Summary
Females develop less age-dependent loss of renal function, in part because of cardiorenal protective effects of estrogens. The low androgen level in women also may be protective, although the animal and clinical data are controversial. Both estrogen and androgens act through multiple mechanisms, sometimes beneficial, sometimes damaging, which makes it difficult to predict the impact of hormone replacement therapy in an aging population. Nitric oxide (NO) deficiency contributes to age-dependent cardiovascular risk and kidney damage in animal models. The increased oxidative stress of aging impacts at multiple sites in the NO biosynthetic pathway to decrease NO production/action. Endothelial dysfunction develops with aging and is delayed in women, in association with a delayed increase in asymmetric dimethylarginine. Animal data suggest that the aging kidney develops NO deficiency because of changes in the neuronal NO synthase. Relative preservation of NO production in females contributes to the better cardiovascular and renal responses to aging.
Keywords: Estrogen, androgen, asymmetric dimethylarginine, cardiovascular events, dimethylarginine dimethylaminohydrolase
With advancing age the glomerular filtration rate (GFR) decreases and structural damage develops.1,2 The contribution of normal aging is difficult to separate from undocumented comorbidities such as hypertension, atherosclerosis, glucose intolerance/diabetes, obesity, dyslipidemias, and chronic kidney disease (CKD).2-8 These factors likely contribute to the variability seen in the rate of loss of GFR with age.1,2,9 Sex is another variable, with females being protected, and this review focuses on some aspects of this sexual dimorphism.
AGE-DEPENDENT CHANGES IN GLOMERULAR FUNCTION
The GFR usually decreases gradually in aging men (Fig. 1) owing to structural damage and to decreases in renal plasma flow (RPF) secondary to renal vasoconstriction.10 In women, however, there is less functional decline with age (Fig. 1). This sex difference is seen in clinical and animal studies and persists irrespective of genetic background/race.2,7,11
Figure 1.
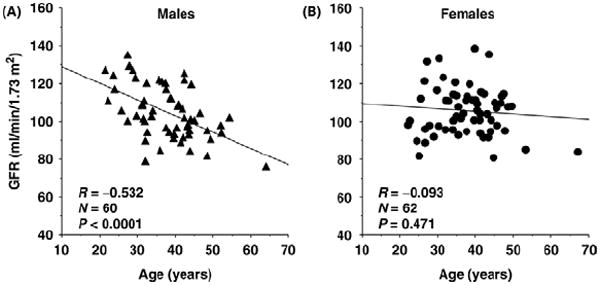
GFR (measured by inulin clearances) in cross-sectional studies in normal men and women of different ages who were evaluated as potential kidney transplant donors. Reprinted with permission from Berg.10
Structural changes in the aging kidney include expansion of the glomerular mesangium and extracellular matrix leading to glomerular sclerosis, glomerular ischemia, arteriolar- and atherosclerosis, loss of peritubular capillaries, and tubulointerstitial injury, all of which contribute to loss of functioning nephrons and declines in GFR.1,2,5,12,13
The normotensive male Munich Wistar (MW) rat develops slowly evolving structural damage, however, this is not associated with increased glomerular blood pressure14 (Fig. 2), which develops only after the injury is established.15 Male rats of the MWF/ZTM strain develop accelerated age-dependent kidney disease but their glomerular blood pressure remains normal.16 Therefore, age-dependent decreases in GFR and glomerular injury can occur in the absence of systemic and glomerular hypertension. However, age-dependent injury and functional declines are accelerated by hypertension1,14,17,18 and glomerular hypertension, as occurs in the sclerosis-prone Sprague-Dawley (SD) rat.19 Structural damage also develops in the kidney of aging man.1,5,13,14
Figure 2.
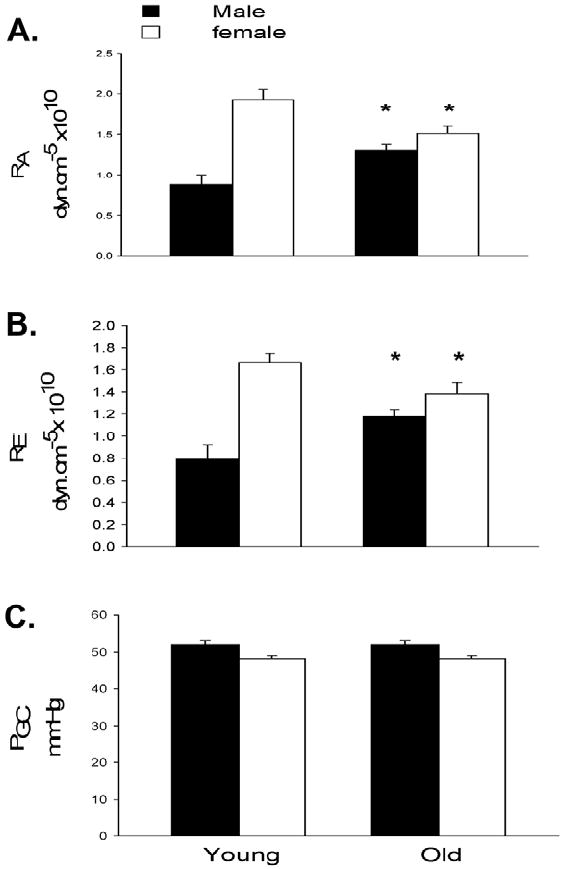
Afferent and efferent arteriolar resistance (RA and RE, respectively) and glomerular blood pressure (PGC) in young adult (~3-4 mo) and older (~18-20 mo) intact male and female MW rats. Data from Baylis.14
In female rats there is considerable protection against age-induced structural damage to the kidney2 and, as shown in Figure 2, glomerular blood pressure remains similar and constant with age in both sexes of the MW rat. Constancy of glomerular blood pressure is maintained in the aging male by parallel increases in afferent and efferent renal arteriolar resistances, whereas parallel relaxation occurs in the initially vasoconstricted female kidney (Fig. 2). In clinical studies Neugarten et al13 reported no sex difference in kidney injury, although a small study by McLachlan et al12 reported a greater vulnerability of aging men to glomerular sclerosis. There is no direct information on what happens to glomerular blood pressure in aging human beings although the exacerbation by systemic hypertension likely reflects development of glomerular hypertension.1,14,17,18
In the aging male MW rat there is progressive renal vasoconstriction leading to decreases in RPF, whereas in the female MW rat preservation of GFR is associated with preserved renal plasma flow, facilitated by gradual relaxation of both afferent and efferent renal arteriolar resistances (Fig. 2). In women, RPF also is maintained10,11 such that by age 70 the values of RPF (factored for surface area) are similar in normotensive men and women,20 reflecting the lower values of GFR and RPF in young women versus men.1,10
Why are males more vulnerable to age-dependent kidney damage and dysfunction? The determinants of sexual dimorphism are complex and in part the result of the gonadal hormones (discussed later).
ESTROGEN AND THE KIDNEY/CARDIOVASCULAR SYSTEM
Estrogens offer cardiovascular protection to premenopausal women, which contribute to the slower rate of progression of chronic renal disease in women versus men.21 Loss of estrogen after menopause may lead to a late decline in renal function and development of structural damage in aging women, although it is difficult to dissociate between aging versus loss of ovarian function.
In aging rodents, injury-resistant female C57Bl6 mice develop age-related glomerulosclerosis after menopause22 and estrogen supplementation reverses glomerular sclerosis in female sclerosis-prone ROP Os/+ mice.23 In addition to protecting the kidney by improving cardiovascular health, estrogens also suppress glomerular mesangial cell growth and extracellular matrix accumulation, thus directly inhibiting development of glomerular sclerosis.22,24-29
Estrogen supplementation in rats and mice often is beneficial, but there have been unfavorable findings in clinical trials on hormone replacement therapy (HRT) in postmenopausal women.30,31 Animal studies routinely use the native 17-β estradiol given subcutaneously whereas clinical trials often use oral conjugated equine estrogens (containing many estrogens, progestins, androgens, and “other substances”),30,31 which have less predictable actions.32 Time of initiation of HRT relative to menopause also may determine outcome because older women showed less benefit and/or increased cardiovascular risk compared with women in whom HRT was initiated at, or close to, menopause.32
Estrogen signaling is very complex and unpredictable with many possible receptor- and non–receptor-mediated actions. The classic nuclear mechanism involves activation of the estrogen receptor (ER), which then binds to estrogen response elements in genes or interacts with transcription factors to control protein synthesis.33 There are now known to be several membrane receptors to estrogen that activate either rapid nongenomic cellular responses or transcription. There is also cross-talk between ER and growth factor receptors and some of the estrogen metabolites exert non–ER-dependent actions.32,33 To date, two ER subtypes, α and β, have been identified that can form homodimers or heterodimers as well as a G-protein–coupled membrane receptor (GPR30). Thus, estrogen can signal through many pathways.33
The kidney contains many ERs and has a large number of estrogen-regulated genes that are mainly under control of the ERα.34 There are sparse and conflicting data on the relative role of the ER subtypes in renal and cardiovascular pathology. Studies by Lane et al35,36 using ER knockout mice (ERKOs) suggest that ERα activation contributes to glomerular hypertrophy and sclerosis after uninephrectomy and with diabetes. Increased renal ERα and a decrease in plasma estradiol occur in type 1 diabetic female rats with evolving renal disease.37 However, ERα is required for vascular repair from atherosclerosis in mice of both sexes,33 ERα increases in old female (protected) mouse kidneys38 and mesangial cells from female glomerular sclerosis-prone mice express decreased ERα and ERβ.39 ERα depletion occurs in high-salt–induced hypertension and renal damage40 and the αERKO female mouse develops albuminuria and glomerular damage with age.41 In some settings ERβ is protective because the βERKO develops age-dependent hypertension42 and βERKO females develop greater cardiac injury after ischemia/reperfusion.43 Observations in knockout animals must be interpreted cautiously given the compensatory adaptations that can occur in nonlethal knockouts.
Although estrogen has many beneficial actions on the kidney21-28 there are a number of experimental situations in which estrogen seems to be damaging. ER-independent actions of estrogen lead to net prosclerotic actions in the db/db (type 2 diabetic) mouse kidney.44 Estrogens worsen renal injury in the stroke-prone SHR,45 and with both chronic NO synthase (NOS) inhibition and ANGII infusion.46 Also, the progression of CKD is faster in the female analbuminemic rat and the Zucker obese rat versus males in association with more severe hypertriglyceridemia.47,48 Given the variety and heterogeneity of signaling mechanisms it is not surprising that the renal/cardiovascular actions of estrogen are complex and variable.
ANDROGENS AND THE KIDNEY/CARDIOVASCULAR SYSTEM
Animal studies suggest that androgens may be damaging. For example, castration of the young adult male prevents age-dependent glomerular sclerosis whereas both intact and ovariectomized females are protected.14 Androgens stimulate mesangial extracellular matrix production and mesangial expansion after subtotal nephrectomy,49 and inhibit glomerular metalloprotease activity,50 and thus are profibrotic. The presence of androgens is associated with greater kidney damage and higher blood pressure (BP) in several hypertensive rat models, perhaps related to enhanced tubular sodium reabsorption and activation of the renin/angiotensin/aldosterone and endothelin systems.51
In normal men the reverse seems true because higher androgen levels correlate with lower BP and overall cardiovascular risk whereas low androgen levels are associated with increased cardiovascular risk and insulin resistance.52,53 Although men with nondiabetic CKD progress more rapidly toward end stage, compared with women21 androgen levels decrease in men with hypertension and renal disease.51 Perhaps the gradual decline in testosterone levels with aging may contribute to age-dependent renal dysfunction in men?
In women the impact of androgen level on cardiovascular risk is controversial. Testosterone levels increase in most postmenopausal women, with aging as cardiovascular risk increases.51 Women with polycystic ovary syndrome have increased androgen levels and increased cardiovascular risk,53 although this may be more related to insulin sensitivity than androgen level.54 However, a low testosterone to bioavailable estrogen ratio correlates with a pro-atherogenic adipocytokine profile,55 and a recent review concluded that there is no clear link between increased testosterone levels and cardiovascular disease in women.56
The classic action of the androgens is via the intracellular androgen receptor (AR), which controls gene transcription. Only one AR gene has been characterized although multiple AR co-regulators can modify responses to testosterone.57 Conversion of testosterone to the more potent dihydrotestosterone (via 5α- reductase) also enhances androgen actions.57 Chronic antagonism of AR lowers BP in the male SHR51 and protects the kidney of the female REN2 rat.58
Androgens also are aromatized to estrogen and the location of aromatase and ERs therefore will impact on actions of testosterone. In fact, vascular and endothelial protection seen with testosterone supplementation is dependent on aromatization to estrogen.59,60 There are also nongenomic actions of androgens including a rapidly occurring vasodilation that does not require AR.53
SUMMARY AND RECOMMENDATIONS
Endogenous estrogens certainly have many positive effects on the cardiovascular system and kidney but estrogen acts via multiple pathways, not all of which are beneficial. There are little data available to allow the development of recommendations for use of HRT. There are 2 clinical studies that suggest that HRT may worsen proteinuria and accelerate the age-dependent decline in renal function in postmenopausal women.61,62 In contrast, Agarwal et al63 reported no deleterious actions of HRT and in fact observed reductions in proteinuria. Both major clinical trials that suggested that HRT increases cardiovascular risk used oral-conjugated equine estrogens, which have less predictable actions than estradiol.30-32,64 Importantly, only oral HRT was associated with accelerated loss of renal function in the study by Ahmed et al.62 Thus, it seems reasonable to favor transdermal or transvaginal administration of 17β-estradiol and to avoid the oral administration route and the use of conjugated equine estrogens. Also, the timing of beginning HRT is important32 and it is wise to avoid beginning HRT in women who are many years postmenopausal.
Androgens often cause cardiovascular risk and exacerbate kidney damage in experimental animals, but in clinical studies higher androgen levels seem to associate with better cardiovascular health in men; the association still is controversial in women. There are likely to be both beneficial and damaging actions of androgens on the cardiovascular system. There are little clinical data on the actions of androgens on the kidney.
SEX STEROIDS, AGING, AND THE NO SYSTEM
Vascular NO is vasodilatory, inhibits growth of contractile cells as well as extracellular matrix production, and also inhibits renal sodium reabsorption.65,66 Chronic NO deficiency results in hypertension, a profibrotic state, and contributes to injury progression in many types of CKD.65 The contribution of NO deficiency to the development of age-dependent CKD is discussed later.
Total NO production (estimated from the urinary excretion of NOX = NO2 + NO3; the stable oxidation products of NO from UNOXV) decreases in the aging male SD rat as CKD develops whereas in the aging female there is little CKD and total NO production is maintained (Fig. 3).67,68 NO production also correlates inversely with kidney damage in the aging male Fischer 344 rat; on normal protein intake, UNOXV decreases and kidney damage develops while protein restriction is associated with preserved UNOXV and structure.69 In man UNOXV decreases with age,70,71 although this effect is lost on both low or high sodium intake.70 The decrease in UNOXV correlates with developing endothelial dysfunction in men71 and although there is no clinical data on sex differences in UNOXV with aging, endothelial dysfunction is delayed in aging women versus men.72 Premenopausal women make more NO than similarly aged men,73 and young adult female rat kidney contains more NO synthase than males.74
Figure 3.
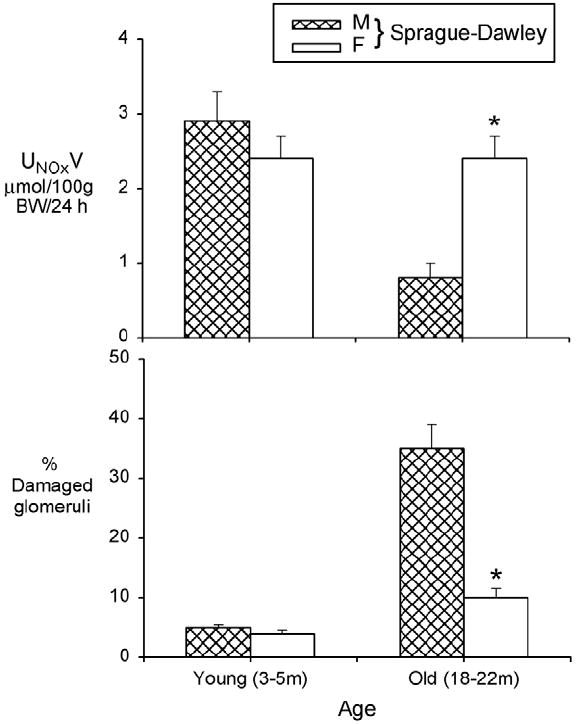
The 24-hour urinary excretion of NO2 + NO3 (NOX), UNOXV (upper panel) and the percentage of damaged glomeruli (ie, those showing segmental and global sclerosis) in young adult (3-5 mo) and old (18-22 mo) male (M) and female (F) SD rats. Data from Erdely et al67 and reprinted with permission from Baylis.66
Some of these sex differences are caused by estrogen, which stimulates NO directly at genomic and nongenomic levels acting on ERs, stimulation of NO indirectly by metabolites and non–ER-dependent actions, reduction of endogenous inhibitors, and probably by the antioxidant actions of estrogens, which prolong the active life of NO.24,25,75,76 Androgens have variable actions on NO production with testosterone-induced inhibition, stimulation, and no effect on NO-dependent vasodilation being reported.57,76 However, androgen-deficient men have enhanced NO-dependent flow-mediated dilation and testosterone supplementation reduces flow-mediated dilation to the normal male value.57 The male rat is more vulnerable to hypertension and proteinuria produced by low-dose NOS inhibition, in part owing to androgens.77 In addition, male rats are more susceptible to fructose feeding and hypercholesterolemia-induced vascular and kidney injury; both states associated with NO deficiency.78,79 It is likely that the vulnerability of the male kidney to NO deficiency contributes to the sexual dimorphism of kidney aging.
POSSIBLE MECHANISMS OF AGE-DEPENDENT DECREASES IN NO PRODUCTION
There are many possible mechanisms by which NO deficiency can occur65 and several probably contribute to the age-dependent loss of NO production (discussed later).
SUBSTRATE (L-ARGININE) AVAILABILITY AND CIRCULATING NOS INHIBITORS
L-arginine is the rate-limiting substrate for NO synthesis and in normal rodent and human beings L-arginine availability is well maintained,65 although fasting plasma L-arginine concentration decreases in the aging male SD rat.80 This suggests that L-arginine becomes an essential amino acid with aging, but as long as an adequate dietary source is available this should not impair NO production.
Increases in the plasma level of the endogenous NOS inhibitor, asymmetric dimethylarginine (ADMA), have been reported in healthy aging human beings, correlating with declines in renal plasma flow.20 There is no difference between the sexes in plasma ADMA levels at 69 and 25 years of age,20 but the increase in women is delayed until approximately 50 years of age81 and correlates with the delayed development of endothelial dysfunction.72 Oral arginine supplementation reverses endothelial dysfunction in healthy aged human beings,82 which may reflect competitive inhibition of the increased ADMA.
Plasma ADMA level probably increases with age owing in part to reduced renal clearance. However, the predominant method of ADMA removal is by metabolism by dimethylarginine dimethylaminohydrolase (DDAH), and the kidney has the highest levels of DDAH of any organ. It is possible that renal and extrarenal DDAH activity decreases with age. This would explain the delayed increase in plasma ADMA in women because estrogen stimulates DDAH activity83 and oral 17-β estradiol replacement in postmenopausal women reduces plasma ADMA.84
NOS PROTEIN ACTIVITY/ABUNDANCE
Reactive oxygen species are a prime determinant of NOS activity, and oxidative stress occurs with aging. By reducing the availability of tetrahydrobiopterin (BH4), an essential cofactor for all the NOS, oxidative stress causes the NOS to become superoxide, rather than NO generators.85 Furthermore, increased ADMA will enhance superoxide production by the uncoupled NOS.86
In addition to decreased activity with age, declines occur in abundance of vascular endothelial eNOS85 and renal cortical neuronal NOS (nNOS)α isoform.67 This is associated with kidney injury rather than aging per se because the female SD rate shows neither age-dependent kidney damage nor a decrease in renal cortical nNOSα levels.67
In summary, endothelial NO production is preserved longer in aging women, probably owing to protective actions of the estrogens. The age-dependent decrease in total NO production probably is caused by increases in circulating ADMA. This causes endothelial dysfunction, which accelerates age-induced CKD. The declining abundance of the renal nNOS also may contribute to the age-dependent CKD. Delays in appearance of endothelial dysfunction and loss of renal nNOS probably protect the female kidney from age-dependent damage and dysfunction. In addition to reduction in NO production, the increased oxidative stress attenuates NO signaling and increased deposition of advanced glycation end products,87 and also prevents access or egress of NO, further promoting the net NO deficiency.
Figure 4.
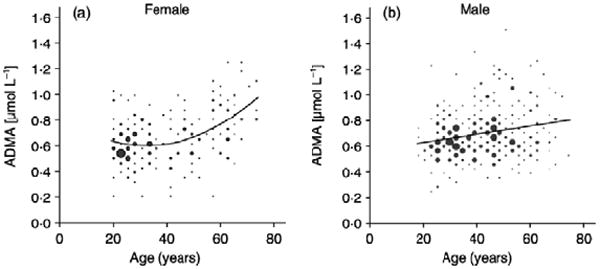
A regression analysis of ADMA levels and age, based on sex, was performed on data from 500 healthy, nonsmoking, nonobese subjects ages 19 to 75 years. Reprinted with permission from Schulze et al.81
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levi M, Rowe JW. Renal function and dysfunction in aging. In: Seldin DW, Giebisch G, editors. The kidney: physiology and pathophysiology. New York: Raven Press; 1992. pp. 3433–3456. [Google Scholar]
- 2.Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol. 1998;9:699–709. doi: 10.1681/ASN.V94699. editorial. [DOI] [PubMed] [Google Scholar]
- 3.Bleyer AJ, Shemanski LR, Burke GL, Hansen KJ, Appel RG. Tobacco, hypertension and vascular disease: risk factors for renal functional decline in an older population. Kidney Int. 2000;57:2072–9. doi: 10.1046/j.1523-1755.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 4.Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006;69:2155–61. doi: 10.1038/sj.ki.5000270. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL. Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int. 1987;31:1153–9. doi: 10.1038/ki.1987.122. [DOI] [PubMed] [Google Scholar]
- 6.Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 2007;71:159–66. doi: 10.1038/sj.ki.5002017. [DOI] [PubMed] [Google Scholar]
- 7.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ. Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged Medicare beneficiaries. J Am Soc Nephrol. 2007;18:1299–306. doi: 10.1681/ASN.2006050524. [DOI] [PubMed] [Google Scholar]
- 8.Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl. 2005;97:S68–77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 9.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–85. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 10.Berg UB. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant. 2006;21:2577–82. doi: 10.1093/ndt/gfl227. [DOI] [PubMed] [Google Scholar]
- 11.Wesson LG., Jr . Physiology of the human kidney. New York: Grune and Stratton; 1969. Renal hemodynamics in physiological states; pp. 96–108. [Google Scholar]
- 12.McLachlan MS, Guthrie JC, Anderson CK, Fulker MJ. Vascular and glomerular changes in the ageing kidney. J Pathol. 1977;121:65–78. doi: 10.1002/path.1711210202. [DOI] [PubMed] [Google Scholar]
- 13.Neugarten J, Gallo G, Silbiger S, Kasiske B. Glomerulosclerosis in aging humans is not influenced by gender. Am J Kidney Dis. 1999;34:884–8. doi: 10.1016/S0272-6386(99)70046-6. [DOI] [PubMed] [Google Scholar]
- 14.Baylis C. Age-dependent glomerular damage in the rat: dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest. 1994;94:1823–9. doi: 10.1172/JCI117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson S, Rennke HG, Zatz R. Glomerular adaptations with normal aging and with long-term converting enzyme inhibition in rats. Am J Physiol. 1994;267:F35–43. doi: 10.1152/ajprenal.1994.267.1.F35. [DOI] [PubMed] [Google Scholar]
- 16.Remuzzi A, Puntorieri S, Mazzoleni A, Remuzzi G. Sex related differences in glomerular ultrafiltration and proteinuria in Munich Wistar rats. Kidney Int. 1988;34:481–6. doi: 10.1038/ki.1988.206. [DOI] [PubMed] [Google Scholar]
- 17.Fliser D, Franek E, Joest M, Block S, Mutschler E, Ritz E. Renal function in the elderly: impact of hypertension and cardiac function. Kidney Int. 1997;51:1196–204. doi: 10.1038/ki.1997.163. [DOI] [PubMed] [Google Scholar]
- 18.Schmieder RE, Schachinger H, Messerli FH. Accelerated decline in renal perfusion with aging in essential hypertension. Hypertension. 1994;23:351–7. doi: 10.1161/01.hyp.23.3.351. [DOI] [PubMed] [Google Scholar]
- 19.Fujihara CK, Limongi DMZP, DeOliveira HCF, Zatz R. Absence of focal glomerulosclerosis in aging analbuminemic rats. Am J Physiol. 1992;262:R947–54. doi: 10.1152/ajpregu.1992.262.6.R947. [DOI] [PubMed] [Google Scholar]
- 20.Kielstein JT, Bode-Boger SM, Frolich JC, Ritz E, Haller H, Fliser D. Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation. 2003;107:1891–5. doi: 10.1161/01.CIR.0000060496.23144.A7. [DOI] [PubMed] [Google Scholar]
- 21.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–29. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 22.Zheng F, Plati AR, Potier M, Schulman Y, Berho M, Banerjee A, et al. Resistance to glomerulosclerosis in B6 mice disappears after menopause. Am J Pathol. 2003;162:1339–48. doi: 10.1016/S0002-9440(10)63929-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliot SJ, Karl M, Berho M, Potier M, Zheng F, Leclercq B, et al. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am J Pathol. 2003;162:1441–8. doi: 10.1016/S0002-9440(10)64277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubey RK, Gillespie DG, Keller PJ, Imthurn B, Zacharia LC, Jackson EK. Role of methoxyestradiols in the growth inhibitory effects of estradiol on human glomerular mesangial cells. Hypertension. 2002;39:418–24. doi: 10.1161/hy0202.103297. [DOI] [PubMed] [Google Scholar]
- 25.Dubey RK, Jackson EK. Estrogen-induced cardiorenal protection: potential cellular, biochemical and molecular mechanisms. Am J Physiol Renal Physiol. 2001;280:F365–88. doi: 10.1152/ajprenal.2001.280.3.F365. [DOI] [PubMed] [Google Scholar]
- 26.Guccione M, Silbiger S, Lei J, Neugarten J. Estradiol upregulates mesangial cell MMP-2 activity via the transcription factor AP-2. Am J Physiol Renal Physiol. 2002;282:F164–9. doi: 10.1152/ajprenal.0318.2000. [DOI] [PubMed] [Google Scholar]
- 27.Kwan G, Neugarten J, Sherman M, Ding Q, Fotadar U, Lei J, et al. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int. 1996;50:1173–9. doi: 10.1038/ki.1996.425. [DOI] [PubMed] [Google Scholar]
- 28.Neugarten J, Medve I, Lei J, Silbiger SR. Estradiol suppresses mesangial cell type I collagen synthesis via activation of the MAP kinase cascade. Am J Physiol. 1999;77:F875–81. doi: 10.1152/ajprenal.1999.277.6.F875. [DOI] [PubMed] [Google Scholar]
- 29.Xiao S, Gillespie DG, Baylis C, Jackson EK, Dubey RK. Effects of estradiol and its metabolites on glomerular endothelial no synthesis and mesangial cell growth. Hypertension. 2001;37:645–50. doi: 10.1161/01.hyp.37.2.645. [DOI] [PubMed] [Google Scholar]
- 30.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 31.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 32.Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovasc Res. 2005;66:295–306. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–26. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- 34.Jelinsky SA, Harris HA, Brown EL, Flanagan K, Zhang X, Tunkey C, et al. Global transcription profiling of estrogen activity: estrogen receptor regulates gene expression in the kidney. Endocrinology. 2003;144:701–10. doi: 10.1210/en.2002-220728. [DOI] [PubMed] [Google Scholar]
- 35.Sun J, Langer WJ, Devish K, Lane PH. Compensatory kidney growth in estrogen receptor-alpha null mice. Am J Physiol Renal Physiol. 2006;290:F319–23. doi: 10.1152/ajprenal.00271.2005. [DOI] [PubMed] [Google Scholar]
- 36.Lovegrove AS, Sun J, Gould KA, Lubahn DB, Korach KS, Lane PH. Estrogen receptor-mediated events promote sex-specific diabetic glomerular hypertrophy. Am J Physiol Renal Physiol. 2004;287:F586–91. doi: 10.1152/ajprenal.00414.2003. [DOI] [PubMed] [Google Scholar]
- 37.Wells CC, Riazi S, Mankhey RW, Bhatti F, Ecelbarger C, Maric C. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med. 2005;2:227–37. doi: 10.1016/s1550-8579(05)80052-x. [DOI] [PubMed] [Google Scholar]
- 38.Sharma PK, Thakur MK. Estrogen receptor a expression in mice kidney shows sex differences during aging. Biogerontology. 2004;5:375–81. doi: 10.1007/s10522-004-3191-6. [DOI] [PubMed] [Google Scholar]
- 39.Potier M, Karl M, Zheng F, Elliot SJ, Striker GE, Striker LJ. Estrogen-related abnormalities in glomerulosclerosis-prone mice reduced mesangial cell estrogen receptor expression and prosclerotic response to estrogens. Am J Pathol. 2002;160:1877–85. doi: 10.1016/S0002-9440(10)61134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esqueda D, Craig T, Hinojosa-Laborde C. Effect of ovariectomy on renal estrogen receptor-alpha and renal estrogen receptor-beta in young salt-sensitive and resistant rats. Hypertension. 2007;50:768–72. doi: 10.1161/HYPERTENSIONAHA.107.095265. [DOI] [PubMed] [Google Scholar]
- 41.Elliot SJ, Berho M, Korach K, Doublier S, Lupia E, Striker GE, et al. Gender-specific effects of endogenous testosterone: female alpha-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int. 2007;72:464–72. doi: 10.1038/sj.ki.5002328. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–8. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 43.Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38:289–97. doi: 10.1016/j.yjmcc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Potier KMM, Schulman IH, Rivera A, Werner H, Fornoni A, Elliot SJ. Autocrine activation of the local insulin-like growth factor I system is up-regulated by estrogen receptor (ER)-independent estrogen actions and accounts for decreased ER expression in type 2 diabetic mesangial cells. Endocrinology. 2005;146:889–900. doi: 10.1210/en.2004-1121. [DOI] [PubMed] [Google Scholar]
- 45.Stier CT, Jr, Chander PN, Rosenfeld L, Powers CA. Estrogen promotes microvascular pathology in female stroke-prone spontaneously hypertensive rats. Am J Physiol Endocrinol Metab. 2003;285:E232–9. doi: 10.1152/ajpendo.00029.2003. [DOI] [PubMed] [Google Scholar]
- 46.Oestreicher EM, Guo C, Seely EW, Kikuchi T, Martinez-Vasquez D, Jonasson L, et al. Estradiol increases proteinuria and angiotensin II type 1 receptor in kidneys of rats receiving L-NAME and angiotensin II. Kidney Int. 2006;70:1759–68. doi: 10.1038/sj.ki.5001897. [DOI] [PubMed] [Google Scholar]
- 47.Joles JA, van Goor H, Koomans HA. Estrogen induces glomerulosclerosis in analbuminemic rats. Kidney Int. 1998;53:862–8. doi: 10.1111/j.1523-1755.1998.00825.x. [DOI] [PubMed] [Google Scholar]
- 48.Gades MD, Stern JS, van Goor H, Nguyen D, Johnson PR, Kaysen GA. Estrogen accelerates the development of renal disease in female obese Zucker rats. Kidney Int. 1998;53:130–5. doi: 10.1046/j.1523-1755.1998.00746.x. [DOI] [PubMed] [Google Scholar]
- 49.Lombet JR, Adler SG, Anderson PS, Nast CC, Olsen DR, Glassock RJ. Sex vulnerability in the subtotal nephrectomy model of glomerulosclerosis in the rat. J Lab Clin Med. 1989;114:66–74. [PubMed] [Google Scholar]
- 50.Reckelhoff JF, Baylis C. Glomerular metalloprotease activity is suppressed by androgens in the ageing kidney. J Am Soc Nephrol. 1993;3:1835–8. doi: 10.1681/ASN.V3111835. [DOI] [PubMed] [Google Scholar]
- 51.Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, Granger JP. Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am J Physiol Renal Physiol. 2005;289:F941–8. doi: 10.1152/ajprenal.00034.2005. [DOI] [PubMed] [Google Scholar]
- 52.Littleton-Kearney M, Hurn PD. Testosterone as a modulator of vascular behavior. Biol Res Nurs. 2004;5:276–85. doi: 10.1177/1099800403262927. [DOI] [PubMed] [Google Scholar]
- 53.Rahman F, Christian HC. Non-classical actions of testosterone: an update. Trends Endocrinol Metab. 2007;18:371–8. doi: 10.1016/j.tem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Brinkworth GD, Noakes M, Moran LJ, Norman R, Clifton PM. Flow-mediated dilatation in overweight and obese women with polycystic ovary syndrome. BJOG. 2006;113:1308–14. doi: 10.1111/j.1471-0528.2006.01090.x. [DOI] [PubMed] [Google Scholar]
- 55.Laughlin GA, Barrett-Connor E, May S. Sex-specific association of the androgen to oestrogen ratio with adipocytokine levels in older adults: the Rancho Bernardo Study. Clin Endocrinol. 2006;65:506–13. doi: 10.1111/j.1365-2265.2006.02624.x. [DOI] [PubMed] [Google Scholar]
- 56.Rizzo M, Rini GB, Carmina E. Androgen excess and cardiovascular risk. Minerva Endocrinol. 2007;32:67–71. [PubMed] [Google Scholar]
- 57.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–40. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 58.Baltatu O, Cayla C, Iliescu R, Andreev D, Jordan C, Bader M. Abolition of hypertension-induced end-organ damage by androgen receptor blockade in transgenic rats harboring the mouse ren-2 gene. J Am Soc Nephrol. 2002;13:2681–7. doi: 10.1097/01.asn.0000033327.65390.ca. [DOI] [PubMed] [Google Scholar]
- 59.Nathan L, Shi W, Dinh H, Mukherjee TK, Wang X, Lusis AJ, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci U S A. 2001;98:3589–93. doi: 10.1073/pnas.051003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukherjee TK, Dinh H, Chaudhuri G, Nathan L. Testosterone attenuates expression of vascular cell adhesion molecule 1 by conversion to estradiol in endothelial cells: implications in atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:4055–60. doi: 10.1073/pnas.052703199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monster TB, Janssen WM, de Jong PE, de Jong-van den Berg LT Prevention of Renal and Vascular End Stage Disease Study Group. Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med. 2001;161:2000–5. doi: 10.1001/archinte.161.16.2000. [DOI] [PubMed] [Google Scholar]
- 62.Ahmed SB, Culleton BF, Tonelli M, Klarenbach SW, Macrae JM, Zhang J, et al. Alberta Kidney Disease Network. Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int. 2008;74:370–6. doi: 10.1038/ki.2008.205. [DOI] [PubMed] [Google Scholar]
- 63.Agarwal M, Selvan V, Freedman BI, Liu Y, Wagenknecht LE. The relationship between albuminuria and hormone therapy in postmenopausal women. Am J Kidney Dis. 2005;45:1019–25. doi: 10.1053/j.ajkd.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 64.Silbiger S. The effects of hormone replacement therapy on renal function. Nat Clin Pract Nephrol. 2009;5:6–7. doi: 10.1038/ncpneph0993. [DOI] [PubMed] [Google Scholar]
- 65.Baylis C. Nitric oxide deficiency in chronic kidney disease. Gottschalk Lecture 2007. Am J Physiol Renal Physiol. 2008;294:F1–9. doi: 10.1152/ajprenal.00424.2007. [DOI] [PubMed] [Google Scholar]
- 66.Baylis C. Mini-review: changes in renal hemodynamics and structure in the aging kidney; sexual dimorphism and the nitric oxide system. Exp Gerontol. 2005;40:271–8. doi: 10.1016/j.exger.2005.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erdely A, Greenfeld Z, Wagner L, Baylis C. Sexual dimorphism in the aging kidney; inverse relationship between injury and nitric oxide system. Kidney Int. 2003;63:1021–6. doi: 10.1046/j.1523-1755.2003.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill C, Lateef AM, Engels K, Samsell L, Baylis C. Basal and stimulated nitric oxide in control of kidney function in the aging rat. Am J Physiol. 1997;272:R1747–53. doi: 10.1152/ajpregu.1997.272.6.R1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sonaka I, Futami Y, Maki T. L-arginine-nitric oxide pathway and chronic nephropathy in aged rats. J Gerontol Biol Sci. 1990;49:B157–61. doi: 10.1093/geronj/49.4.b157. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt RJ, Beierwaltes WH, Baylis C. Aging and alterations in dietary sodium intake on total nitric oxide production in normal man. Am J Kidney Dis. 2001;37:900–8. doi: 10.1016/s0272-6386(05)80004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lyons D, Roy S, Patel M, Benjamin N, Swift CG. Impaired nitric oxide-mediated vasodilatation and total body nitric oxide production in healthy old age. Clin Sci. 1997;93:519–25. doi: 10.1042/cs0930519. [DOI] [PubMed] [Google Scholar]
- 72.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–6. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 73.Forte P, Kneale BJ, Milne E, Chowienczyk PJ, Johnston A, Benjamin N, et al. Evidence for a difference in nitric oxide biosynthesis between healthy women and men. Hypertension. 1998;32:730–4. doi: 10.1161/01.hyp.32.4.730. [DOI] [PubMed] [Google Scholar]
- 74.Neugarten J, Ding Q, Friedman A, Le J, Silbiger S. Sex hormones and renal nitric oxide synthases. J Am Soc Nephrol. 1997;8:1240–6. doi: 10.1681/ASN.V881240. [DOI] [PubMed] [Google Scholar]
- 75.Holden DP, Cartwright JE, Nussey SS, Whitley GS. Estrogen stimulates dimethylarginine dimethylaminohydrolase activity and the metabolism of asymmetric dimethylarginine. Circulation. 2003;108:1575–80. doi: 10.1161/01.CIR.0000091083.61609.DF. [DOI] [PubMed] [Google Scholar]
- 76.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–49. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 77.Verhagen AM, Attia DM, Koomans HA, Joles JA. Male gender increases sensitivity to proteinuria induced by mild NOS inhibition in rats: role of sex hormones. Am J Physiol Renal Physiol. 2000;279:F664–70. doi: 10.1152/ajprenal.2000.279.4.F664. [DOI] [PubMed] [Google Scholar]
- 78.Vasudevan H, Nagareddy PR, McNeill JH. Gonadectomy prevents endothelial dysfunction in fructose-fed male rats, a factor contributing to the development of hypertension. Am J Physiol Heart Circ Physiol. 2006;291:H3058–64. doi: 10.1152/ajpheart.00598.2005. [DOI] [PubMed] [Google Scholar]
- 79.Attia DM, Goldschmeding R, Attia MA, Boer P, Koomans HA, Joles JA. Male gender increases sensitivity to renal injury in response to cholesterol loading. Am J Physiol Renal Physiol. 2003;284:F718–26. doi: 10.1152/ajprenal.00009.2002. [DOI] [PubMed] [Google Scholar]
- 80.Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley EJ, Kruckeberger WC. Changes in nitric oxide precursor, L-arginine and metabolites, nitrate and nitrite, with aging. Life Sci. 1994;55:1895–902. doi: 10.1016/0024-3205(94)00521-4. [DOI] [PubMed] [Google Scholar]
- 81.Schulze F, Maas R, Freese R, Schwedhelm E, Silberhorn E, Böger RH. Determination of a reference value for N(G), N(G)-dimethyl-L-arginine in 500 subjects. Eur J Clin Invest. 2005;35:622–6. doi: 10.1111/j.1365-2362.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 82.Bode-Boger SM, Muke J, Surdacki A, Brabant G, Boger RH, Frolich JC. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8:77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- 83.Holden DP, Cartwright JE, Nussey SS, Whitley GS. Estrogen stimulates dimethylarginine dimethylaminohydrolase activity and the metabolism of asymmetric dimethylarginine. Circulation. 2003;108:1575–80. doi: 10.1161/01.CIR.0000091083.61609.DF. [DOI] [PubMed] [Google Scholar]
- 84.Verhoeven MO, Hemelaar M, Teerlink T, Kenemans P, van der Mooren MJ. Effects of intranasal versus oral hormone therapy on asymmetric dimethylarginine in healthy postmenopausal women: a randomized study. Atherosclerosis. 2007;195:181–8. doi: 10.1016/j.atherosclerosis.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 85.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–44. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- 86.Druhan LJ, Forbes SP, Pope AJ, Chen CA, Zweier JL, Cardounel AJ. Regulation of eNOS-derived superoxide by endogenous methylarginines. Biochemistry. 2008;47:7256–63. doi: 10.1021/bi702377a. [DOI] [PubMed] [Google Scholar]
- 87.Vlassara H, Palace MR. Glycoxidation: the menace of diabetes and aging. Mt Sinai J Med. 2003;70:232–41. [PubMed] [Google Scholar]


