Abstract
Kidney function declines with age in association with the development of age-associated glomerulosclerosis. The well established structural and functional changes with age are briefly reviewed. The modification of aging pathology by calorie restriction is discussed. The role of the podocyte as a critical cell in the aging process is considered, using animal models and human biopsy material. Newer data on changes in gene expression and possible changes in biology in the glomerulus are discussed. There is speculation on the implications of this change in biology for human disease and progression.
Keywords: Aging, Glomerulus, Podocyte, Calorie Restriction
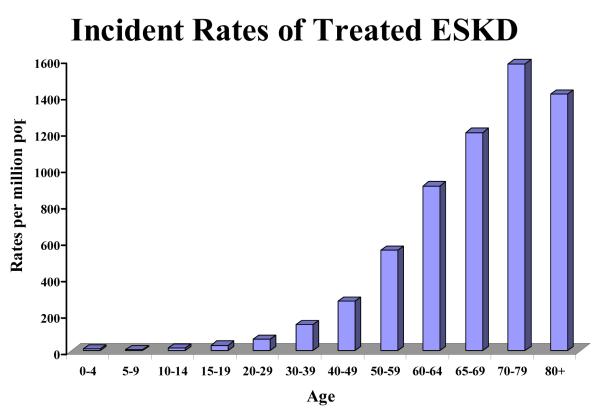
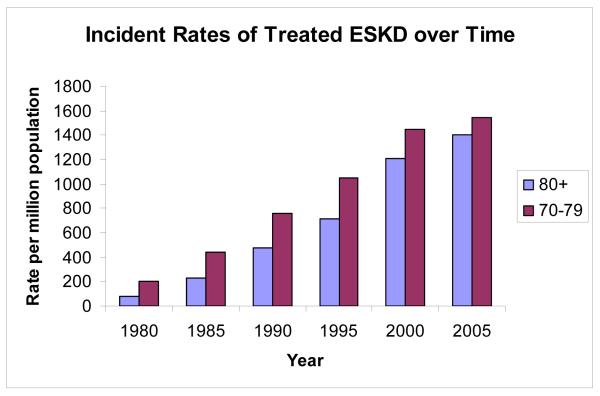
End Stage kidney disease (ESKD) is a disease of the aging population (Figure 1). The mean age at the start of renal replacement therapy is 62.3 years for men and 63.4 years for women (1). Peak incident counts of treated ESKD occur in the 70-79 age group at over 15,000 patients per year. Peak incident rates of treated ESKD occur in the 70-79 year old age group at 1,543 per million population (1) and have been increasing steadily over time (Figure 2). Glomerulosclerosis (GS) is the underlying cause of 90% of ESKD (1,2). GS is associated with aging itself and with diabetes, hypertension and other glomerular diseases including FSGS and inflammatory glomerulonephropathies (2). GS identified in autopsy studies is present in more than 70% of people over the age of 40 years, with increasing prevalence and percentage of glomeruli involved by glomerulosclerosis with age (3). Renal function declines with age in association with well described changes in structure and function (4-6). There is a strong correlation between age and worse outcome for most glomerular diseases (2). Diseases such as diabetes and hypertension are more common in older age, and are known to accelerate GS. However this does not seem to be sufficient to account for the rates of ESKD or the increase in susceptibility to injury that occurs with age. Understanding how aging affects the glomerulus will help us to recognize who is at risk for kidney damage and how to prevent progression to ESKD.
Figure 1. Incident Rates of Treated ESKD.
Incident rates per million population per decade. Data obtained from U.S. Renal Data System, USRDS 2008 Annual Data Report: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2008.
Figure 2. Incident Rates of Treated ESKD over Time.
Incident rates of treated ESKD per million population from 1980 to 2005. Blue bars represent patients over 80 years of age. Red bars represent the 70-79 year old age group. Data from the USRDS annual report for 2007.
Previous studies on age-related kidney injury are restricted to classical histologic and functional analysis in humans and in rodents, with a focus on the impact of diet on these changes in rodents (3-12). They include marked widening of the glomerular basement membrane with age, expansion of the mesangial compartment, enlargement of the glomerulus, development of glomerulosclerosis in an increasing proportion of glomeruli with age, reduction in glomerular filtration rate, reduction in capacity of the kidney to concentrate urine, worsening outcome following glomerular injury (Figure 3).
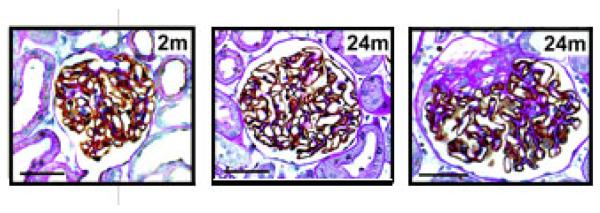
Figure 3. Aging Glomeruli.
Photomicrographs of glomeruli at different ages of ad lib fed and calorie-restricted rats developed with peroxidase GLEPP1 as a podocyte marker and counterstained with PAS and hematoxylin. The panels are all taken at the same magnification. Left: 2 month glomerulus. Center: 24 month calorie restricted. Right: an example of a partially sclerosed glomerulus at 24 months of age in the ad lib fed group. Note the increase in glomerular size and the expanded mesangial compartment in the ad lib fed older rats compared with calorie-restricted rats. The size bar in each photomicrograph is 50 μm long.
A key cell involved in glomerulosclerosis is the podocyte, a highly differentiated neuron-like epithelial cell with limited capacity for cell division and replacement (13-18). Podocytes function to support and maintain the glomerular basement membrane filtration mechanism (17). Genetic diseases that manifest a glomerulosclerotic phenotype include mutations in proteins expressed by the podocyte, thereby providing support for a link between podocyte dysfunction and glomerulosclerosis (19-23). Experimental models suggest that podocyte injury and loss from the glomerulus may be a key component of the process driving glomerulosclerosis (24-27). Using a transgenic model where podocytes express the human diphtheria toxin receptor, we were able to duplicate glomerulosclerosis by titrating the dose of diphtheria toxin to knock out varying percentages of podocytes (27). Loss of podocyte markers from glomeruli is also associated with glomerulosclerosis in human biopsy samples from patients with FSGS (28,29). Several investigators have shown increased appearance of podocytes and podocyte constituents in urine in association with glomerulosclerosis and more rapid deterioration of renal function in FSGS, lupus nephropathy, IgA nephropathy and diabetes (30-32). Together these data support the idea that the podocyte is a key component for the development of glomerulosclerosis and that a failure of podocytes to cover the available glomerular basement membrane filtration surface area results in denuded areas of GBM, which in turn triggers matrix accumulation and glomerulosclerosis. A key question to be addressed is whether the acceleration of ESKD prevalence in older age could somehow also be related to podocyte injury and loss. Floege et al (33) have provided data to support the concept that the glomerulosclerosis of aging is “a podocyte disease” although the mechanism by which this might occur was not elucidated. We have addressed this question using a rat model (Fischer 344) that does not develop diabetes or hypertension so as to avoid these confounding variables, but does develop glomerulosclerosis with age when fed an ad-lib diet (34).
In our model we also looked at how high calorie intake might interact with age-associated glomerulosclerosis (Figure 3). It has been known since the early 90s that calorie restriction not only results in extension of life expectancy, but also moderates the pathologies of aging (34). Cynthia Kenyon, working in c. elegans, showed that the increase in longevity associated with calorie restriction occurs through interference in the insulin signaling pathway (35). Since then similar data has become available for drosophila and mice (36-38). Cai et al showed in mice that restricting AGE (advanced glycation end products) intake was as important as restricting calories in preventing renal pathology with age (39). Early work on Fischer 344 rats had shown that calorie restriction prevents the development of age-associated glomerulosclerosis (34). Calorie restricted rats are fed 60% of the calories consumed by their ad-lib fed litter mates. Their chow is supplemented with vitamins and minerals to avoid nutritional deficiencies. On this diet they live 6 – 8 months longer than their ad-lib fed litter mates, and do not develop renal pathologies even at the end of their extended lives.
With aging, the glomerulus undergoes significant enlargement with robust mesangial expansion (Figure 3). There are appropriate increases in mesangial and endothelial cell numbers, such that the ratio between glomerular volume and cell number stays constant. We have shown that there is a relative rather than an absolute depletion of podocytes that occurs with age (40). Podocytes undergo hypertrophy rather than hyperplasia in association with glomerular enlargement. However, as hypertrophy proceeds it is associated with changed podocyte biology and eventually failed further hypertrophy such that there is relative podocyte depletion and associated glomerulosclerosis. Calorie-restriction prevents these events from occurring (Table 1). Our morphometric data shows that in ad-lib fed animals, glomerular volume increases 3-fold by 24 months, while podocytes do not increase in number (40). Through hypertrophy, podocyte mass is only able to increase 2.6-fold. In calorie restricted animals, podocyte mass increases 1.3-fold while glomerular volume increases 1.5-fold (Table 1).
Table 1. Fold-changes in ad-lib fed and calorie-restricted rats in relation to baseline.
The parameters listed are Glomerular Volume (Glom Vol), Total glomerular cell number (Total Cells), Podocyte number per glomerulus (Podocyte #), Podocyte size (Podocyte size), The numerical data provided are the fold-increase above the common 2 month time point measured in the ad-lib fed and calorie-restricted rat groups at 24 months of age.
| Glom Vol | Total Cells | Podocyte # | Podocyte Size | |
|---|---|---|---|---|
| 2m | 1 | 1 | 1 | 1 |
| 24m Ad-lib | 3.1 | 2.6 | 1.2 | 2.2 |
| 24m CalRes | 1.5 | 1.0 | 1.1 | 1.2 |
Accompanying these morphometric changes, we also see changes in glomerular gene expression with age, using the same model. We also performed DNA expression profiling using microarray analysis of isolated glomerular RNA preparations from both ad-lib and calorie-restricted rats during aging. To identify genes of potential importance to the glomerulosclerotic process and to minimize the age affect we examined the differences in gene expression between ad-lib fed and calorie-restricted rats throughout life (40). There were 497 out of approximately 30,000 probe sets (1.6%) which were significantly different with aging. Of these, 302 probe sets coded for known genes. Several podocyte marker molecules were present in the selected probe sets. The most striking result was that in each case the difference (ad-lib minus calorie-retricted) was negative at 24 months. The probe sets identified coded for the podocyte transcription factor WT1, mutations of which cause glomerulosclerosis in man (41); another podocyte transcription factor Pod1 (42); nephrin, the key podocyte slit diaphragm protein which is mutated in Congenital Nephrotic Syndrome (43); the podocyte apical membrane protein tyrosine phosphatase PTPro/GLEPP1 (44); and alpha 5 type IV collagen, mutations of which are responsible for abnormalities of GBM in Alport’s syndrome (45). Thus, by 24 months ad- lib fed rat glomeruli had significantly less mRNA per total glomerular mRNA for these podocyte molecules than did the calorie-restricted rats of the same age.
To confirm the finding that podocyte mRNAs were relatively decreased at the 24 month time point in ad-lib fed rats we performed quantitative real time PCR for nephrin. As was seen in the nephrin DNA expression microarray, the major difference was at 24 months when the ad-lib fed rat glomeruli contained significantly less mRNA for nephrin (59%) than did calorie-restricted rats (40). This was confirmed at the protein level by measuring nephrin protein in glomerular protein extracts from the ad-lib fed rats. There was a statistically significant 47% decrease in nephrin protein concentration relative to total glomerular protein at 24 months in ad lib fed rats compared with the 17 month time point (P<0.05). This result is consistent with the morphometric and glomerular mRNA data presented above. This would support the idea that podocyte biology changes in major ways with aging, including the loss of markers that characterize the podocyte phenotype.
Not all the gene expression changes represent decline or loss of podocyte molecules. Floege et al previously demonstrated that desmin, an intermediate filament, was increased in podocytes in association with aging in a rat model (33). Desmin was one of the molecules that increased on the DNA microarray lists and was significantly higher in ad-lib fed than in calorie-restricted glomeruli. We therefore examined desmin expression over time in ad-lib and calorie-restricted rats. Glomerular desmin mRNA was increased significantly in ad-lib fed rats by 17 months and 24 months as assessed by real time PCR measurements. Desmin protein was also increased at these time points as assessed by protein quantitation in glomerular extracts. Desmin mRNA levels remained low in calorie-restricted rats (40). This result suggests that even by 17 months in ad-lib fed rats, before proteinuria was present and glomerulosclerosis had appeared, the biology of the podocyte had changed in a significant way.
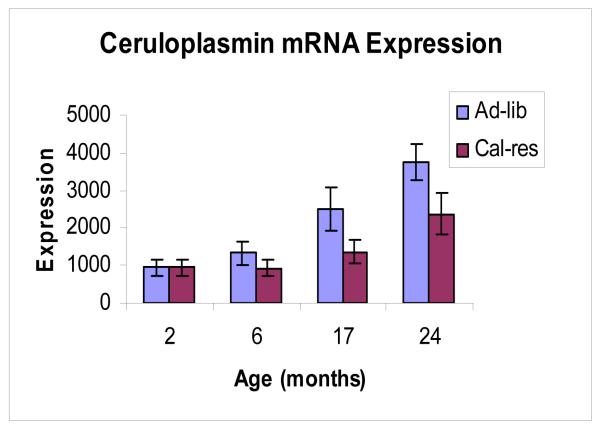
These changes in biology are not limited to podocytes. Data from our affymetrix DNA expression profiling encouraged us to look at genes expressed elsewhere in the glomerulus. One of the most robust changes with aging in ad-lib fed rats is the increase in ceruloplasmin expression (46). Ceruloplasmin (Cp) is a copper containing ferroxidase which functions as an anti-oxidant in part by oxidizing toxic ferrous iron to non-toxic ferric iron (47-49). High calorie intake increases free radical production and oxidation of key biomolecules. Fischer 344 rats maintained on an ad-lib diet develop oxidant injury and age-associated glomerulosclerosis by 24 months (50,51). Calorie restriction prevents both oxidant injury and glomerulosclerosis. In ad-lib fed rats Cp mRNA expression increased 6-fold (P<0.01) and protein expression increased 5-fold (P=0.01) between 2 and 24 months of age (Figure 4). In calorie restricted rats Cp mRNA expression increased 3-fold (P<0.01) and protein expression increased 1.6-fold (NS) between 2 and 24 months of age (46). Both the cell—associated alternately spliced variant and secreted variant were expressed. This expression was localized to the parietal epithelial cells lining the inner aspect of Bowman’s capsule of the glomerulus (Figure 5). Cp was also present in urine, particularly of old ad-lib fed rats with high tissue Cp expression (46). We speculate that Cp expression at this site may be part of the repertoire of the glomerular parietal epithelial cell to protect the glomerular podocytes and downstream nephron from toxic effects of filtered molecules including ferrous iron, and is driven by the aging process in the glomerulus.
Figure 4. Glomerular mRNA expression for Ceruloplasmin.
qRT-PCR for ceruloplasmin mRNA in arbitary units showing a steady increase in expression throughout adult life in isolated glomeruli from Fischer 344 rats. Blue bars represent ad-lib fed rats; red bars represent calorie restricted rats.
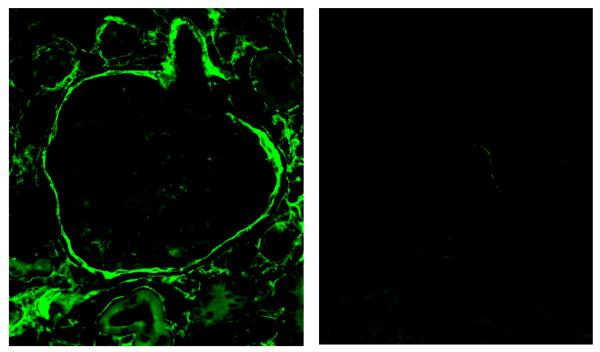
Figure 5. Glomerular Ceruloplasmin Expression.
Ceruloplasmin expression by immunofluorescence microscopy using a mouse monoclonal antibody and a FITC-conjugated goat anti-mouse secondary antibody. Left panel shows an ad-lib fed 24 month glomerulus with staining around Bowman’s capsule and the interstitial compartment. Right panel shows a 2 month glomerulus.
The gene expression changes discussed above, and the probable resulting changes in biology, are all increased by ad-lib feeding and reduced by calorie restriction. The affymetrix gene expression profiling however, identified another group of genes. In this group the level of expression changed linearly (either up or down) throughout the period of adult aging from 2 to 24 months and was not different between calorie restricted and ad-lib fed animals. We identified 163 genes in this group. We have started to work with these genes and found that they represent proteins that are characteristic of each of the different cell types in the glomerulus. Although the podocyte is a key cell in the development of age-associated glomerulosclerosis, it would appear that the biology of the entire glomerulus changes with age. This suggests that there is a coordinated aging process in the kidney, some of which is influenced by environmental factors such as diet, but part of which is genetic. We are currently working to identify possible factors that may be driving and/or coordinating this aging process. We hope that in this process, we are able to identify which patients with impaired kidney function are likely to progress to end stage and who may be more vulnerable to renal failure among those with diabetes, hypertension and other diseases impacting on kidney function.
In conclusion, we have shown that, in addition to the well established morphometric changes, there are important changes in gene expression and probably biology in the glomerulus as animals age. We continue to work on identifying the players in this aging process and how they interact. We hope that this will have meaningful parallels for human aging and disease in the kidney.
Acknowledgments
The work was supported by grants from the National Institutes of Health KO8 AG022019, DK46073 and P50 DK39255, and by pilot grant AG08808 from the Claude Pepper Older Americans Independence Center. Funding was also received from the Geriatric Research, Educational and Clinical Center from the Veterans Administration. We also received technical assistance from Ronald Koenig, MD Director of the Michigan National Institute of Diabetes and Digestive and Kidney Disease Biotechnology Center (DK58771)
Footnotes
Work was performed at University of Michigan
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: 2008. U.S. Renal Data System, USRDS 2008 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. [Google Scholar]
- 2.Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s Pathology of the Kidney. 6th Edition Lippincott-Raven; Philadelphia, NY: 2006. [Google Scholar]
- 3.Kaplan C, Pasternack B, Shah H, et al. Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol. 1975;80:227–234. [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal VK. Changes with age in the human kidney. Exp Gerontol. 1982;17:321–331. doi: 10.1016/0531-5565(82)90032-8. [DOI] [PubMed] [Google Scholar]
- 5.Lindeman RD, Tobin J, Shock NW. Longitudinal Studies on the Rate of Decline in Renal Function with Age. J. Am Geriatric Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson S, Brenner BM. The aging kidney: structure, function, mechanisms and therapeutic implications. J. Am Ger Soc. 1987;35:590–593. doi: 10.1111/j.1532-5415.1987.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 7.Coleman GL, Barthold SW, Osbaldiston GW, Foster SJ, Jonas AM. Pathological changes during aging in barrier-reared Fischer 344 male rats. J. Gerontol. 1977;32(3):258–278. doi: 10.1093/geronj/32.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindeman RD, Goldman R. Anatomic and physiologic age changes in the kidney. Exp Geront. 1986;21:379–406. doi: 10.1016/0531-5565(86)90044-6. [DOI] [PubMed] [Google Scholar]
- 9.Brandis A, Bianchi G, Reale E, Helmchen U, Kuhn K. Age-dependant glomerulosclerosis and proteinuria occurring in rats of the Milan normotensive strain and not in rats of the Milan hypertensive strain. Lab Invest. 1986;55:234–243. [PubMed] [Google Scholar]
- 10.Kleinknecht C, Laouari D, Hinglais N, Habib R, Dodu C, Lacour B, et al. Role of amount and nature of carbohydrates in the course of experimental renal failure. Kidney Int. 1986;30:687–693. doi: 10.1038/ki.1986.241. [DOI] [PubMed] [Google Scholar]
- 11.Tapp DC, Wortham WG, Addison JF, Hammonds DN, Barnes JL, Venkatachalam MA. Food restriction retards body growth and prevents end-stage renal pathology in remnant kidneys of rats regardless of protein intake. Lab Invest. 1989;60:184–195. [PubMed] [Google Scholar]
- 12.Keenan KP, Coleman JB, McCoy CL, Hoe CM, Soper KA, Laroque P. Chronic nephropathy in ad libitum overfed Sprague-Dawley rats and its early attenuation by increasing degrees of dietary (caloric) restriction to control growth. Toxicol Pathol. 2000;28:788–798. doi: 10.1177/019262330002800604. [DOI] [PubMed] [Google Scholar]
- 13.Pabst R, Sterzel RB. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983;24:626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- 14.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 15.Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc. Res Tech. 2002;15:189–195. doi: 10.1002/jemt.10072. [DOI] [PubMed] [Google Scholar]
- 16.Griffin SV, Petermann AT, Durvasula RV, Shankland SJ. Podocyte proliferation and differentiation in glomerular disease: role of cell-cycle regulatory proteins. Nephrol Dial Transplant. 2003;18(Suppl 6):vi8–13. doi: 10.1093/ndt/gfg1069. [DOI] [PubMed] [Google Scholar]
- 17.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 18.Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc. Res Tech. 2002;15:189–195. doi: 10.1002/jemt.10072. [DOI] [PubMed] [Google Scholar]
- 19.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 20.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 21.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 23.Laurens WE, Vanrenterghem YF, Steels PS, Van Damme BJ. A new single nephron model of focal and segmental glomerulosclerosis in the Munich-Wistar rat. Kidney Int. 1994;45:143–149. doi: 10.1038/ki.1994.17. [DOI] [PubMed] [Google Scholar]
- 24.Kretzler M, Koeppen-Hagemann I, Kritz W. Podocyte damage is a critical step in the development of glomerulosclerosis in the uninephrectomized-desoxycorticosterone hypertensive rat. Virchows Arch. 1994;425:181–193. doi: 10.1007/BF00230355. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, et al. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- 26.Schiffer M, Bitzer M, Roberts IS, Kopp JB, Dijke P, Mundel P, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wharram BL, Sanden SK, Filipiak WE, Goyal M, Wiggins JE, Saunders TL, et al. Podocyte depletion causes focal segmental glomerulosclerosis (FSGS) in rats [Abstract] J Am Soc Nephrol. 2004;15:240A. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 28.Sharif K, Goyal M, Kershaw D, Kunkel R, Wiggins R. Podocyte phenotypes as defined by expression and distribution of GLEPP1 in the developing glomerulus and in nephrotic glomeruli from MCD, CNF, and FSGS. A dedifferentiation hypothesis for the nephrotic syndrome. Exp Nephrol. 1998;6:234–244. doi: 10.1159/000020528. [DOI] [PubMed] [Google Scholar]
- 29.Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 30.Hara M, Yanagihara T, Kihara I. Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron. 2001;89:342–347. doi: 10.1159/000046097. [DOI] [PubMed] [Google Scholar]
- 31.Kanno K, Kawachi H, Uchida Y, Hara M, Shimizu F, Uchiyama M. Urinary sediment podocalyxin in children with glomerular diseases. Nephron Clin Pract. 2003;95:c91–c99. doi: 10.1159/000074322. [DOI] [PubMed] [Google Scholar]
- 32.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40–F48. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floege J, Hackman B, Kliem V, Kriz W, Alpers CE, Johnson RJ, et al. Age-related glomerulosclerosis and interstitial fibrosis in Milan normotensive rats: a podocyte disease. Kidney Int. 1997;51:230–243. doi: 10.1038/ki.1997.28. [DOI] [PubMed] [Google Scholar]
- 34.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 35.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993 Dec 2;366:461–4. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 36.Mukhopadhyay A, Tissenbaum HA. Reproduction and longevity: secrets revealed by C. elegans. Trends Cell Biol. 2007 Feb;17(2):65–71. doi: 10.1016/j.tcb.2006.12.004. Epub 2006 Dec 21. Review. [DOI] [PubMed] [Google Scholar]
- 37.Shaw P, Ocorr K, Bodmer R, Oldham S. Drosophila aging 2006/2007. Exp Gerontol. 2008 Jan;43(1):5–10. doi: 10.1016/j.exger.2007.10.008. Epub 2007 Dec 3. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartke A. New findings in transgenic, gene knockout and mutant mice. Exp Gerontol. 2006 Dec;41(12):1217–9. doi: 10.1016/j.exger.2006.09.001. Epub 2006 Oct 17. Review. [DOI] [PubMed] [Google Scholar]
- 39.Cai W, He JC, Zhu L, Chen X, Zheng F, Striker GE, et al. Oral Glycotoxins Determine the Effects of Calorie Restriction on Oxidant Stress, Age-Related Diseases, and Lifespan. Am J. Path. 2008;173(2):327. doi: 10.2353/ajpath.2008.080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, et al. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005 Oct;16(10):2953–66. doi: 10.1681/ASN.2005050488. Epub 2005 Aug 24. [DOI] [PubMed] [Google Scholar]
- 41.Ruf RG, Schultheiss M, Lichtenberger A, Karle SM, Zalewski I, Mucha B, et al. APN Study Group: Prevalence of WT1 mutations in a large cohort of patients with steroid-resistant and steroid-sensitive nephrotic syndrome. Kidney Int. 2004;66:564–570. doi: 10.1111/j.1523-1755.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 42.Quaggin SE. Transcriptional regulation of podocyte specification and differentiation. Microsc Res Tech. 2002;57:208–211. doi: 10.1002/jemt.10076. [DOI] [PubMed] [Google Scholar]
- 43.Patrakka J, Tryggvason K. Nephrin--a unique structural and signaling protein of the kidney filter. Trends Mol Med. 2007 Sep;13(9):396–403. doi: 10.1016/j.molmed.2007.06.006. Epub 2007 Sep 4. [DOI] [PubMed] [Google Scholar]
- 44.Thomas PE, Wharram BL, Goyal M, Wiggins JE, Holzman LB, Wiggins RC. GLEPP1, a renal glomerular epithelial cell (podocyte) membrane protein-tyrosine phosphatase. Identification, molecular cloning, and characterization in rabbit. J Biol Chem. 1994;269:19953–19962. [PubMed] [Google Scholar]
- 45.Hudson BG. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J Am Soc Nephrol. 2004;15:2514–2527. doi: 10.1097/01.ASN.0000141462.00630.76. [DOI] [PubMed] [Google Scholar]
- 46.Wiggins JE, Goyal M, Wharram BL, Wiggins RC. Antioxidant ceruloplasmin is expressed by glomerular parietal epithelial cells and secreted into urine in association with glomerular aging and high-calorie diet. J Am Soc Nephrol. 2006 May;17(5):1382–7. doi: 10.1681/ASN.2005111239. Epub 2006 Apr 5. [DOI] [PubMed] [Google Scholar]
- 47.Patel BN, Dunn RJ, Jeong SY, Julien J-P, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J. Neuroscience. 2002;22:6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein IM, Kaplan HB, Edelson HS, Weissmann G. Ceruloplasmin: an acute phase reactant that scavenges oxygen-derived free radicals. Ann N Y Acad Sci. 1982;389:368–79. doi: 10.1111/j.1749-6632.1982.tb22150.x. [DOI] [PubMed] [Google Scholar]
- 49.Gutteridge JM. Iron and oxygen radicals in the brain. Ann Nuerol. 1992;32:S16–S21. doi: 10.1002/ana.410320705. [DOI] [PubMed] [Google Scholar]
- 50.Ferrucci L, Cavazzini C, Corsi A, Bartali B, Russo CR, Lauretani F, et al. Biomarkers of frailty in older persons. J Endocrinol Invest. 2002;25:S10–S15. [PubMed] [Google Scholar]
- 51.Cunningham J, Leffell M, Mearkle P, Harmatz P. Elevated plasma ceruloplasmin in insulin-dependent diabetes mellitus: evidence for increased oxidative stress as a variable complication. Metabolism. 1995;44:996–999. doi: 10.1016/0026-0495(95)90095-0. [DOI] [PubMed] [Google Scholar]