapid movements. For instance, immediately before the rapid phase of mitosis the cytoskeleton of the dividing cell depolymerizes and repolymerizes after telophase (Alberts et al, 1983). The very ability of the outer hair cell to change its length requires that it be mechanically flexible. Yet, the structural integrity of the organ of Corti requires that the cell possess considerable compressive rigidity along its major axis. Mammalian hearing imposes on the outer hair cell what seem to be conflicting requirements of mechanical stiffness while at the same time being able to move at acoustic frequencies. By segregating the cytoskeletal structures at the base and the apex of the cell, the outer hair cell leaves the region between the nucleus and the cuticular plate free to move in response to the force generator for the electromotile response.
Ultrastructural and histochemical studies reveal organelles and a structural organization that distinguish the outer hair cell not only from other hair cells but from any other cell so far described. It has a distinctive cylindrical shape with an eccentrically placed nucleus (Fig. 3). But is is in the distribution of its cytoskeletal molecules that it displays a dramatic difference with other animal cells. The stereocilia and cuticular plate are densely packed with actin and other long chain structural molecules. Intermediate filaments and microtubules can be found in the infranuclear region as well. The same studies that demonstrate the presence of structural proteins at the two poles of the cell reveal the axial cytoplasm between the cuticular plate and the nucleus to be remarkably free of cytoskeletal elements (Flock, 1988; Flock, Bretscher, & Weber, 1982; Flock, Flock, & Ulfendahl, 1986; Hackney & Furness, 1989; Slepecky & Chamberlain, 1985; Zenner, 1980). It is the portion of the cell between the nucleus and the cuticular plate that undergoes deformation in response to electrical stimuli. It is unlikely an accident of nature that no significant cytoskeletal organization can be found here.
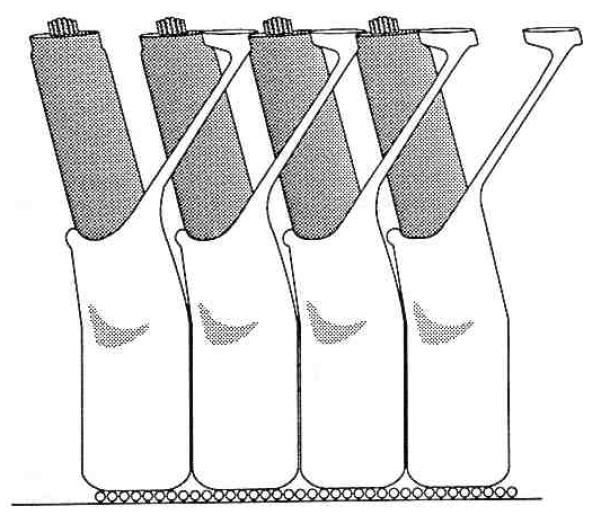
Figure 3.
Outer hair cell morphology. The general organization of the outer hair cell is shown on the left and a blowup of the cell’s cortex is portrayed on the right. The smooth unbroken membranes of the laminated cisternal system are based on the recent description of Evans (1990). Filamentous structures (not portrayed) that may contain actin (Flock, 1988) can be found in the space between the lateral cytoplasmic membrane and the outermost subsurface cistern (see Fig. 4). Stereociliar actin filaments are embedded in the cuticular plate at the top of the cell. Cytoskeletal elements are also found in the intranuclear or synaptic region but not in the central cytoplasmic core of the cell between the cuticular plate and the nucleus.
HYDRAULIC SUPPORT IN THE OUTER HAIR CELL
Nature arrived at a novel solution to the mechanical requirements imposed on the outer hair cell by replacing the cytoskeleton with an hydraulic skeleton. The outer hair cell’s subplasma lamina (Bannister, Dodson, Astbury, & Douek, 1988; Lim, Hanamure, & Ohashi, 1989) immediately below the lateral plasma membrane provides a flexible elastic cortex that is stabilized by the positive hydrostatic pressure of the cell’s cytoplasm. The formation of a “hydroskeleton” is a structural solution to the problem of how to achieve stiffness with flexible, tensile elements. A hydrostatic supportive system consists of a fluid under pressure in the container wherein the fluid acts as the compression-resisting component and the container alone resists tension. Wainwright (1970) has summarized the functional morphology of hydraulic support systems in animals. The six features that he discusses are easily recognized features of the outer hair cell’s morphology (see Fig. 4).
Figure 4.
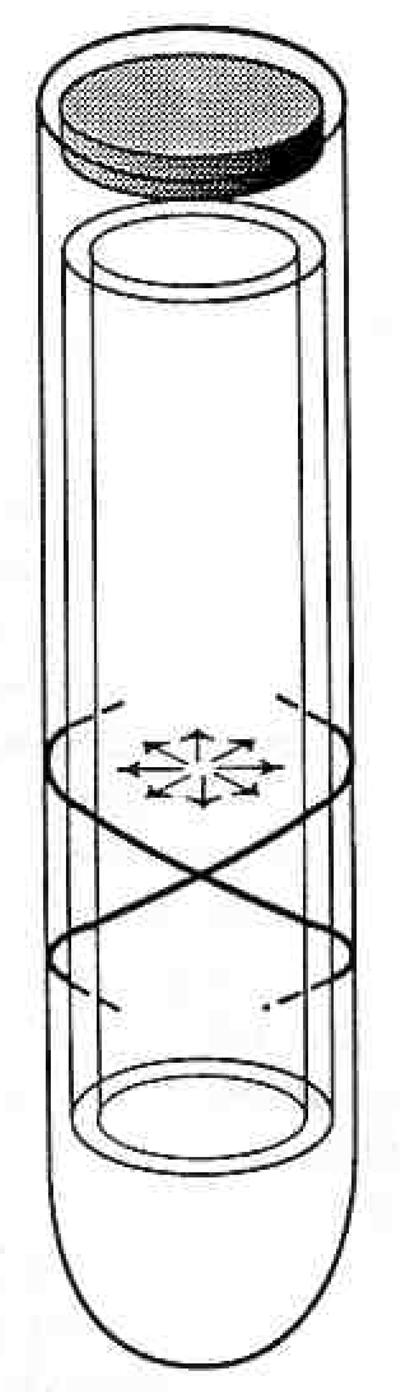
Schematic of outer hair cell as a pressure vessel. “Circumferential” tensile elements are indicated by oppositely oriented helices in the lateral walls of the cell (Bannister et al, 1988; Flock, 1988; Holley & Ashmore, 1986; Lim et al, 1989). The radially oriented arrows indicate the cytoplasm’s positive hydrostatic pressure. The outer hair cell’s turgor pressure is most likely based on a slightly hyperosmotic cytoplasm that “inflates” the cell.
The outer hair cell has tension and compression resisting elements. The subplasma lamina (Bannister et al, 1988; Flock, 1988; Lim et al, 1989) together with the laminated cisternal system represent the tension resisting elements. The cell’s cytoplasm is the compression resisting element.
The compression component (the cytoplasm) is the most volumous component in the cell and it extends the length of the cell. The conventional cytoskeletal elements of the outer hair cell are segregated into specific intracellular domains in the base and apex of the cell and do not extend the length of the cell. This contrasts with the cytoskeletal elements that serve as compression units in other cells [e.g., the Deiter’s and pillar cells (Hackney & Furness, 1989; Slepecky & Chamberlain, 1983)].
A cornpressive resisting filament free cytoplasm is located at the outer hair cell’s central core. An additional structure is found centrally only in the guinea pig outer hair cells and only in cells from the apical region of the cochlea (Carlisle et al, 1989). These cells have actin containing filaments in their central core that span the distance between the cuticular plate and the nucleus. The organization of these filaments does not favor a compression resisting role.
The circumferentially oriented (Bannister et al, 1988; Holly & Ashmore, 1988b; Lim et al, 1989) tension resisting component (Fig. 4) extends from the base to the apex in a specialized area of the cell cortex. Additional tension resisting material is found in the cuticular plate-stereociliar complex and infranuclear synaptic region.
The tensile material is peripheral.
The outer hair cell undergoes 3-dimensional changes in shape. The organization of the subplasma lamina and laminated cisternal system (Bannister et al, 1988; Flock, 1988; Holley & Ashmore, 1988b; Lim et al, 1989) have a crossed-helical arrangement of fibrils that allows for flexibility and strength. There is even evidence for the presence of polysaccharide moieties in the cortical structures of the outer hair cell (Gil-Loyzaga & Brownell, 1988). Animal structures with high tensile strength are made up of high molecular weight protein and polysaccharide moieties whose strongly anisodiametric molecules impart strength along their major axis (Wainwright, 1970).
One consequence of a hydraulic support system based on cylinders is that as the cylinder becomes longer the hydrostatic pressures required to resist longitudinal compressive forces must become larger. This is because of the greater tendency for long structures to shear (bend) while shorter, proportionately wider, structures do not. When the tendency to shear is considered, the effective compressive strength of a cylinder is inversely related to its length. Long, cylindrical, pressure vessels must be stabilized by larger hydrostatic pressures in order to resist the shear that might result from longitudinal compressive forces. Greater hydrostatic pressure in the longer, more apical, outer hair cells may begin to exceed the ability of the “circumferentially” oriented tensile elements in the lateral wall to withstand the longitudinal component of the turgor pressure. Additional tensile elements may be required to withstand the longitudinal stress. The central, actin containing, core described in the longer outer hair cells found closer to the apex of the guinea pig cochlea (Carlisle et al, 1989) may provide the requisite longitudinal tensile strength to resist the increased turgor pressure.
ELECTROMOTILITY AND CELL TURGOR
Cell turgor is required in order that the pressure gradients responsible for the electromotile response can be communicated to the ends of the cell (Brownell & Winston, 1989). Some mechanism must render the outer hair cell’s cytoplasm slightly hyperosmotic (Brownell, Imredy, & Shehata, 1989a; Brownell, Shehata, & Imredy, 1989b). This mechanism may be related to the unusually high concentration of glycogen in its cytoplasm (Duval & Hukee, 1976; Thalmann, 1975; Thalmann, 1972; Thalmann, Thalmann, & Comegys, 1972). There is a uniform increase in outer hair cell glycogen concentration from the base to the apex of the cochlea. If glycogen contributes to the osmotic pressure of the cytoplasm then outer hair cells near the apex may have a higher turgor pressure.
The outer hair cell can be viewed as a pressure vessel (Fig. 4) containing a mechanism for generating pressure gradients at acoustic frequencies. Evidence for the existence of pressure gradients within the axial cytoplasm comes from direct observation of the movement of cellular organelles (Brownell & Kachar, 1986; Holley & Ashmore, 1988a; Kachar, Brownell, Altschuler, & Fex, 1986) in this region. Their movement can only result from pressure gradients particularly in the absence of cytoskeletal elements that might communicate movement directly (Fig. 5). The hydrostatic pressure of the outer hair cell makes it a mechanical unit. Changes in the pressure most likely provide the force needed for locomotion. Evidence for the importance of cell turgor for electromotility and otoacoustic emissions comes from manipulations that change hydrostatic pressure of the ouler hair cell’s cytoplasm.
Figure 5.
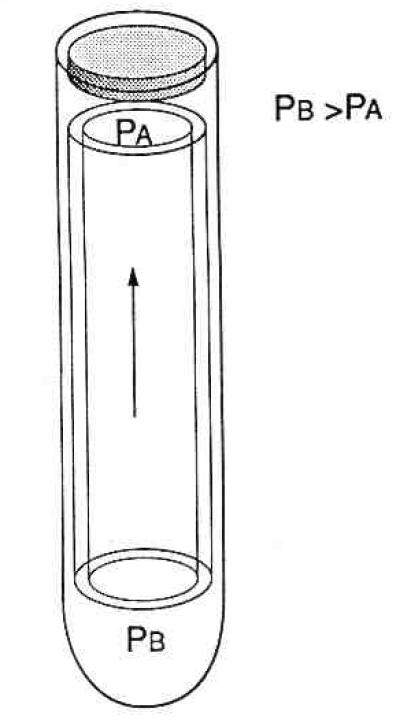
Pressure gradients in the axial portion of the cytoplasm are required to explain the movement of cytoplasm associated with rapid electromotility. Pressure effects could be transmitted to the cochlear partition even if the cell is isometrically constrained in situ.
SLOW CHANGES IN OUTER HAIR CELL VOLUME AND TURGOR
Volume Increases
Goldstein and Mizukoshi (1967) were the first to isolate outer hair cells and demonstrate their ability to change shape. They replaced the bathing media, which resembled perilymph, with a high potassium “artificial endolymph” and described the resulting shape change as an increase in cell volume that resembled the increase observed on reducing the osmolarity of the bathing media by dilution with distilled water. Goldstein and Mizukoshi’s observations have been confirmed by a number of laboratories which also demonstrated the volume increase to be reversible (Brownell et al, 1989a; Brownell el al, 1989b; Dulon, Aran, & Schacht, 1987; Flock et al, 1986; Ulfendahl, 1988; Ulfendahl & Slepecky, 1988; Zenner, 1986; Zenner, Wolfgang, & Gitter, 1988; Zenner et al, 1985). These chemically induced shape changes follow a time course that is considerably longer than that of rapid electromotility and for this reason they are thought to represent a different type of motile process. Several laboratories have speculated that they result from conventional contractile events triggered by the depolarization of the membrane potential that occurs when the bathing media (with its low potassium concentration) is replaced by the high potassium media. We have examined reversible volume increases stimulated by low ionic strength sugar solutions (Brownell et al, 1989a; Brownell et al, 1989b). The magnitude and time course of the sugar induced shape changes are virtually indistinguishable from those elicited by high extracellular potassium. Morphometric analysis reveals that both the sugar and potassium manipulations lead to increased cell volume with no change in the cell surface area. The low ionic strength sugar solutions hyperpolarize the cells (Brownell et al, 1989b) demonstrating that the slow volume increase need not result from membrane depolarization. The length changes associated with all forms of outer hair cell volume increase is most likely a passive event associated with the solid geometry of cylinders. The only way a cylinder can increase its volume without increasing its surface area is to move toward a spherical shape, so the cylinder becomes shorter and fatter.
The volume increase is most likely the result of an increase in the hydrostatic pressure of the cytoplasm. An increase in the concentration of a solute in the extracellular media leads to an increase in the intracellular concentration of that substance, if the cell’s cytoplasmic membrane is permeable to that substance. Unless another substance exits the cell, the cytoplasmic osmolarity will increase and with it the hydrostatic pressure of the cytoplasm. The circumferential tensile strength of the outer hair cell’s lateral conex restores the cell to its elongated shape as the increased hydrostatic pressure that causes the volume increase is reduced on restoring the cell to its normal bathing media. While high potassium environments often lead to volume increases in animal cells with conventional cytoskeletons, osmotically balanced sugar solutions do not. These observations suggest the outer hair cell may possess a unique mechanism for cell volume control that involves permeability changes to nonelectrolytes. The possibility that the outer hair cell membrane can be made permeable to relatively large molecules may explain their non vesicular uptake of exogenous horseradish peroxidase (Leake-Jones & Snyder, 1987; Siegel & Brownell, 1986).
Volume Decreases
Pharmacological and electrical manipulations can also result in the outer hair cell losing volume. Slow electrically induced changes may be observed with voltage clamp manipulations using tight-seal, whole-cell electrodes. Sustained depolarization leads to a loss of fluid and, once depleted, sustained hyperpolarization leads to an increase of fluid as the cell regains its original shape (Brownell et al, 1989a; Brownell et al, 1989b; Brownell & Winston, 1989). The cells crenulate and flatten as they lose turgor. Rapid movements, evoked by voltage pulses, eventually disappear as the cells lose their turgor during sustained depolarization but return after moving the holding potential to hyperpolarizing values. The dependence of the rapid movements on a normal cytoplasmic pressure is consistent with a mechanism that requires hydraulic transmission of cytoplasmic pressure changes.
Extracellular and intracellular application or salycilates (the active ingredient in aspirin) interferes with the maintenance of outer hair cell turgidity (Brownell et al, 1989a; Brownell et al, 1989b). The cells crenulate and flatten in the same way they do when losing volume with sustained depolarization in voltage clamp. Sustained hyperpolarization is often not able to return the cells to their turgid appearance. This may be associated with the fact that many cells also show an increase in their membrane conductance during aspirin toxicity. Ototoxic doses of aspirin are known to block otoacoustic emissions (Long & Tubis, 1988; Mcfadden & Plattsmier, 1984). The fact that aspirin blocks both outer hair cell electromotility and otoacoustic emissions is one of the strongest arguments for the role of the outer hair cell in the generation or otoacoustic emissions.
SUMMARY
Analyzing the architectonics of the organ of Corti in light of new knowledge about both the static and dynamic mechanics of the outer hair cell suggest that we should no longer view the outer hair cell as being mechanically supported by Deiter’s cells. An integrative view of the structure suggests the compressive strength of the outer hair cell works with the tensile strength of the Deiter’s cell process to stabilize and integrate the structure. The outer hair cell supports the Deiter’s cell as much as the Deiter’s cell supports the outer hair cell. The organ of Corti contains the structural features of the geodesic dome in which short rods with compressive strength are integrated with cables possessing tensile strength. Such structures have tensile integrity or tensegrity (Buckminster Fuller, 1975; Roland & Frei, 1965). Structures organized around such interactions are low in mass and can be easily deformed without being damaged.
The measurement of otoacoustic emissions followed by the demonstration of outer hair cell electromotility occurred at a pivotal moment for the evolution of our understanding of the inner ear. The ability of a source of acoustic energy in the organ of Corti to explain a variety of experimental observations on cochlear mechanics, including the generation of otoacoustic emissions, helped to establish a role for bidirectional transduction in the cochlea and permitted the hearing science comunity to embrace the motor capabilities of the inner ear.
Acknowledgments
The editorial review of Drs. D. K. Ryugo, W. E. Shehata, E. D. Young, and M. Zidanic is greatly appreciated.
Footnotes
Work supported by Office of Naval Research, task 441k704.
References
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. Garland; New York: 1983. [Google Scholar]
- Ashmore JF, Brownell WE. Kilohertz movements induced by electrical stimulation in outer hair cells isolated from the guinea pig cochlea. J Physiol. 1986;377:41P. [Google Scholar]
- Ashmore JF, Meech RW. Ionic basis of membrane potential in outer hair cells of guinea pig cochlea. Nature. 1986;322:368–371. doi: 10.1038/322368a0. [DOI] [PubMed] [Google Scholar]
- Assad JA, Hacohen N, Corey DP. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci USA. 1989;86:2918–2922. doi: 10.1073/pnas.86.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister LH, Dodson HC, Astbury AF, Douek EE. The cortical lattice: a highly order system of subsurface filaments in guinea pig cochlear outer hair cells. Prog Brain Res. 1988;74:213–219. doi: 10.1016/s0079-6123(08)63016-2. [DOI] [PubMed] [Google Scholar]
- Békésy G. Experiments in Hearing. McGraw-Hill; New York: 1960. [Google Scholar]
- Brown MC, Nuttal AL. Efferent control of cochlear inner hair cell responses in the guinea pig. J Physiol. 1984;354:625–646. doi: 10.1113/jphysiol.1984.sp015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Nuttall AL, Masta RI. Intracellular recordings from cochlear inner hair cells: effects of stimulation of the crossed olivocochlear efferents. Science. 1983;222:69–72. doi: 10.1126/science.6623058. [DOI] [PubMed] [Google Scholar]
- Brownell WE. Cochlear transduction: An integrative model and review. Hear Res. 1982;6:335–360. doi: 10.1016/0378-5955(82)90064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE. Observations on a motile response in isolated outer hair cells. In: Webster WR, Aitken LM, editors. Mechanisms of Hearing. Monash University Press; 1983. pp. 5–10. [Google Scholar]
- Brownell WE. Microscopic observation of cochlear hair cell motility. Scanning Electron Microsc. 1984;III:1401–1406. [PubMed] [Google Scholar]
- Brownell WE. Outer hair cell motility and cochlear frequency selectivity. In: Moore BCJ, Patterson RD, editors. Auditory Frequency Selectivity. Plenum Press; New York: 1986. pp. 109–120. [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Brownell WE, Imredy JP, Shehata W. Stimulated volume changes in mammalian outer hair cells. Proc Ann Int Conf IEEE-Eng Med Biol Soc. 1989a;11:1344–1345. [Google Scholar]
- Brownell WE, Kachar B. Outer hair cell motility: A possible electro-kinetic mechanism. In: Allen JB, Hall JL, Hubbard AE, Neely ST, Tubis A, editors. Peripheral Auditory Mechanisms. Springer-Verlag; New York: 1986. pp. 369–376. [Google Scholar]
- Brownell WE, Manis PB, Zidanic M, Spirou GA. Acoustically evoked radial current densities in scala tympani. J Acoust Soc Am. 1983;74:792–800. doi: 10.1121/1.389866. [DOI] [PubMed] [Google Scholar]
- Brownell WE, Shehata W, Imredy JP. Slow electrically and chemically evoked volume changes in guinea pig outer hair cells. In: Akkas N, editor. Biomechanics of Active Movement and Deformation of Cells. Springer-Verlag; New York: 1989b. pp. 493–498. [Google Scholar]
- Brownell WE, Winston JB. Slow electrically evoked volume changes in guinea pig outer hair cells. Abstracts of the Midwinter Research Meeting of the Association for Research in Otolaryngology. 1989;12:138–139. [Google Scholar]
- Brownell WE, Zidanic M, Spirou GA. Standing currents and their modulation in the cochlea. In: Altschuler R, Hoffman D, Bobbin R, editors. Neurobiology of Hearing: The Cochlea. Raven Press; New York: 1986. pp. 91–107. [Google Scholar]
- Buckminster Fuller R. Synergetics. MacMillan; New York: 1975. [Google Scholar]
- Carlisle L, Thorne PR, Zajic G, Altschuler RA, Schacht J. A comparative study of actin filaments in cochlear hair cells: outer hair cells in the apex of guinea pig cochlea contain a unique ultrastructural feature. In: Wilson JP, Kemp DT, editors. Cochlear Mechanisms Structure, Function and Models. Plenum Press; New York: 1989. pp. 21–28. [Google Scholar]
- Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol (Lond) 1985;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Santos-Sacchi J, Flock Å. Intracellular recordings from cochlear outer hair cells. Science. 1982;218:582–584. doi: 10.1126/science.7123260. [DOI] [PubMed] [Google Scholar]
- Drescher DG, Kerr TP. Na+,K+-activated adenosine triphosphatase and carbonic anhydrase: Inner ear enzymes of ion transport. In: Dresher DG, editor. Auditory Biochemistry. Charles C Thomas; Springfield: 1985. pp. 436–472. [Google Scholar]
- Dulguerov P, Zidanic M, Brownell WE. Potassium gradients in scala tympani of the guinea pig in the absence of sound stimulation. Soc. Neurosci. Abst. 1985;15:245. [Google Scholar]
- Dulon D, Aran J-M, Schacht J. Osmotically induced motility of outer hair cells: implications for Meniere’s, disease. Arch Otorhinolaryngol. 1987;244:104–107. doi: 10.1007/BF00458558. [DOI] [PubMed] [Google Scholar]
- Duval AJ, III, Hukee MJ. Delineation of cochlear glycogen by electron microscopy. Ann Otol Rhinol Laryngol. 1976;85:234–246. doi: 10.1177/000348947608500208. [DOI] [PubMed] [Google Scholar]
- Evans BN. Asymmetries in outer hair cell electro-mechanical responses. Abstracts of the Midwinter Meeting of the Association for Research in Otolaryngology. 1988;11:29. [Google Scholar]
- Evans BN. In vitro correlates of outer hair cell vulnerability: Electro-mechanical and ultrastructural observations. Hear Res. 1990 (in press) [Google Scholar]
- Evans B, Dallos P, Hallworth R. Asymmetries in motile responses of outer hair cells in simulated in vivo conditions. In: Wilson JP, Kemp DT, editors. Cochlear Mechanisms Structure Function and Models. Plenum Press; New York: 1989. pp. 205–206. [Google Scholar]
- Flock Å. Do sensory cells in the ear have a motile function? Prog Brain Res. 1988;74:297–304. doi: 10.1016/s0079-6123(08)63028-9. [DOI] [PubMed] [Google Scholar]
- Flock Å, Bretscher A, Weber K. Immunohistochemical localization of several cytoskeletal proteins in inner ear sensory and supporting cells. Hear Res. 1982;6:75–89. doi: 10.1016/0378-5955(82)90082-x. [DOI] [PubMed] [Google Scholar]
- Flock Å, Flock B, Ulfendahl M. Mechanisms of movement in outer hair cells and a possible structural basis. Arch Otorhinolaryngol. 1986;243:83–90. doi: 10.1007/BF00453755. [DOI] [PubMed] [Google Scholar]
- Geisler CD. A model of the effect of outer hair cell motility on cochlear vibrations. Hear Res. 1986;24:125–132. doi: 10.1016/0378-5955(86)90056-0. [DOI] [PubMed] [Google Scholar]
- Gil-Loyzaga PE, Brownell WE. Wheat germ agglutinin and Helix Pomatia agglutinin lectin binding on cochlear hair cells. Hear Res. 1988;34:149–156. doi: 10.1016/0378-5955(88)90102-5. [DOI] [PubMed] [Google Scholar]
- Gitter AH, Zenner HP, Fromter E. Membrane potential and ion channels in isolated outer hair cells of guinea pig cochlea. ORL. 1986;48:68–75. doi: 10.1159/000275848. [DOI] [PubMed] [Google Scholar]
- Gold T. The physical basis of the action of the cochlea. Proc R Soc Lond B Biol Sci. 1948;135:492–498. [Google Scholar]
- Goldstein AJ, Mizukoshi O. Separation of the organ of Corti into its component cells. Ann Otol Rhinol Laryngol. 1967;76:414–426. doi: 10.1177/000348946707600210. [DOI] [PubMed] [Google Scholar]
- Hackney CM, Furness DN. Observations on the cytoskeleton and related structures of mammalian cochlear hair cells. In: Wilson JP, Kemp DT, editors. Cochlear Mechanisms Structure, Function and Models. Plenum Press; New York: 1989. pp. 11–20. [Google Scholar]
- Holley MD, Ashmore JF. On the mechanism of a high-frequency force generator in outer hair cells isolated from the guinea pig cochlea. Proc R Soc Lond B Biol Sci. 1988a;232:413–429. doi: 10.1098/rspb.1988.0004. [DOI] [PubMed] [Google Scholar]
- Holley MD, Ashmore JF. A cytoskeletal spring in cochlear outer hair cells. Nature. 1988b;335:635–637. doi: 10.1038/335635a0. [DOI] [PubMed] [Google Scholar]
- Jen DH, Steele CR. Electrokinetic model of cochlear hair cell motility. J Acoust Soc Am. 1987;82:1667–1678. doi: 10.1121/1.395158. [DOI] [PubMed] [Google Scholar]
- Johnstone BM, Patuzzi RB, Syka J, Sykova E. Stimulus-related potassium changes in the organ of Corti of guinea-pig. J Physiol (Lond) 1989;408:77–92. doi: 10.1113/jphysiol.1989.sp017448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B, Brownell WE, Altschuler RA, Fex J. Electro-kinetic shape changes of cochlear outer hair cells. Nature. 1986;322:365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Kerr TP, Ross MD, Ernst SA. Cellular localization of Na+,K+-ATPase in the mammalian cochlear duct: Significance for cochlear fluid balance. Am J Otolaryngol. 1982;3:332–338. doi: 10.1016/s0196-0709(82)80006-9. [DOI] [PubMed] [Google Scholar]
- Khanna SM, Leonard DGB. Basilar membrane tuning in the cat cochlea. Science. 1982;215:305–306. doi: 10.1126/science.7053580. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Watanabe T, Thomas EC, Clark LF. Discharge Patterns of Single Fibers in the Cat’s Auditory Nerve. MIT Press; Cambridge: 1965. [Google Scholar]
- Klinke R, Smolder J. Hearing mechanisms in caiman and pigeon. In: Bolis L, Keynes R, Maddrell S, editors. Comparative Physiology of Sensory Systems. Cambridge University Press; 1984. [Google Scholar]
- Leake-Jones P, Snyder R. Uptake of horseradish peroxidase from perilymph by cochlear hair cells. Hear Res. 1987;25:153–171. doi: 10.1016/0378-5955(87)90088-8. [DOI] [PubMed] [Google Scholar]
- LePage EL. Frequency-dependent self-induced bias of the basilar membrane and its potential for controlling sensitivity and tuning in the mammalian cochlea. J Acoust Soc Am. 1987;82:139–154. doi: 10.1121/1.395557. [DOI] [PubMed] [Google Scholar]
- Lim DJ, Hanamure Y, Ohashi Y. Structural organization of the mammalian auditory hair cells in relation to micromechanics. In: Wilson JP, Kemp DT, editors. Cochlear Mechanisms Structure, Function and Models. Plenum Press; New York: 1989. pp. 3–10. [Google Scholar]
- Long GR, Tubis A. Modification of spontaneous and evoked otoacoustic emissions and associated psychoacoustic microstructure by aspirin consumption. J Acoust Soc Am. 1988;84:1343–1353. doi: 10.1121/1.396633. [DOI] [PubMed] [Google Scholar]
- Manley GA, Schulze M, Oeckinghaus H. Otoacoustic emissions in a song bird. Hear Res. 1987;26:257–266. doi: 10.1016/0378-5955(87)90062-1. [DOI] [PubMed] [Google Scholar]
- McFadden D, Plattsmier HS. Aspirin abolishes spontaneous oto-acoustic emissions. J Acoust Soc Am. 1984;76:443–448. doi: 10.1121/1.391585. [DOI] [PubMed] [Google Scholar]
- Mountain DC. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science. 1980;210:71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Mountain DC. Active filtering by hair cells. In: Allen JB, Hall JL, Hubbard AE, Neely ST, Tubis A, editors. Peripheral Auditory Mechanisms. Springer-Verlag; New York: 1986. [Google Scholar]
- Neely ST, Kim DO. An active cochlear model showing sharp tuning and high sensitivity. Hear Res. 1983;9:123–130. doi: 10.1016/0378-5955(83)90022-9. [DOI] [PubMed] [Google Scholar]
- Palmer AR, Wilson JP. Spontaneous and evoked acoustic emissions in the frog Rana esculenta. J Physiol. 1981;324:66P. [Google Scholar]
- Pujol R, Sans A, Calas A. High resolution radioautographic study of the inner ear following in vivo tritiated deoxyglucose administration. Eur Neurol. 1981;20:157–161. doi: 10.1159/000115225. [DOI] [PubMed] [Google Scholar]
- Rhode WS. Observations on the vibration of the basilar membrane in squirrel monkeys using the Mössbauer technique. J Acoust Soc Am. 1971;49:1218–1231. doi: 10.1121/1.1912485. [DOI] [PubMed] [Google Scholar]
- Rhode WS. Some observations on cochlear mechanics. J Acoust Soc Am. 1978;64:158–176. doi: 10.1121/1.381981. [DOI] [PubMed] [Google Scholar]
- Robles L, Ruggero MA, Rich NC. Basilar membrane mechanics at the base of the chinchilla cochlea. I. Input-output functions, turning curves, and phase responses. J Acoust Soc Am. 1986;80:1364–1374. doi: 10.1121/1.394389. [DOI] [PubMed] [Google Scholar]
- Roland C. Frei Otto: Tension Structures. Praeger; New York: 1965. [Google Scholar]
- Russell IJ, Sellick PM. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ, Sellick PM. Low frequency characteristics of intracellularly recorded receptor potentials in mammalian hair cells. J Physiol. 1983;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AF, Goodwin P, Nigel KW, Sharp F. Auditory stimulation alters the pattern of 2-deoxyglucose uptake in the inner ear. Brain Res. 1982;234:213–225. doi: 10.1016/0006-8993(82)90863-0. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Sharp FR. Localization of (3H)2-deoxyglucose at the cellular level using freeze-dried tissue and dry-looped emulsion. Brain Res. 1982;252:177–180. doi: 10.1016/0006-8993(82)90994-5. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Asymmetry in voltage-dependent movements of isolated outer hair cells from the organ of Corti. J Neurosci. 1989;9:2954–2962. doi: 10.1523/JNEUROSCI.09-08-02954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Dilger JP. Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res. 1988;35:143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Schulte BA, Adams JC. Distribution of immunoreactive Na+, K+-ATPase in gerbil cochlea. J Histochem Cytochem. 1989;37:127–134. doi: 10.1177/37.2.2536055. [DOI] [PubMed] [Google Scholar]
- Sellick PM, Patuzzi R, Johnstone BM. Measurement of basilar membrane motion in the guinea pig using the Mössbauer technique. J Acoust Soc Am. 1982;72:131–141. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Brownell WE. Synaptic and golgi membrane recycling in cochlear hair cells. J Neurocytol. 1986;15:311–328. doi: 10.1007/BF01611434. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Kim DO. Efferent neural control of cochlear mechanics? Olivo-cochlear bundle stimulation affects cochlear biomechanical nonlinearity. Hear Res. 1982;6:171–182. doi: 10.1016/0378-5955(82)90052-1. [DOI] [PubMed] [Google Scholar]
- Slepecky N, Chamberlain SC. Distribution and polarity of actin in inner ear supporting cells. Hear Res. 1983;10:359–370. doi: 10.1016/0378-5955(83)90098-9. [DOI] [PubMed] [Google Scholar]
- Slepecky N, Chamberlain SC. Immuno-electron-microscopic and immunofluorescent localization of cytoskeletal and muscle-like contractile proteins in inner ear sensory cells. Hear Res. 1985;20:245–260. doi: 10.1016/0378-5955(85)90029-2. [DOI] [PubMed] [Google Scholar]
- Steele CR, Jen DH. Mechanical analysis of hair cell microstructure and motility. In: Wilson JP, Kemp DT, editors. Cochlear Mechanisms Structure Function and Models. Plenum Press; New York: 1988. pp. 67–74. [Google Scholar]
- Strack G, Klinke R, Wilson JP. Evoked cochlear response in Caiman crocodilus. Pfluegers Arch. 1981;(Suppl 391):R43. [Google Scholar]
- Thalmann R. Biochemical studies of the auditory system. In: Tower DB, editor. Human Communication and Its Disorders. Vol 3. Raven Press; New York: 1975. pp. 31–44. [Google Scholar]
- Thalmann R. Recent refinements of quantitative microchemical analysis of tissues and cells of the inner ear. Acta Otolaryngol. 1972;73:160–174. doi: 10.3109/00016487209138926. [DOI] [PubMed] [Google Scholar]
- Thalmann R, Thalmann I, Comegys TH. Quantitative cytochemistry of the organ of corti. Dissection, weight determination and analysis of single outer hair cell. Laryngoscope. 1972;11:2059–2078. doi: 10.1288/00005537-197211000-00008. [DOI] [PubMed] [Google Scholar]
- Traynelis ST, Dingledine R. Role of extracellular space in hyperosmotic suppression of potassium-induced electrographic seizures. J Neurophysiol. 1989;61:927–938. doi: 10.1152/jn.1989.61.5.927. [DOI] [PubMed] [Google Scholar]
- Ulfendahl M. Volume and length changes in outer hair cells of the guinea pig after pottassium-induced shortening. Arch Otorhinolaryngol. 1988;245:237–243. doi: 10.1007/BF00463935. [DOI] [PubMed] [Google Scholar]
- Ulfendahl M, Slepecky N. Ultrastructural correlates of inner ear sensory cell shortening. J Submicrosc Cytol Pathol. 1988;20:47–51. [PubMed] [Google Scholar]
- Wainwright SA. Design in hydraulic organisms. Die Naturwissenschaften. 1970;57:321–326. [Google Scholar]
- Whitehead ML, Wilson JP, Baker RJ. The effects of temperature on otoacoustic emission tuning properties. In: Moore BCJ, Patterson RD, editors. Auditory Frequency Selectivity. Plenum Press; New York: 1986. pp. 39–48. [Google Scholar]
- Wilson JP. Subthreshold mechanical activity within the cochlea. J Physiol. 1979;298:32–33P. [PubMed] [Google Scholar]
- Wit HP, van Dijk P, Segenhaut JM. An eletrical correlate of spontaneous otoacoustic emission in a frog, a preliminary report. In: Wilson JP, Kemp DT, editors. Cochlear Mechanisms Structure, Function and Models. Plenum Press; New York: 1988. pp. 341–347. [Google Scholar]
- Zenner HP. Cytoskeletal and muscle like elements in cochlear hair cells. Arch Otorhinolaryngol. 1980;230:81–92. doi: 10.1007/BF00665383. [DOI] [PubMed] [Google Scholar]
- Zenner HP. Motile responses in outer hair cells. Hear Res. 1986;22:83–90. doi: 10.1016/0378-5955(86)90082-1. [DOI] [PubMed] [Google Scholar]
- Zenner HP, Wolfgang A, Gitter AH. Outer hair cells as fast and slow cochlear amplifiers with a bidirectional transduction cycle. Acta Otolaryngol. 1988;105:457–462. doi: 10.3109/00016488809119501. [DOI] [PubMed] [Google Scholar]
- Zenner HP, Zimmerman U, Gitter AH. Fast motility of isolated mammalian auditory sensory cells. Biochem Biophys Res Commun. 1987;149:304–308. doi: 10.1016/0006-291x(87)91639-1. [DOI] [PubMed] [Google Scholar]
- Zenner HP, Zimmerman U, Schmitt U. Reversible contraction of isolated mammalian cochlear hair cells. Hear Res. 1985;18:127–133. doi: 10.1016/0378-5955(85)90004-8. [DOI] [PubMed] [Google Scholar]
- Zidanic M, Brownell WE. Low-frequency modulation of the intracochlear potential field: An energy source for the cochlear amplifier. Il Valsalva. 1990 (in press) [Google Scholar]
- Zidanic M, Brownell WE. The fine structure of the intracochlear potential field. I. The silent current. Biophys J. 1990 doi: 10.1016/S0006-3495(90)82644-8. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker E, Manley GA. Acoustical responses and suppression-period patterns in guinea pigs. Hear Res. 1981;4:43–52. doi: 10.1016/0378-5955(81)90035-6. [DOI] [PubMed] [Google Scholar]