Abstract
Objectives:
Unhelpful beliefs about sleep have been linked to insomnia, and increasing one's cognitive flexibility about sleep has been linked to post-treatment sleep improvement. This study evaluated if levels of such beliefs differ across insomnia groups, and whether there are particular beliefs that differ for specific insomnia subtypes.
Methods:
Participants (N = 1384) were people with insomnia and good sleepers ranging from 18 to 89 years old (M = 42.6, SD = 19.4). Data from previous studies at five insomnia clinical sites were pooled to examine responses on the Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS-16) across differing insomnia groups.
Results:
Group analyses revealed that those from community-based insomnia clinics and those who are hypnotic-dependent generally had the highest levels of unhelpful sleep-related beliefs. With the exception of beliefs about sleep needs (wherein only community sleep clinic patients had high scores relative to good sleepers), all insomnia groups had higher scores on the DBAS-16 than good sleepers. A validity analysis suggested that a DBAS-16 index score > 3.8 was the level of unhelpful beliefs associated with clinically significant insomnia, although a slightly lower cutoff may be useful to identify an unhelpful degree of sleep-related beliefs in highly screened PI and medical patient groups.
Conclusions:
This study offers descriptive data for the use of the DBAS-16 across insomnia subgroups, which will help the user understand what degree of maladaptive sleep beliefs are most strongly associated with clinically significant levels of insomnia. Results also may have implications for cognitive targeting during treatment for particular insomnia groups.
Keywords: Beliefs about sleep, cognitive behavior therapy, insomnia, sensitivity and specificity
Introduction
Having rigidly-held beliefs or unrealistic expectations about sleep is thought to be important in the maintenance of some insomnias 1-5. Several studies have established a greater propensity to have an unhelpful degree of sleep-incongruent beliefs in those with poor sleep relative to those with good sleep 1-3, 5. For example, a belief that 8 hours of sleep is needed to function can increase anxiety when the desired sleep amount is not met, and can lead to increased attention to daytime deficits to confirm the belief that a certain amount of sleep is needed. In an attempt to compensate for real or perceived sleep loss, patients can engage in sleep-interfering behaviors such as spending an excessive amount of time in bed. Given the presumed mechanistic role in the maintenance of insomnia, these sleep-related beliefs are targeted for modification in Cognitive Behavior Therapy (CBT) for insomnia. Clinical trials of CBT with Primary Insomnia patients have demonstrated that elevated maladaptive beliefs about sleep decline with treatment and are related to other indices of clinical improvement 2, 3, 6. Given the role of these beliefs in the protraction of insomnias, clinicians regularly assess for unhelpful sleep-related cognitions as part of their remedial efforts with these patients.
The most widely used tool for assessing the purported maladaptive beliefs in insomnia is the Dysfunctional Beliefs and Attitudes about Sleep scale (DBAS). The newest version of the DBAS (DBAS-16) 7 consists of 16 statements relating to maladaptive sleep-related beliefs, such as “One poor night's sleep will ruin the rest of the week.” The DBAS-16 has a 10-point Likert scale ranging from 0-10. For each of the 16 beliefs, the number corresponding to the degree of belief (i.e., 10=agree completely) is circled. The DBAS-16 index score is a mean-item score (i.e., the item scores are summed and divided by 16). The authors of the DBAS-16 propose a four-factor structure with subscales that assess: 1) sleep-related worry and helplessness; 2) beliefs about sleep medications; 3) expectations about sleep need; and 4) beliefs about the consequences/effects of insomnia. Scores for the subscales are calculated by summing the item responses and dividing by the number of items in the scale. The shorter length reduces administration time and appears to maintain the psychometrics of the original 30-item version of the DBAS (DBAS-30). Furthermore, several of the DBAS-16 items have demonstrated clinical utility in that they discriminate good sleepers from those with insomnia, decline with beliefs-targeted treatment (i.e., Cognitive Behavior Therapy for insomnia), and/or relate to several other indices of clinical improvement 1. Although there is preliminary support for this version of the instrument 7, evaluating the instrument in a large sample of heterogeneous insomnia subtypes would help us to further understand this measure's properties. Moreover, it would be helpful to evaluate this instrument across a range of insomnia types (e.g., those with medical and psychiatric comorbidities, hypnotic dependence, and those presenting at a community insomnia clinic) that may complete the measure.
Providing descriptive statistics across varied types of insomnia patients as well as determining a cut-score most associated with clinically relevant levels of maladaptive sleep beliefs, would increase the usefulness of the DBAS-16. Supportive of the validity of this measure are two previous studies conducted with the 30-item version of the DBAS. The first of these 8 showed a moderately high correlation between the DBAS-30 and both the Pittsburgh Sleep Quality Index (PSQI) 9 and the Sleep Impairment Index (SII) 5. The second study 10 examined the ability of the DBAS-30 total score to detect the presence of insomnia, and compared it with three other sleep-related measures. The study employed a relatively small sample (N = 38) of young, mainly female university students. Receiver Operating Characteristic (ROC) curve analyses suggested a cut-off score of 34.9 (3.5 for a 10-point Likert format); yielding high accuracy (area under the curve = .92, p <0.001) and good sensitivity (89%) and specificity (78%). Unfortunately, research of this nature has yet to be conducted with the DBAS-16, so similar studies to describe the properties of this scale across a variety of insomnia sufferers are needed. The stability of the results will be enhanced if such studies include samples with a wider age range, better representation of males and inclusion of treatment-seeking people with insomnia.
Recognizing the importance of assessing beliefs across insomnia subtypes , we initiated this investigation to examine the DBAS-16's properties among various insomnia sufferers. In the first set of analyses we examined its internal consistency (i.e., Cronbach's alpha) and inter-item reliability (i.e., mean inter-item correlations) of the full scale and its subscales across insomnia groups. Our main aim was to explore whether patient groups differed on specific beliefs about sleep so we conducted a multivariate analysis of covariance of the DBAS and subscales. Lastly, a ROC curve analysis was employed to determine the level of beliefs associated with clinically significant levels of insomnia (i.e., those with an insomnia diagnosis).
Methods
Participants
These analyses used previously collected data from five major centers for insomnia treatment (Duke University Insomnia and Sleep Research Program, Laval University Sleep Disorders Center, Rush University Medical Center, Stanford University Medical Center Sleep Clinic and Flinders University in Australia). The samples were divided into the following groups: good sleepers (GS), Primary Insomnia only (PI), those with insomnia and comorbid medical conditions (MED), those with insomnia and hypnotic dependence (HYP), and a group of outpatients presenting for insomnia treatment at community sleep clinics (CSC). Data were collected from all sites with Institutional Review Board approval and informed consent was obtained from all participants. Table I contains a summary of the assessment and recruitment characteristics for each site and Table II contains demographic data for each group.
Table I.
Site-specific summary of sample recruitment and assessment
| Group/site | Source of diagnosis |
Sleep interview |
Medical evaluation |
Psychological evaluation |
Research- respondent |
Clinic- referred |
Treatment- seeking |
Exclusionary conditions |
|---|---|---|---|---|---|---|---|---|
| Good Sleepers | ||||||||
| Duke/Durham VA | Self- identified |
Clinical interview; SIS-D |
Yes | SCID | Yes | No | No | Self-reported insomnia or any other sleep disorder; AHI≥15; terminal or sleep-interfering medical conditions; past or current Axis I; current psychotropic medication; |
| Flinders University | Self- identified + sleep diary SOL and WASO<30 minutes |
Clinical interview |
No | No | Yes | No | No | Self-reported insomnia or other sleep disorders, medical conditions interfering with sleep. |
| Primary Insomnia | ||||||||
| Duke/Durham VA | Sleep specialist |
Clinical interview; SIS-D |
Yes | SCID | Yes | Yes | Some | Terminal or sleep-interfering medical conditions; past or current Axis I; current psychotropic medication; hypnotic dependent; self-reported history of sleep disorder other than insomnia; AHI ≥ 15; PLMI ≥ 15 |
| Flinders University | Sleep specialist |
Clinical interview |
No | Self-report (CES-D and STAI) |
Yes | No | Yes | Current psychopathology; self- reported history of sleep disorder other than insomnia |
| Hypnotic Dependent | ||||||||
| Laval University | Sleep specialist |
Clinical interview |
Yes | SCID | Yes | Yes | Yes | Insomnia was directly related to a medical or psychiatric disorder; presence of severe psychopathology (e.g., uni- or bipolar disorder, psychosis); use of psychotropic drugs other than benzodiazepines for sleep; AHI > 15; PLMI > 15; |
| Medical Insomnia | ||||||||
| Rush Medical Center | Self- Identified + at least 6 episodes of SOL > 30, WASO > 60 or TST < 6.5 hrs. on a 2- week diary |
Clinical interview |
Yes | Self-report Brief Symptom Inventory (BSI) and Geriatric Depression Scale (GDS) |
Yes | No | No | Self-reported history of sleep disorder other than insomnia; AHI > 15; PLMI > 30; clinically elevated Brief Symptom Inventory scores; GDS >15; taking greater than a standard dose of a hypnotic |
|
Community Sleep Clinic |
||||||||
| Stanford Insomnia Clinic |
Sleep specialist |
Clinical interview |
Some | Yes | No | Yes | Yes | Any active untreated severe medical or psychiatric pathology |
| Repatriation General Hospital Adelaide |
Sleep specialist |
Clinical interview |
Some | CES-D, STAI | No | Yes | Yes | Any active untreated severe medical or psychiatric pathology |
AHI=apnea hypopnea index; CES-D=Center for Epidemiologic Studies Depression Scale, PLMI=periodic limb movement index; SCID=Structured Clinical Interview for DSM Axis I Disorders; SIS-D = Structured Interview for Sleep Disorders; SOL=sleep onset latency; STAI=State-Trait Anxiety Scale; TST=total sleep time; WASO=wakefulness after sleep onset
Table II.
Demographic information for each of the groups (N = 1384)
| Variable | Good Sleeper N=335 |
Primary Insomnia N=329 |
Medical Comorbidity N=114 |
Hypnotic dependent N=76 |
Community Sleep Clinic N=530 |
|---|---|---|---|---|---|
| Female % within group |
60% | 55% | 70% | 50% | 63% |
| Age range (years) | 20-79 | 18-79 | 55-89 | 55-82 | 18-83 |
| Mean age (SD) | 30.2 (15.9) | 47.0 (16.0) | 68.8 (8.4) | 62.5 (6.3) | 44.02 (16.2) |
| Self-reported Sleep* | |||||
| Mean TST (SD) | 462.2 (69.1) | 363.6 (63.7) | 345.6 (70.8) | 358.5 (74.4) | 347.7 (89.9) |
| Mean SOL (SD) | 19.0 (13.95) | 37.9 (34.4) | 41.5 (39.9) | 34.9 (26.4) | 48.8 (32.5) |
| Mean WASO (SD) | 14.4 (20.2) | 73.8 (50.3) | 55.3 (39.5) | 51.1 (39.2) | 96.0 (61.7) |
| Mean SE% (SD) | 93.2 (5.15) | 76.2 (10.9) | 72.0 (12.9) | 71.4 (13.8) | 68.8 (17.3) |
Note. SD = standard deviation; TST = total sleep time; SOL = sleep onset latency; WASO = time awake after sleep onset; SE = sleep efficiency (time in bed/time spent asleep*100).
Good sleeper self-reported sleep estimates for the Duke/Durham VA site only (N=104) were the mean values of prospective two-week sleep logs and the self-reported sleep estimates for the Flinders University Clinic (N=231) were retrospective mean sleep estimates; Self-reported sleep values for the Primary Insomnia, Medical Comorbidity and Hypnotic Dependent groups were mean values of prospective two-week sleep logs; Community Sleep Clinic self-reported sleep estimates include data for the Stanford site only (N =360), as data for the Flinders University Clinic were unavailable.
Good Sleepers (GS)
There were a total of 104 good sleeper participants from Duke University and Durham VA Medical Centers and 231 good sleeper participants from Flinders University. The GS group from Duke University and Durham VA Medical Centers were selected from a study which was originally designed to compare home and laboratory sleep indices in insomnia sufferers. They were screened for the presence of insomnia, psychiatric disorders or medical disorders with associated sleep disruption using the Structured Interview for Sleep Disorders (SIS-D)11, Structured Clinical Interview for DSM-III R (SCID)12 and a medical evaluation. These data have been published in both peer-reviewed manuscripts 13, 14 and abstract format 15. The GS subgroup participants from Flinders University were undergraduate students (18 years or older) each of whom self-identified as a “good sleeper.” In addition to self-identifying as a good sleeper, they also had to have a mean estimated Sleep Diary SOL and WASO that were less than 30 minutes.
Primary Insomnia (PI)
The PI group (n = 329) were respondents to advertisements for insomnia research or clinic-referred patients at Duke University and the Durham VA Medical Center (n = 243), 13, 16, 17 or Flinders University (n = 86) 18. All participants underwent screening procedures to establish a diagnosis of primary insomnia and to rule-out other causes of insomnia such as psychiatric disorders, sleep disruptive medical conditions or other primary sleep disorders. As the nature of these screening procedures and inclusion/exclusion criteria used in selecting each of these samples have been reported elsewhere, 13, 16-18 they will not be reiterated here.
Hypnotic Dependent Insomnia (HYP) group
The participants for the HYP group (N = 76) were research participants at the Laval University Sleep Clinic. These participants have been described elsewhere 19. Briefly, they were treatment-seeking older adult (55 years or older) outpatients with prolonged use of BZ medication for sleep on more than half the nights for at least three months and a subjective complaint of difficulty initiating and/or maintaining sleep for at least three nights per week for at least six months accompanied by marked distress over subjective daytime impairment.
Insomnia with Comorbid Medical Conditions (MED) group
The participants for the MED group (N = 114) were part of a clinical trial of CBT for insomnia in those with comorbid medical conditions (e.g., Coronary Artery Disease – CAD, Chronic Obstructive Pulmonary Disease – COPD, or Osteoarthritis – OA) at Rush Medical Center (see Rybarczyk, 2005 and Table I for details of inclusion/exclusion criteria) 20.
Community Sleep Clinic (CSC) group
Participants for the CSC sample (n = 530) were treatment-seeking community based sleep clinic patients at either Stanford University Medical Center (n = 360) or the Sleep Disorders Clinic at the Repatriation General Hospital, Adelaide, Australia (n = 170). Patients at both sites were diagnosed by a sleep clinician as having a Disorder of Initiating and/or Maintaining Sleep (DIMS). Participants could have a variety of comorbid medical or psychiatric conditions so long as they were not requiring imminent treatment of their comorbid condition. Participants also could have primary insomnia alone. All participants in this cohort were initially evaluated by a sleep specialist by means of clinical interview and subsequently were referred to CBT for insomnia.
Measures
The 16 items of the DBAS were extracted from the 30-item version of the DBAS that participants had completed at their respective study sites prior to the initiation of treatment. At sites except Flinders University (Australia), DBAS-16 item scores were converted from the original 100-point scale to a 10-point Likert by dividing the item score by 10 and rounding to the nearest whole number. However, at the Flinders University site, a different conversion procedure was needed since a 5-point Likert scale for responding to each item was used. Scores from this site were converted to a 10-point Likert by employing the following linear transformation: (x−1)*2.5. This linear transformation produced 5 possible scores ranging from 0 to 10 with a 2.5 point distance between adjacent points on the transformed scale. Such a transformation, thus, allowed for distribution of scores across a 10 point range, yet preserved the intended responses of those subjects who chose the lowest score on the original 5 point scale. It was important to preserve the lower endpoint, as we were including samples of good sleepers who frequently select the lowest score (i.e., if a doubling procedure were used, the lowest possible score would be a 2). Following these various score conversion procedures, a total DBAS-16 score was calculated as the mean across all 16 items for each participant. The subscales were computed by taking the mean of the items of the scale.
Analyses
Internal Consistency
To provide users of the instrument information about its properties in various insomnia subtypes, we computed Cronbach's alpha for the total score and the four subscales as well as item and subscale means and standard deviations, and corrected item-total correlations. The item-total correlation is an index of the utility of an item, as it is the correlation between the item and the total score after controlling for the item of interest. Analyses of covariance (ANCOVA) tested whether the group means differed on individual items using Bonferroni correction (.05/16 = .003) to reduce the likelihood of a type I error. Because two of our groups included older insomnia sufferers and a MANOVA revealed a statistically significant age effect (p=.001), we entered age as a covariate.
Construct Validity/Group Differences
Insomnia sufferers have been described as a group with elevated levels of unhelpful beliefs that contribute to sleep difficulties, and previous studies have shown that persons with insomnia obtain a significantly higher DBAS-30 total score than do matched normal sleepers. Assuming the DBAS-16 remains a valid measure of unhelpful sleep related beliefs, the total score on this instrument should discriminate those with and without insomnia. Hence, as a test of the DBAS-16's validity, we conducted ROC Curve analyses with membership in any of the insomnia groups as the criterion variable. We derived cut-points by plotting the relation between the sensitivity and 1-specificity of the DBAS-16 total score over all possible values on a ROC curve and selecting a clinical cut-off score that maximized both values. Sensitivity represents the probability of detecting insomnia when it is present, and specificity represents the probability of not detecting insomnia when it is indeed not present. The further the ROC curve lies above a reference line, the more accurately a chosen cut-off score classifies positive and negative cases in a chosen sample. 21 We also computed the area under the curve (AUC) to determine the probability that the DBAS score for a randomly chosen insomnia case would exceed the result for a good sleeper case. We calculated the AUC using SPSS software and tested it for statistical significance. Classification accuracy was evaluated using the following recommended ranges: low accuracy = AUC < 0.7, moderate accuracy = AUC between 0.7 and 0.9, and high accuracy = AUC > 0.9 22.
Although the CSC group did not employ standardized diagnostic tools, we opted to include this group in our ROC analyses because they were referred for insomnia treatment, verified via subjective self-report of insomnia and clinical interview, and were subsequently treated for insomnia. We also thought it important to include this sample, as it is a community based sleep clinical sample, and the DBAS-16 is intended for both clinical and research settings. Similarly, although the GS subgroup from Flinders University was not screened with standardized diagnostic tools, we opted to include them in our classification analysis because, in addition to not complaining of insomnia, they did not have pathological levels of SOL or WASO. Thus the criterion used to define an insomnia case was a sleep specialist DSM insomnia diagnosis and the criterion used to define a non-insomnia case was self-identification as a normal sleeper and either: 1) failure to meet DSM diagnostic criteria for an insomnia diagnosis, or 2) mean SOL and WASO below 30 minutes.
As the main purpose of the study was to describe beliefs across differing insomnia groups, means for each group for the total DBAS-16 score and subscales were compared using multivariate analysis of covariance (age was entered as a covariate).
Results
Figure I shows the distribution for DBAS score in the good sleeper and insomnia group. Scores ranged from 0.2 to 9.44 and the figure shows that the DBAS scores approximated normal distributions within each group. Although, as we would expect, the distribution of the good sleeper group reflected their lower levels of maladaptive sleep beliefs, thus there appears to be some slight flattening of the distribution and a slight skew towards the left. There were no outliers and no apparent problems with restriction of range.
Fig. 1.
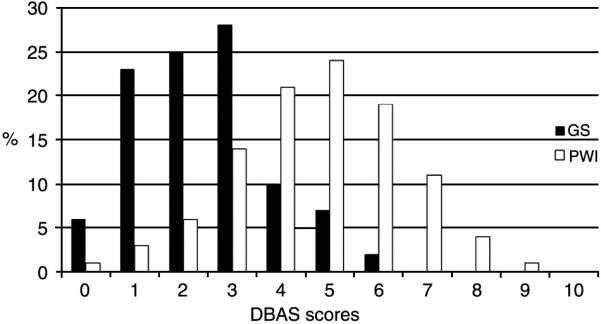
Distribution of DBAS scores across the GS and PI groups.
Internal Consistency
Table III depicts the item means, standard deviations and item-total correlation coefficients. Item-total correlation coefficients (ITCs) for the pooled insomnia groups ranged from .073 to .647. All ITCs were greater than the suggested minimum value for ITCs of .30 (Nunnally & Bernstein, 1994) 23with the exception of items 10 and 13. Results for good sleepers were similar: ITC ranged from .127 to .554 and all ITCs were greater than .30 except items 10, 13 and 15. After controlling for Age, there was a group effect on all ANCOVAs at p<.003 except for items 1, 2 and 13. Table IV depicts the unadjusted group means, standard deviation, and Cronbach's alpha for the four scales and total score. The internal consistency estimates for the total DBAS-16 score (Cronbach's alpha = .821) and Effects subscale (.750) for the pooled insomnia groups were acceptable, but the Cronbach's alpha for the pooled insomnia groups for the Worry/Helplessness (.660), Expectations (.581) and Medication (.470) subscales were poor.
Table III.
Group item means, standard deviations and mean item correlations
| Groups |
|||
|---|---|---|---|
| DBAS-16 items | Good sleepers |
All insomnia groups |
|
| 1. I need 8 hours | Mean | 5.63 (2.94) | 6.06 (3.19) |
| Item-total correlation | .316 | .325 | |
| 2. Need to catch-up on sleep loss | Mean | 4.84 (2.83) | 5.03 (3.21) |
| Item-total correlation | .342 | .329 | |
| 3. Concerned about health consequences |
Mean | 4.62 (3.32) | 6.57 (2.98) |
| Item-total correlation | .391 | .476 | |
| 4. Worried I may lose control over my abilities to sleep |
Mean | 1.65 (2.22) | 5.02 (3.28) |
| Item-total correlation | .328 | .537 | |
| 5. A poor night's sleep will interfere with my activities the next day |
Mean | 5.36 (2.77) | 6.80 (2.82) |
| Item-total correlation | .400 | 579 | |
| 6. Better off taking a sleeping pill | Mean | 1.78 (2.27) | 4.35 (3.52) |
| Item-total correlation | .265 | .428 | |
| 7. Negative mood is due to poor sleep |
Mean | 3.48 (2.66) | 6.10 (2.95) |
| Item-total correlation | .470 | .543 | |
| 8. One night will disturb the whole week |
Mean | 1.73 (2.38) | 2.85 (2.67) |
| Item-total correlation | .423 | .429 | |
| 9. Without adequate sleep, I can hardly function the next day |
Mean | 2.53 (2.39) | 4.46 (2.92) |
| Item-total correlation | .554 | .590 | |
| 10. Can't predict whether I'll have a good or poor night's sleep |
Mean | 4.23 (3.09) | 7.00 (3.02) |
| Item-total correlation | .216 | .073 | |
| 11. Little ability to manage the negative consequences of disturbed sleep |
Mean | 3.70 (2.63) | 5.49 (2.91) |
| Item-total correlation | .412 | .406 | |
| 12. Feeling tired, no energy, or not functioning well is due to poor sleep |
Mean | 5.25 (2.67) | 6.99 (2.60) |
| Item-total correlation | .449 | .505 | |
| 13. I think insomnia is due to a chemical imbalance |
Mean | 4.25 (2.05) | 4.31 (2.59) |
| Item-total correlation | .127 | .271 | |
| 14. Insomnia is ruining my life | Mean | 2.43 (2.57) | 5.23 (3.34) |
| Item-total correlation | .515 | .647 | |
| 15. Medication is the only solution | Mean | 1.23 (1.85) | 3.29 (3.10) |
| Item-total correlation | .235 | .391 | |
| 16. I avoid/cancel obligations after a poor night's sleep |
Mean | 1.67 (2.35) | 3.27 (3.05) |
| Item-total correlation | .477 | .339 | |
Table IV.
Group means, standard deviations and Cronbach's alpha for DBAS-16 summary score and subscales
| DBAS-16 scores |
Groups |
||||||
|---|---|---|---|---|---|---|---|
| Good sleepers (GS) |
All insomnia groups |
Primary Insomnia (PI) |
Medical Comorbid (MED) |
Hypnotic Dep. (HYP) |
Insomnia Clinic (CSC) |
||
| n | 335 | 1049 | 329 | 114 | 76 | 530 | |
| Expectations scale (items 1, 2) |
Mean | 5.17 | 5.50 | 5.06 | 4.55 | 5.51 | 6.18 |
| SD | 2.41 | 2.65 | 2.70 | 2.69 | 2.29 | 2.38 | |
| alpha | .485 | .581 | .560 | .517 | .570 | .578 | |
| Effects scale (items 5, 7, 9, 12, 16) |
Mean | 3.45 | 5.57 | 4.85 | 4.77 | 5.06 | 6.23 |
| SD | 1.74 | 2.04 | 2.05 | 2.16 | 2.05 | 1.60 | |
| alpha | .708 | .750 | .787 | .749 | .812 | .689 | |
| Worry scale (items 3, 4, 8, 10, 11, 14) |
Mean | 2.15 | 5.46 | 4.55 | 4.47 | 5.39 | 6.41 |
| SD | 1.56 | 1.87 | 1.62 | 1.70 | 1.87 | 1.58 | |
| alpha | .635 | .660 | .581 | .609 | .754 | .595 | |
| Medication scale (items 6, 13, 15) |
Mean | 2.28 | 4.05 | 2.83 | 2.84 | 4.60 | 5.52 |
| SD | 1.31 | 2.20 | 1.76 | 1.89 | 1.71 | 1.95 | |
| alpha | .304 | .470 | .466 | .318 | .140 | .387 | |
|
DBAS-16 score |
Mean | 2.96 | 5.23 | 4.38 | 4.27 | 5.14 | 6.16 |
| SD | 1.26 | 1.60 | 1.42 | 1.47 | 1.43 | 1.32 | |
| alpha | .797 | .821 | .798 | .786 | .802 | .772 | |
Note. SD = standard deviation; alpha = Cronbach's alpha
Construct Validity/Group Differences
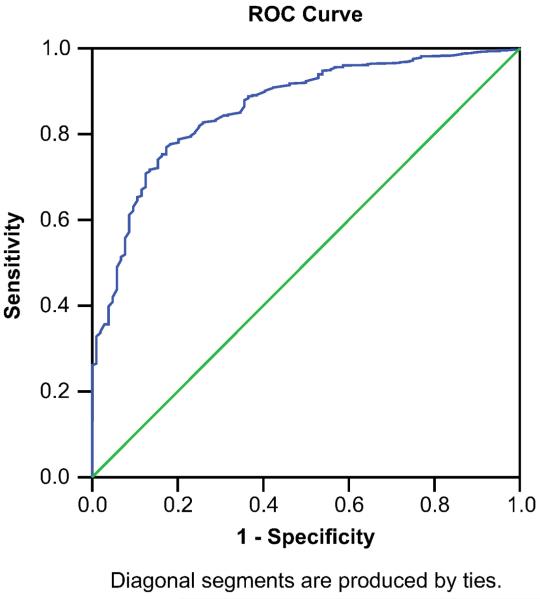
Figure II shows the ROC curve for combining all insomnia groups and testing against the good sleepers. The AUC (0.86) was moderately high, and was statistically significant at p < .001. The 95% confidence interval was .82 to .89 (SE = .02). The ROC curve suggested that a clinical cut-off of 3.8 on a 10-point Likert scale maximized sensitivity (80%) and specificity (76%) for group classification. If we evaluate the cutoff derived from the overall sample of 3.8 (true positive rate=80% and false positive rate=24%) in each group: 1) sensitivity for the CSC group increases to 92% and specificity remains the same at 76% (AUC is improved to .93, SE=.012); 2) for the hypnotic-dependent group sensitivity increases slightly to 84% and specificity remains the same at 76% (AUC is slightly improved to 87%, SE=.028); 3) the medical group sensitivity decreases to 65% and specificity remains about the same at 75% (AUC is .76, SE=.033) and 4) PI sensitivity decreases to 66% and specificity remains the same at 76% (AUC is .78, SE=.027). Since the overall cutoff resulted in much lower sensitivity (i.e., a 3.8 cutoff would have a false negative rate of 35% for the medical group and 25% for the PI group) we examined other possible cutoffs. Lowering the cutoff to 3.5 for each of these groups resulted in a slightly improved sensitivity rate of 70% for the medical group and 73% for the PI group; although some specificity is lost too (69% and 70% respectively).
Fig. 2.
ROC curve for DBAS-16.
We hypothesized that Good Sleepers would differ from those with insomnia on the DBAS-16 mean item score and the subscales, but it was unknown as to whether the insomnia subgroups would differ from one another. Thus, we conducted a multivariate analysis of covariance (MANCOVA), entering Age as a covariate on the DBAS index score and the four themes of the DBAS-16 to evaluate any group differences. The MANCOVA was statistically significant, F(20, 4561) = 22.3, p < .001; thus we followed-up the significant group effect with analyses of variance (ANCOVAs) and pairwise comparisons to understand the differences. After controlling for age, the ANCOVAs for the DBAS [F(4, 1378) = 69.3, p<.001]; Effects [F(4, 1378) = 40.5, p<.001]; Worry/Helplessness [F(4, 1378) = 97.3, p<.001]; Expectations [F(4, 1378) = 3.3, p=.01]; and Medication [F(4, 1378) = 33.9, p<.001] were all statistically significant. Table IV presents the unadjusted group means. Follow-up group comparisons on the DBAS index score revealed that CSC=HYP>MED=PI>GS (p < .001). This same result was observed for the pairwise comparison for the Worry/Helplessness subscale (i.e., CSC had the highest score, followed by HYP, followed by not significantly different scores in MED and PI groups; although both MED and PI groups had higher scores than GS). On the Effects subscale: 1) CSC was significantly higher than all groups, 2) MED, HYP and PI groups did not differ significantly from each other, although 3) these three groups were significantly higher than GS. On the Medication scale, the CSC and HYP were similarly high and were significantly higher than GS, PI, and MED (who did not statistically differ from each other). Lastly, the CSC group had significantly higher scores on the Expectation subscale, and GS, PI, HYP, and MED groups did not differ from each other.
Site Differences
In addition to these analyses we conducted a series of ANCOVAs (covarying age) to assess differences within our sub-samples due to study sites. Results of these analyses were all significant after controlling for age (p < .05) suggesting site effects. Good sleepers at Flinders University (M=3.98, SD=1.27) had higher scores than good sleepers at Duke University (M=2.96, SD=1.26). Those at Flinders in the Primary Insomnia group had higher DBAS-16 scores (M=4.96, SD=1.55) than those with PI at Duke (M=4.18, SD=1.31). Those at Stanford in the CSC group had higher DBAS-16 scores (M=6.16, SD=1.34) than those in the CSC group at Flinders (M=5.59, SD=1.44). The effect sizes for the comparison of good sleepers, PI and CSC groups were very small (eta squared = 0.05, 0.05, and 0.03 respectively).
Discussion
Overall, this large, multi-site study of unhelpful sleep beliefs suggests that the DBAS-16 is a reliable and valid tool for use across a range of insomnia patient groups; although it is important to understand that insomnia subtypes differ from one another in such beliefs. Reliability estimates (Cronbach's alpha and item-total correlation coefficients) confirmed the previously reported acceptable reliability of the full DBAS-16 scale and overall, Cronbach's alpha was similar across each study group. In addition, there were acceptable item correlations with the total for all but two items. Thus, the majority of item means both significantly correlated with the total score and discriminated those with insomnia from those without. In contrast to these findings, internal consistency indices for the various subscales, as originally conceived by Morin 7, were less promising. In fact, only the Effects scale had acceptable alpha values across the various samples included in this study. One consideration for the reliability of the subscales is the short scale length for the Medication and Expectations subscales. Longer scales tend to have higher reliability, and the Medication and Expectations subscales with poor internal consistency have 3 and 2 items respectively. In general, the lack of support for the subscales (with the exception of the Effects scale) would suggest their use should be avoided.
The validity analyses confirmed that the DBAS taps into sleep beliefs that discriminate those with insomnia from good sleepers. These results should not imply that the DBAS-16 is a diagnostic or screening tool for insomnia. It is important to view this instrument as a measure for identifying clinically significant levels of unhelpful beliefs related to sleep, rather than as a screen for insomnia. The ROC curve analysis suggested that a DBAS-16 total score above 3.8 is associated with the degree of unhelpful beliefs found in those with clinical insomnia. This cutoff is slightly higher, but consistent with a previously reported cut-off (>3.5) in a smaller sample of young university students completing the DBAS-30 10. The false negative rate of 20% and the fact that the ROC analyses in the PI and medical groups had poorer sensitivity suggest that this cutoff may be too stringent for all insomnia groups. Indeed these analyses do not take into consideration that the base rates for insomnia may be considerably lower for predominantly research-recruited people with insomnia than those solely from a sleep clinic. . It would have been interesting to examine these issues in those recruited solely for research with those from treatment centers only; unfortunately with the exception of the CSC group, our groups have a combination of each referral source and thus preclude such testing. Future studies that provide normative data would be useful to determine the extent to which the current findings extend to positive cases and “controls” in the general population, as well as other types of insomnias from a variety of settings.
Generally, community sleep clinic patients tended to exhibit higher levels of maladaptive sleep beliefs than all other groups. This group is different from the others in several ways: 1) the clinics are tertiary settings, 2) there are no research respondents, and 3) there are multiple medical and psychiatric comorbidities. Hypnotic-dependent patients (HYP) had similarly high levels of overall unhelpful beliefs, and were comparable to those with PI and medical comorbidities on beliefs about the consequences/effects of insomnia, and expectations about sleep needs. The HYP group had the highest (along with the CSC group) level of maladaptive beliefs about medications. Interestingly, only the CSC patient group had higher expectations about sleep needs than Good Sleepers; a finding reported elsewhere 24. This could mean that beliefs relating to sleep needs may need only be challenged in clinical settings. However, given that modifications in these beliefs (i.e., decreases in scores on this scale) are associated with other indices of clinical improvement 1, it may be more likely that this difference is attributable to the unique characteristics of this group described above. For example, there were no research respondents and it has been reported elsewhere that those volunteering for research studies tend to report less distress and preoccupation with sleep than those in clinics 25. With this in mind, the fact that those who were hypnotic dependent were mainly research-respondents and they had the most (or second most) strongly held beliefs of all insomnia groups suggest that cognitive restructuring might need to be of particular clinical focus for such patients. One other consideration is whether daytime symptoms (e.g., fatigue) could account for differences between the groups; this might be an interesting future area of exploration. While it is interesting to speculate on the reasons for the differences, because the groups differed on several characteristics including comorbidity and these were not statistically controlled, one must exercise caution in interpreting the results. In addition, as with many cognitive factors, the effect sizes are fairly small so it is difficult to ascertain the full clinical significance of such results. Future studies could answer these questions more definitively.
A final point to mention is that our results also showed some within-group differences attributable to study site. Site differences were noted within our groups of normal sleepers, PI sufferers and those composing the CSC sample. Each of these comparisons included one Australian sample and one sample obtained from a site in the United States. Arguably these noted site differences could be attributed to differences in sample selection methods and cultural differences between the two countries from whence the samples were selected. However, it is also possible that the difference in response options (i.e., a 5-point Likert scale in Australia vs. a 10- or 100 point scale in the United States) could have contributed to these differences as well. Although we attempted to convert all data into a standard 10-point scale, there are admittedly limitations to the mathematical algorithm we used. Further, this was an archival study and it is not certain if the same results would have occurred if all participants had completed the DBAS-16 (as opposed to completing the full version and extracting the 16 items). Thus, future cross-cultural studies with the DBAS-16 would benefit by consistent use of the standard 10-point Likert scale that is now standard on this instrument.
Overall these results provide important information for how subgroups of people with insomnia score on this instrument. There was evidence of some poor item-specific results, although this may be attributable to the fact that some items selected for inclusion in the DBAS-16 were chosen largely based on their presumed clinical usefulness rather than on any empirical basis. 5 It is important to note that although the total score of the DBAS-16 is used as an index of a problematic level of sleep-disruptive beliefs, the individual items of the DBAS-16 can also be used as a tool for modification of specific beliefs. For example, some clinicians use responses to particular DBAS-16 items in therapy to orient the patient to possible overvalued ideation and to modify such sleep disruptive beliefs 5. Although some items lack strong individual psychometric support, their therapeutic value and relation to treatment outcome should not be underestimated. This was shown in a study finding that some DBAS items that do not discriminate insomnia sufferers from normal sleepers, decline significantly during CBT and/or show treatment-related changes that correlate with other measures of insomnia improvement 1. This suggests utility for these items, although they could benefit from further psychometric work. Nonetheless, the main index is reliable and is useful for establishing a level of unhelpful beliefs characteristic of those with differing types of insomnia and demographic characteristics. More future large-scale collaborations to provide normative and psychometric data for other insomnia measures would greatly advance the field.
Acknowledgements
This research was supported by the following grants: Dr. Edinger - Department of Veteran's Affairs Grant #VA0009 and National Institute of Mental Health Grant #MH48187; Dr. Morin - National Institute of Mental Health Grant #MH-60413 and Canadian Institutes of Health Research #MT42504. Dr. Rybarczyk - National Institute on Aging Grant# AG17491-04; Dr. Lack – National Health and Medical Research Council of Australia Grant #NHMRC950017
References
- 1.Carney CE, Edinger JD. Identifying critical dysfunctional beliefs about sleep in primary insomnia. Sleep. 2006;29:440–53. [Google Scholar]
- 2.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Does cognitive-behavioral insomnia therapy alter dysfunctional beliefs about sleep? Sleep. 2001;24:591–9. doi: 10.1093/sleep/24.5.591. [DOI] [PubMed] [Google Scholar]
- 3.Espie CA, Inglis SJ, Harvey L, Tessier S. Insomniacs' attributions: Psychometric properties of the Dysfunctional Beliefs about Sleep Scale and the Sleep Disturbance Questionnaire. Journal of Psychosomatic Research. 2000;48:141–8. doi: 10.1016/s0022-3999(99)00090-2. [DOI] [PubMed] [Google Scholar]
- 4.Harvey AG. A cognitive model of insomnia. Behaviour Research & Therapy. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 5.Morin CM. Insomnia: Psychological assessment and management. Guilford Press; New York: 1993. [Google Scholar]
- 6.Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes related to sleep improvements in the treatment of insomnia? Behaviour Research & Therapy. 2002:40. doi: 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 7.Morin CM, Vallières A, Ivers H. Dysfunctional Beliefs and Attitudes About Sleep (DBAS): Validation of a Brief Version (DBAS-16) Sleep. 2007;30:1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blais FC, Gendron L, Mimeault V, Morin CM. Assessment of insomnia: Validation of three questionnaires. Encephale. 1997;23:447–53. [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 10.Smith S, Trinder J. Detecting insomnia: Comparison of four self-report measures of sleep in a young adult population. Journal of Sleep Research. 2001;10:229–35. doi: 10.1046/j.1365-2869.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 11.Schramm E, Hohagen P, Grasshoff M, et al. Test-retest reliability and validity of the Structured Interview for Sleep Disorders according to the DSM-III-R. American Journal of Psychiatry. 1993;150:867–72. doi: 10.1176/ajp.150.6.867. [DOI] [PubMed] [Google Scholar]
- 12.Spitzer RL, Williams JBW, Gibbons M, First MB. Instruction manual for the Structured Clinical Interview for DSM-III-R. Biometrics Research Department, New York State Psychiatric Institute; New York: 1990. [Google Scholar]
- 13.Edinger JD, Fins AI, Sullivan RJ, et al. Sleep in the laboratory and sleep at home II: Comparison of older insomniacs and normal sleepers. Sleep. 1997;20:1119–26. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- 14.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic Primary Insomnia: A randomized controlled trial. JAMA. 2001;285:1856–64. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 15.Gehrman P, Edinger JD, Means MK, Husain AM. Measurement of sleep in young insomniacs: A multi-trait, multi-method approach. Sleep. 2003;26(Suppl):A310. [Google Scholar]
- 16.Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-Response Effects of Cognitive-Behavioral Insomnia Therapy: A Randomized Clinical Trial. Sleep. 2007;30:203–12. doi: 10.1093/sleep/30.2.203. [DOI] [PubMed] [Google Scholar]
- 17.Edinger JD, Glenn DM, Bastian LA, et al. Sleep in the laboratory and sleep at home II: Comparison of middle-aged insomnia sufferers and normal sleepers. Sleep. 2001:24. doi: 10.1093/sleep/24.7.761. [DOI] [PubMed] [Google Scholar]
- 18.Wright HK, Lack L, Bootzin RR. Relationship between dim light melatonin onset and the timing of sleep in sleep onset insomniacs. Sleep and Biological Rhythms. 2006;4:78–80. [Google Scholar]
- 19.Morin CM, Bastien CH, Guay B, Radouco-Thomas M, Leblanc J, Vallieres A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. American Journal of Psychiatry. 2004;161:332–42. doi: 10.1176/appi.ajp.161.2.332. [DOI] [PubMed] [Google Scholar]
- 20.Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, Davis A. A placebo-controlled test of Cognitive-Behavioral Therapy for comorbid insomnia in older adults. Journal of Consulting and Clinical Psychology. 2005;73:1164–74. doi: 10.1037/0022-006X.73.6.1164. [DOI] [PubMed] [Google Scholar]
- 21.Mossman D, Somoza E. ROC curves, test accuracy and the description of diagnostic tests. Journal of Neuropsychiatry and Clinical Neuroscience. 1991;3:330–3. doi: 10.1176/jnp.3.3.330. [DOI] [PubMed] [Google Scholar]
- 22.Swets J. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–93. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 23.Nunnally J, Bernstein IH. Psychometric theory. 3rd ed. McGraw-Hill; New York: 1994. [Google Scholar]
- 24.Carney CE, Edinger JD, Manber R, Garson CS, Segal ZV. Beliefs about sleep in disorders characterized by sleep and mood disturbance. Journal of Psychosomatic Research. 2007;62:179–88. doi: 10.1016/j.jpsychores.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Stepanski E, Korshorek G, Zorick F, Glinn M, Roehrs T, Roth T. Characteristics of individuals who do or not seek treatment for chronic insomnia. Psychosomatics. 1989;30:421–7. doi: 10.1016/S0033-3182(89)72248-9. [DOI] [PubMed] [Google Scholar]