SUMMARY
Neuroimaging studies using diffusion tensor imaging (DTI) have revealed regions of cerebral white matter with decreased microstructural organization (lower fractional anisotropy or FA) among poor readers. We examined whether 100 hours of intensive remedial instruction affected the white matter of 8–10-year-old poor readers. Prior to instruction, poor readers had significantly lower FA than good readers in a region of the left anterior centrum semiovale. The instruction resulted in a change in white matter (significantly increased FA), and in the very same region. The FA increase was correlated with a decrease in radial diffusivity (but not with a change in axial diffusivity), suggesting that myelination had increased. Furthermore, the FA increase was correlated with improvement in phonological decoding ability, clarifying the cognitive locus of the effect. The results demonstrate for the first time the capability of a behavioral intervention to bring about a positive change in cortico-cortical white matter tracts.
INTRODUCTION
A major challenge of cognitive neuroscience is to understand how changes in the structural properties of the brain underpin the plasticity exhibited whenever a person develops, ages, learns a new skill, or adapts to a neuropathology. Longitudinal studies have shown regional changes in the volume of gray matter that co-occur with skill acquisition or learning (Draganski et al., 2004; Draganski et al., 2006), but there is also growing acknowledgement that higher-level cognition is based on co-functioning of a set of cortical areas in a dynamic large-scale network, highlighting the central role of cortical communication. Improved anatomical connectivity in motor tracts as measured by fractional anisotropy (FA) has been associated with enriched experience (extensive childhood piano practice) in a correlational study using diffusion tensor imaging (DTI) (Bengtsson et al., 2005). (FA, which measures the anisotropy of the diffusion of water molecules (Basser and Pierpaoli, 1996), is sensitive to axonal density, size, myelination, and the coherence of organization of fibers within a voxel, and thus provides an index of the structural integrity of white matter).
Functional imaging studies have consistently demonstrated that children with reading disability display under-activation of a network of left-lateralized areas during reading, including occipito-temporal, temporo-parietal, and inferior frontal cortical regions (Hoeft et al., 2006; Hoeft et al., 2007; Meyler et al., 2007; Shaywitz et al., 2002; Simos et al., 2000a; Simos et al., 2000b), and that effective remedial reading interventions lead to increases in the activation in these same areas (Aylward et al., 2003; Meyler et al., 2008; Shaywitz et al., 2004; Simos et al., 2002; Temple et al., 2003), indicating that effective remediation can lead to a change in the brain functioning of poor readers. However, skilled reading depends not only on the activation of a set of relevant cortical areas, but also on communication among them. Reading difficulty has also been associated with lower functional connectivity (the synchronization of neural activity) across areas of the reading cortical network (Hampson et al., 2006; Horwitz et al., 1998; Pugh et al., 2000). This suggests that reading disability might be associated with structural properties of the white matter that provides the anatomical connectivity among the individual nodes of the reading network. Consistent with this view, several DTI studies of poor readers have found white matter regions with lower FA compared to controls (Beaulieu et al., 2005; Deutsch et al., 2005; Klingberg et al., 2000; Niogi and McCandliss, 2006; Odegard et al. 2009; Richards et al., 2008; Rollins et al., 2009). FA may be reduced in poor readers due to a number of possible differences in the microstructural properties of white matter, including reduced myelination, reduced axonal packing density, decreased axonal diameter, or reduced coherence of the orientation of axons within the region (Beaulieu, 2002; Ben-Shachar et al., 2007), all of which might impact the efficiency of communication (bandwidth) among cortical areas.
Here we report a longitudinal DTI study indicating that intensive remedial reading instruction (approximately 100 hours) can change the structural integrity of the cortical white matter of children who are poor readers. The children’s DTI data was first assessed prior to when instruction began and then a second time after the instruction ended, approximately six months later. At the pre-remediation scan, the poor readers showed significantly reduced fractional anisotropy (FA) in the anterior left centrum semiovale region, relative to a control group of good readers. Subsequent to the instruction, the remediated poor readers had not only made substantial gains in their reading ability, but also showed significantly increased FA in the anterior left centrum semiovale, in contrast to good readers and to a control group of untreated poor readers.
To help determine which microstructural properties had changed during remediation, we also examined the diffusivity in directions that are perpendicular to the principal axis of diffusion in anisotropic regions of white matter (radial diffusivity, (λ2+λ3)/2), or parallel to it (axial diffusivity, λ1). For example, changes in radial diffusivity in the absence of changes in axial diffusivity have been associated with changes in myelin (Beaulieu et al. 2002; Song et al. 2002; Song et al., 2005), whereas changes in axial diffusivity in the absence of changes in radial diffusivity have been associated with an increase in axon diameter (Dougherty et al., 2007). The results analyzed this way indicate for the first time that a behavioral intervention can bring about a positive change in the microstructure of human cortico-cortical white matter tracts, demonstrating the malleability of the anatomical connectivity that supports human cortical network function.
RESULTS
Forty-seven children (eight to twelve years old) who were poor readers were randomly assigned to either an intensive 100-hour program of systematic and explicit remedial reading instruction focused primarily on developing word-level decoding skills (n=35), or they were assigned to a control group which received normal classroom instruction (n=12). There was also a control group of good readers (n=25) of the same age. The remedial instruction was distributed over about six months of schooling, with instruction occurring in groups of three children with one teacher. (Although the remedial instruction came in one of four alternative forms (see Experimental Procedures), there were no reliable differences among the children assigned to the different forms in either initial behavioral measures or DTI measures, nor in the impacts of the instruction (see Supplemental Results and Discussion, available online). Hence the data reported here are collapsed across the children in the four forms of remedial reading instruction.) The remediated and unremediated poor readers scored equivalently at the pre-instruction scan on multiple measures of reading ability, whereas the group of good readers scored significantly better than both groups of poor readers on every reading ability measure (see Table S1, available online). The behavioral results indicated that the poor readers who received the remedial instruction showed significant improvement on most of the age-standardized Woodcock Reading Mastery Test - Revised (WRMT-R, Woodcock et al. 1998) reading ability measures when retested following the instruction period, but that the control poor readers did not show improvement on these measures, indicated by a reliable overall group by time effect (F1, 45 = 4.36, p < .05), with means shown in Table 1. Individual ANOVAs for each measure indicated that the interaction between group and time was reliable only for the subtest measuring non-word reading ability (Word Attack scores, F1, 45 = 5.22, p < .05), but not for the subtests measuring real word reading ability (Word Identification) or passage comprehension ability (Passage Comprehension). This pattern of outcomes suggests that the instruction specifically improved phonological decoding skills more than the standard reading curricula did. This conclusion was also supported by an analysis of changes in raw scores on all ability measures collected from the poor readers before and after the treatment phase (See Supplemental Results and Discussion and Table S2).
Table 1.
Changes in age-standardized Woodcock Reading Mastery Test - Revised scores between the pre-remediation and post-remediation scans
| Change in Scores | Group x Time ANOVA |
||||
|---|---|---|---|---|---|
| Group | |||||
| Measure | Poor Readers (PR) |
Poor Reader Controls (PC) |
Interaction effect |
||
| Time 2 - Time1 | t(34) | Time 2 - Time1 | t(11) | F(1, 45) | |
| WRMT-R Word Attack | 5.5 | 3.98** | −0.3 | −0.17 | 5.22* |
| WRMT-R Word Identification | 2.5 | 2.50* | 2.3 | 0.65 | 0.01 |
| WRMT-R Passage Comprehension | 1.1 | 0.86 | −3.0 | −1.04 | 2.18 |
| WRMT-R Basic Skills Cluster | 4.2 | 5.06*** | 2.6 | 0.87 | 0.48 |
| WRMT-R Total Reading Cluster | 2.2 | 2.51* | 1.5 | 0.47 | 0.08 |
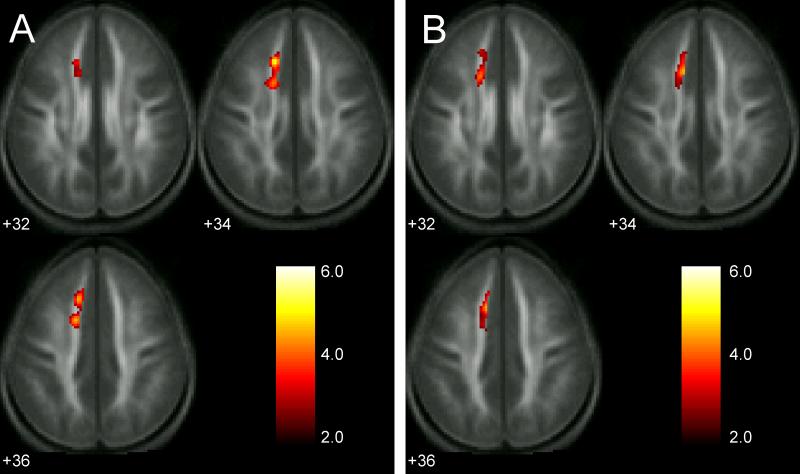
The DTI results indicated that poor readers who received the remedial instruction showed a reliable increase in FA between the pre-remediation and post-remediation scans, with a peak difference in the left anterior centrum semiovale, as shown in Figure 1A. Corresponding contrasts conducted for the two control groups that received no remedial instruction found no areas showing either an increase or decrease in FA between the two scans, indicating that the change in FA among the remediated poor readers was not due to maturational changes over the six-month interval between the two scans. This same region also showed significantly reduced FA at the pre-remediation scan among all poor readers relative to the group of good readers (Figure 1B). The reliable increase in FA between the two scans among the poor readers, but no change in FA between the scans among the good readers, nor among the unremediated poor reader controls, resulted in a significant group (3) by time (2) interaction with a peak F-value in the same region of the left anterior centrum semiovale (Figure 2A), strongly suggesting that intensive remedial reading instruction led to changes in some microstructural property of white matter in a region of left frontal white matter, a region that differed between good and poor readers prior to the treatment. Additional analyses presented in the Supplemental Results confirmed that these findings were not due to the particular voxel-based analysis methods that were used; essentially identical results were obtained using unsmoothed data and non-parametric statistical inference methods (See Supplemental Results and Discussion and Figures S1 and S2).
Figure 1.
(A) Region where the poor reader group showed an increase in FA between the pre- remediation and post-remediation scans (peak t(34) = 5.12, at Montreal Neurological Institute (MNI) coordinates −12 28 36, spatial extent = 450 voxels, p < .05 corrected for multiple comparisons). There were no areas where poor readers showed a decrease in FA between phases, nor were there any areas where the control group of good readers or the control group of unremediated poor readers showed either an increase or decrease in FA. (B) Region showing a significant difference in FA between good readers and all poor readers at the first scan (peak t(70) = 4.66, at MNI coordinates −10 20 38, spatial extent = 418 voxels, p < .05 corrected for multiple comparisons). Statistical maps are overlaid on a normalized FA image averaged across all participants and both scans. The MNI z-coordinate is shown at the bottom left of each axial slice. Color scale represents t-values.
Figure 2.
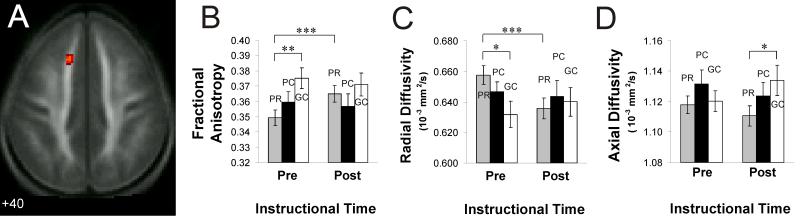
(A) Location of the cluster of voxels with the maximum F-value (peak F2, 69 = 9.66, spatial extent = 49 voxels, p < .0005 uncorrected, at MNI coordinates −12 26 40) for a test of the group by time interaction. (B) Mean FA for this cluster in each group at each phase of the study. (C) Mean radial diffusivity for this cluster in each group and at each phase. (D) Mean axial diffusivity for this cluster in each group and at each phase. Error bars represent the standard error of the mean. PR = poor readers who received remediation, PC = poor reader control group, GC = good reader control group. * = p < .05, ** = p < .005, *** = p < .0005.
Because increased FA in highly organized white matter can occur due to either a relative decrease in radial diffusivity or a relative increase in axial diffusivity (or both), a further analysis examined the remediation effect in each of these components separately in the region shown in Figure 2A. It was the radial diffusivity that had changed in the remediated poor readers subsequent to the instruction. There was a reliable group by time interaction for radial diffusivity in this same region (F2, 69 = 5.92, p < .005); this measure reliably decreased among the remediated poor readers (t(34) = 3.98, p < .0005), but showed no change in either the good readers or the poor reader controls, as shown in Figure 2C. This pattern of radial diffusivity effects mirrors the findings for FA (a reliable increase in FA among poor readers who received remedial instruction but no reliable change in FA among the two unremediated groups (Figure 2B). By contrast, the other component of FA, axial diffusivity, showed no significant changes between phases for any group at this location, nor was there a reliable interaction (Figure 2D). The pattern of diffusivity effects indicates that the difference in FA between poor and good readers before remediation is due to initially higher radial diffusivity in the poor readers, and that the change in FA results from a change in some microstructural feature (e.g. myelination, packing density, or axon diameter) that affects radial diffusivity. The pattern of results also argues against the pre-remediation differences in FA between good and poor readers being due to the existence of more crossing fibers or smaller diameter axons in the poor readers in the area, and argues against the proposition that the changes in FA resulting from remediation were due to changes in either of these microstructural features, both of which would be expected to affect axial diffusivity. This leaves increased myelination as a plausible mechanism of the microstructural change.
The findings of increased reading ability and increased FA strongly suggest that the remedial instruction brought about a change in both variables, but say little about the relation between the two variables. To investigate this relation in more detail and to assess which aspects of reading ability were associated with increased FA, an exploratory stepwise hierarchical multiple regression analysis examined how well the change in raw reading scores of an individual poor reader could account for that individual’s change in FA in the region. This analysis (which also took the change in age between scans into account) indicated that a model including the change in raw scores on two subtests from the Test of Word Reading Efficiency (TOWRE, Torgesen et al. 1998) provided the best fit to the change in FA data among poor readers (R2= 0.10, F2, 43 = 2.36, p = .11). The change in Phonemic Decoding Efficiency (PDE, a measure of non-word reading fluency similar to the WRMT-R WA subtest) was positively associated with change in FA (partial r = .23, p = .06). In contrast, the change in the Sight-Word Efficiency (SWE, a measure of real word reading fluency similar to the WRMT-R Word ID subtest) showed a negative partial correlation with change in FA (pr = −.21). No other variables met the criteria for entry into the model. An identical analysis conducted with radial diffusivity in the region as the dependent measure also showed that these same two measures provided the best fit to the data (R2= 0.13, F2, 43 = 3.41, p < .05) with change in PDE significantly negatively associated with change in radial diffusivity (pr = .23, p < .05) and change in SWE positively associated with the change (pr = .29). In contrast, for axial diffusivity, an identical stepwise regression analysis indicated that no change in any of the raw ability measures explained enough variance for entry into the model (p’s > .15). The outcome of these analyses indicate that there is a coupling between the behavioral change in reading and the anatomical change measured by FA and radial diffusivity, and indicates that increased phonological decoding ability provides the best predictor of increased FA and decreased radial diffusivity.
These results and conclusions are further supported by additional analyses (described in the Supplemental Results and Discussion) of the relationships between individual differences in various reading abilities and various diffusion tensor measures in the entire sample of good and poor readers, (using reading and diffusion measures obtained prior to the remediation), in the cluster that eventually showed an increase in FA among the remediated poor readers. Multiple regression analyses indicated that individual differences in phonological decoding ability (as measured by WRMT-R WA scores) were strongly positively related to FA (see Figure S3), strongly negatively related to radial diffusivity, and only weakly negatively related to axial diffusivity at the time of the pre-remediation scan (see Figure S4). These findings suggest that radial diffusivity drives the positive relationship between FA and individual differences in reading ability measured at the initial scan. In addition, both FA and radial diffusivity were more strongly related to Word Attack scores than to Word ID scores, suggesting that connections passing through the cluster area may be more important for phonological processing than for direct access to meaning via a direct orthographic route (see Supplemental Results and Discussion).
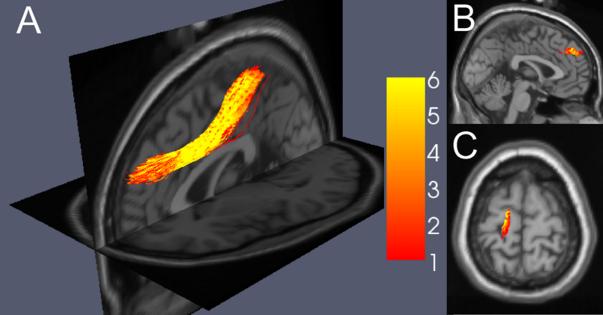
To determine the orientation of the tracts showing the remediation-related change and to identify the cortical areas that they likely connect, fiber tractography was carried out on group-averaged diffusion tensor data, using as a seed region the cluster of voxels showing a reliable group by time interaction. These group-averaged tracts were remarkably similar in their gross morphology between the good and poor readers and also across the two scanning sessions, as shown in Figure 3A, indicating the reliability of the data and the tracking methods. The principal direction of diffusion in the region showing a group difference in FA at the pre-remediation scan remained the same at the follow-up scan, and the fibers identified as passing through the regions were remarkably consistent between the two scans for both groups of subjects, suggesting that microstructural changes in the white matter within the region, rather than changes in the orientation of fibers, are responsible for the remediation effect and for the relationship of reading ability to the diffusion measures. The principal diffusion direction was anterior-posterior in all groups, and fibers passing through this region extended anteriorly and medially to terminate in a medial region of the superior frontal gyrus (Figure 3B) and extended posteriorly and superiorly to terminate in the left paracentral lobule (Figure 3C).
Figure 3.
(A) Consistency of the group-averaged tractography for PR, PC, and GC groups at each of two scans, using a seed region based on the cluster in Figure 2A. Color scale indicates the consistency of the tracking across the groups and phases, with red indicating voxels entered by only one of the groups at one scan, and yellow indicating voxels entered by all three groups at both scans. (B) Location of the anterior termination of the estimated fibers in the medial superior frontal gyrus. (C) Location of the posterior termination of the estimated fibers in the left paracentral lobule.
To check for consistency with previous DTI studies of white matter abnormalities among poorer readers in a left temporo-parietal region (Beaulieu et al. 2005; Deutsch et al. 2005; Klingberg et al. 2000), we tested for group differences and a remediation effect in this region that had shown a relation to reading ability in these previous studies. Although there were no statistically reliable effects in the voxel-wise analyses, the FA was reliably lower among poor readers at the initial scan when the average FA across the entire region of interest was examined and the specific analysis was closely matched to those previous studies. A review of diffusion studies of this region (Ben-Shachar et al., 2007) suggests that the reduced FA among poorer readers is probably due to increased fiber crossings, and if this is indeed case, then intensive reading remediation would not be expected to change the coherence or the orientation of the fibers. Consistent with this expectation, there was no remediation effect in the region (See Supplementary Results and Discussion).
DISCUSSION
The finding of longitudinally-measured, experimentally-mediated changes in the structural properties of left hemisphere white matter in children with reading problems reveals the considerable potential of behavioral remediation and rehabilitation programs, and furthers the understanding of reading disability and brain plasticity. The most important finding is clearly that both reading ability and the structural integrity of left hemisphere white matter can be increased by extensive practice in word decoding skills. This finding suggests that whatever the cause of abnormally low FA among poor readers may be, the abnormality is amenable to behavioral treatment when provided within an age window in which maturation, experience, and development are still capable of influencing FA.
The precise microstructural properties underlying both the initial group differences in FA and radial diffusivity and the remediation-related changes in these measures may be identifiable by further research. Among the factors influencing radial diffusivity are myelination, axonal packing density, and axon diameter (Beaulieu, 2002). One reason that myelination is a particularly attractive potential mechanism for future exploration is that myelin is known to affect primarily radial diffusivity (Song et al., 2002; Song et al., 2005). In addition, neuronal firing has been shown to affect myelination in central nervous system axons (Demerens et al., 1996; Ishabashi et al., 2006; Stevens et al., 2002). Although it is unknown whether such a mechanism could increase myelination in humans at the ages examined in the current study, it is possible that intensive training in word-decoding skills increases the communication among left hemisphere cortical areas, which may in turn increase the myelination along the axons connecting these regions, decrease radial diffusivity along these axons, and increase FA. Methods exist for investigating this hypothesis concerning the role of myelination in the remediation effect using techniques such as magnetization transfer or T2 relaxation imaging for directly measuring myelin content.
It is tempting to ask about the causal directionality between the reading effects and the diffusion effects: does an increase in the efficiency of neural transmission resulting from remediation produce an increase in phonological decoding ability, or does increased phonological decoding ability produce increased reading behavior and consequent increases in the efficiency of the neural transmission? Both alternatives are possible, but it is also possible that the two types of changes develop interactively, as one might expect in a dynamic system like the brain. If the latter is the case, then it may be more fruitful to investigate factors that can accelerate or more finely control both the neuroplastic changes in white matter and the changes in reading processes, rather than attempting to determine the casual directionality.
The functional role in the reading process of the modified left anterior centrum semiovale white matter is not well understood, but it may pertain to the control processes of reading, rather than to word decoding itself. Activation in the left medial superior frontal gyrus occurs in normal children when processing orthographic and phonological forms of stimuli that are mutually inconsistent (Bitan et al., 2007), suggesting a response selection role for this area which may have been repeatedly evoked in the remedial phonological decoding tasks. The paracentral lobule has been found to activate more to phonologically dissimilar items than to similar items in a verbal memory task in adults (Sweet et al., 2008). Another control function associated with the paracentral lobule is as hub controller in the “structural core” of cortico-cortical axonal communication pathways (Hagmann et al., 2008), the nodes of which correspond to the “default mode” network (Raichle et al., 2001). It is possible that the repeated phonological processing in the remediation strengthened inhibitory connections between the paracentral lobule and medial frontal cortex, leading to reduced default network activity during reading. Although our findings do not illuminate the roles of the areas whose connectivity was improved, they nevertheless establish a structural change that could only have been brought about because of changes in activity in these areas or in secondarily connected areas.
The methodological question of how to accurately align the data from different participants for group analysis remains a topic of interest because of inherent limitations in regularizing unsystematic morphological variation. The limitations of the voxel-based approach used in the current study lie in its dependence on the accuracy of the co-registration algorithm and the amount of smoothing subsequently applied to the data to compensate for the inaccuracy (Jones et al. 2005; Smith et al., 2006). Recently-developed alternative methods that attempt to avoid these particular concerns (Lee et al., 2009; Oakes et al. 2007; Smith et al. 2006) merit further evaluation, which is beyond the scope of the present paper. To address these limitations of the voxel-based approach, we have demonstrated that the main conclusions of the present study are also supported by analyses that do not use spatial smoothing or parametric assumptions (see Supplemental Results and Discussion and Figures S1 and S2).
The capability to improve white matter provides a possible remediation not only for reading difficulty but also for other neurological abnormalities believed to be underpinned by deficits in anatomical connectivity, such as autism (Just et al., 2007). Although the basic computing power of the brain surely lies in individual neurons, it is only their collective action, made possible by white matter connectivity, that enables the multi-centered large-scale brain networks that characterize human thought. For this reason, modest modifications in white matter may enable major changes in cognitive ability.
EXPERIMENTAL PROCEDURES
Participants
Seventy-two participants were included in the analyses (35 poor readers that received the treatment, 12 poor readers that did not receive the treatment, and 25 good readers that did not receive the treatment). They were selected from a larger sample on the basis of their having provided functional and behavioral data used in an fMRI study of sentence comprehension (Meyler et al., 2007), and on their having artifact-free DTI data at both the pre-remediation and post-remediation phases. The children gave verbal informed consent in the presence of a parent or guardian, who gave signed informed consent. The children were paid for their participation. A parent questionnaire was used to verify that all participants met inclusion criteria. All protocols were approved by the University of Pittsburgh and Carnegie Mellon University Institutional Review Boards.
The participants were all right-handed, native English-speaking children, with normal vision and hearing. Children were excluded from the study if they had brain injury, sensory disorders, psychiatric disorders, attention deficit disorder, metal in their bodies, were on medication, or were claustrophobic. The poor readers were participants in the Power4Kids Reading Initiative, a randomized-trial field study of remedial instruction for children with reading difficulties varying in severity (Torgesen et al., 2006). Criteria for inclusion in the project were a score at or below the 30th percentile on the combination of the sight word efficiency and phonological decoding subtests of the Test of Word Reading Efficiency (Torgesen et al., 1999) during its initial administration, and a score at or above the 5th percentile on the Peabody Picture Vocabulary Test (Dunn and Dunn, 1997). The good readers (designated as average to above average by their teachers) were recruited from the same schools.
Remedial Instruction
The main goal of the neuroimaging was to determine whether there was a relation between reading improvement and changes in white matter (regardless of the focus of the various remedial instruction programs). The poor readers were randomly assigned to either a control condition that did not include remedial instruction or to one of four reading remedial reading programs: Corrective Reading (n = 9), Wilson Learning System (n = 9), Spell Read Phonological Auditory Training (n = 10), and Failure Free Reading (n = 7). All of these programs provided systematic and explicit instruction in word-level decoding skills. Failure Free Reading focuses on developing recognition of words by sight, while the other three programs emphasize phonemic decoding. Additional detail about the specific instructional approaches and how they were implemented can be found elsewhere (Meyler et al., 2008; Torgesen et al., 2006). The instruction was delivered five days per week for 50 minutes a day to groups of one to three students at a time, for a period of six months, providing a total of approximately 100 hours of intensive reading instruction.
Diffusion Tensor Imaging
Diffusion data were acquired on a 3T Siemens Allegra Scanner at the Brain Imaging Research Center of Carnegie Mellon and the University of Pittsburgh. A diffusion-weighted, double spin-echo, echo-planar imaging (EPI) sequence was used to reduce effects of eddy currents, with TR = 4400 ms, TE = 74 ms, bandwidth = 2,298 Hz/Voxel, FOV = 200 mm, and matrix size = 128 × 128. Thirty-six 3-mm thick slices were imaged (no slice gap) with no diffusion-weighting (b = 0 s/mm2) and with diffusion-weighting gradients applied in six orthogonal directions (b = 850 s/mm2). Twelve images of each slice by gradient direction (and b = 0) combination were acquired and averaged to produce the final diffusion imaging dataset for each participant. The FMRIB Diffusion Toolkit (v. 2.0, part of the FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl/) was used for motion and eddy current correction prior to analysis.
Data Analyses
Diffusion tensors and scalar diffusion parameter maps were calculated for each participant in native space using standard algorithms (Basser and Pierpaoli, 1996; Basser et al., 1994). For voxel-wise analyses, the diffusion tensor data were reduced to fractional anisotropy (FA) maps for each participant. For normalization of the DTI data to a standard space, a custom template was created from the T2-weighted b0 scans of all participants. SPM2 (Wellcome Department of Cognitive Neurology, London, UK) was used to first normalize each participant’s b0 volume to the Montreal Neurological Institute (MNI) EPI template using an affine transformation and 12 iterations of the default SPM2 non-linear normalization algorithm. These normalized T2-weighted images were then averaged across all participants in both reading ability groups to produce a new template customized for the ages and reading abilities of the sample. Each participant’s original, native-space b0 volume was then normalized to this new template using the same algorithm, and the transformation parameters for this normalization were applied to the participant’s FA map and the maps for axial diffusivity (λ1) and radial diffusivity (λ2+λ3/2). For most of the analyses, the normalized maps for the three DTI scalar measures were spatially smoothed with an 8-mm FWHM Gaussian filter to accommodate imprecision of the normalization procedure, to improve signal to noise ratio, and to satisfy assumptions of Gaussian random field theory. Each participant’s DTI data were masked on the basis of their individual FA map at a threshold of 0.2 in order to restrict the analyses to white matter.
Analyses of standardized test scores were carried out in SAS (v. 9.1) software using mixed-effects ANOVAs (PROC MIXED) and paired or two-sample t-tests, with corrections for multiple comparisons made by using a false discovery rate (Benjamini and Hochberg, 1995) of 5%, where appropriate (PROC MULTTEST). Voxel-wise statistical analyses of FA were carried out in SPM2 using the general linear model. Random-effects contrasts of FA data were carried out using Group (Good Reader Controls, Poor Reader Controls, and Remediated Poor Readers) as a between-subject variable and instructional Time (Pre-vs. Post-) as a within-subject variable. Reliable simple effects of Time within groups and Group within each time are reported for clusters of voxels exceeding a voxel-level threshold of p < .005 (uncorrected) and a cluster size threshold of p < .05, corrected for multiple corrections in the context of random Gaussian field theory as implemented in SPM2. Additional random effects multiple regression analyses were carried out within the pre-remediation and post-remediation phases of the experiment and for post-remediation minus pre-remediation difference images, with age and raw reading scores entered as continuous independent predictor variables. Voxel-wise non-parametric tests reported in the Supplemental Results and Discussion were carried out using the Randomise (v.1.2) tool included in version 4.1 of the FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl/) with 5,000 permutations and default neighbourhood connectivity parameters for the threshold-free cluster enhancement option for multiple comparison correction. FA and axial and radial diffusivity were also analyzed by extracting the scalar values from each subject for each voxel showing reliable effects on FA, and the values averaged across voxels were submitted to mixed-effects ANOVAs and multiple regression analyses (SAS PROC MIXED) and to stepwise hierarchical regression analyses (SAS PROC REG). Stepwise regressions were conducted using the default options of the REG procedure for variable entry and removal (p < .15 for both).
To produce an averaged diffusion tensor dataset for each group at each phase of the study, a 12-parameter affine transformation was computed between the b0 scan for each participant and the b0 template created above. This affine transformation was then applied separately to each component of the participant’s diffusion tensor dataset, the spatially-transformed components were recombined for each subject, and the eigenvectors of the resulting tensor data were reoriented using the preservation of principal directions (PPD) method (Alexander et al., 2001) as implemented in the Camino software package (Cook et al., 2006). The individual components were then averaged across participants within each group at each phase, and the resulting averaged and reoriented components were recombined to produce a group-averaged diffusion tensor dataset. Deterministic streamline fiber tracking of group-averaged diffusion tensor data was carried out using a modified version of the FACT algorithm (Mori et al., 1999) as implemented in Camino, using a curvature threshold of 70 degrees and a liberal anisotropy threshold of 0.05 to allow estimated fibers to penetrate gray matter in order to better characterize the possible cortical and subcortical regions connected by the estimated fibers. Tractography was seeded using the cluster showing a group by time interaction for the FA data at the pre-remediation phase shown in Figure 2A.
Supplementary Material
ACKNOWEDGMENTS
This research was supported by grants from the R.K. Mellon Foundation, the National Institute of Mental Health (Grant MH029617), and the William and Flora Hewlett Foundation. Participants were recruited through the Power4Kids program, which is a public-private partnership including the Haan Foundation for Children; Institute of Education Sciences, U.S. Department of Education; Heinz Endowments; Smith Richardson Foundation; W.K. Kellogg Foundation; Grable Foundation; Rockefeller Foundation; Ambrose Monell Foundation; Raymond Foundation; and Barksdale Reading Institute. We thank Anne Meyler for help with data collection and collation, Kwan-Jin Jung and Vladimir Cherkassky for technical assistance, Cindy Haan and Joe Torgesen for leadership of the Power4Kids program, and Donna Durno, Rosanne Javorsky, and the Allegheny Intermediate Unit for their central coordinating efforts throughout the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans. Med. Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Richards TL, Berninger VW, Nagy WE, Field KM, Grimme AC, Richards AL, Thomson JB, Cramer SC. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effect self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissue elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Curr. Opin. Neurobiol. 2007;17:258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B. Stat. Methodol. 1995;57:289–300. [Google Scholar]
- Bitan T, Burman DD, Chou T, Dong L, Cone NE, Cao F, Bigio JD, Booth JR. The interaction between orthographic and phonological information in children: An fMRI study. Hum. Brain Mapp. 2007;28:880–891. doi: 10.1002/hbm.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PA, Bai Y, Nedjati-Gilani S, Seunarine KK, Hall MG, Parker GJ, Alexander DC. Camino: Open-Source diffusion-MRI reconstruction and processing. Proc. Int. Soc. Magn. Reson. Med. 2006;14:2759. [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. USA. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JDE, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox G, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci U S A. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J. Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test-Revised. American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. Neuroimage. 2006;31:513–9. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, Whitfield-Gabrieli S, Gabrieli JD. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, Siok WT, Reiss AL, Whitfield-Gabrieli S, Gabrieli JD. Functional and morphometric dissociation between dyslexia and reading ability. Proc. Natl. Acad. Sci. USA. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc. Natl. Acad. Sci. USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishabashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. NeuroImage. 2005;26:546–54. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Saltz T, Gabrieli JDE, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Gabrieli JDD, Just MA. Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: A longitudinal study of neuroplasticity. Neuropsychologia. 2008;46:2580–2592. doi: 10.1016/j.neuropsychologia.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield-Gabrieli S, Gabrieli JD, Just MA. Brain activation during sentence comprehension among good and poor readers. Cereb. Cortex. 2007;17:2780–2787. doi: 10.1093/cercor/bhm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44:2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Farris EA, Ring J, McColl R, Black J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47:1972–7. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, Skudlarski P, Marchione KE, Jenner AR, Fletcher JM, Liberman AM, Shankweiler DP, Katz L, Lacadie C, Gore JC. The angular gyrus in developmental dyslexia: Task-specific differences in functional connectivity within posterior cortex. Psychol. Sci. 2000;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Schulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T, Stevenson J, Crouch J, Johnson LC, Maravilla K, Stock P, Abbott R, Berninger V. Tract-based spatial statistics of diffusion tensor imaging in adults with dyslexia. Am. J. Neuroradiol. 2008;29:1134–1139. doi: 10.3174/ajnr.A1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins NK, Vachha B, Srinivasan P, Chia J, Pickering J, Hughes CW, Gimi B. Simple developmental dyslexia in children: alterations in diffusion-tensor metrics of white matter tracts at 3 T. Radiology. 2009;251:882–91. doi: 10.1148/radiol.2513080884. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol. Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Bergman E, Papanicolaou AC. Cerebral mechanisms involved in word reading in dyslexic children: A magnetic source imaging approach. Cereb. Cortex. 2000a;10:809–816. doi: 10.1093/cercor/10.8.809. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Bergman E, Fishbeck K, Papanicolaou AC. Brain activation profiles in dyslexic children during non-word reading: a magnetic source imaging study. Neurosci. Lett. 2000b;290:61–65. doi: 10.1016/s0304-3940(00)01322-7. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Berier JI, Foorman BR, Castillo EM, Davis RN, Fitzgerald M, Papanicolaou AC. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: A neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet LH, Paskavitz JF, Haley AP, Gunstad JJ, Mulligan RC, Nyalakanti PK, Cohen RA. Imaging phonological similarity effects on verbal working memory. Neuropsychologia. 2008;46:1114–1123. doi: 10.1016/j.neuropsychologia.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JDE. Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proc. Natl. Acad. Sci.USA. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen JK, Myers D, Schirm ASE, Vartivarian S, Mansfield W, Stancavage F, Durno D, Javorsky R, Haan C. Volume II: National Assessment of Title I: Interim Report to Congress. Institute of Education Sciences; 2006. Closing the Reading Gap: First year findings from a randomized trial of four reading interventions for striving readers. [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency (TOWRE) Pro-ed; Austin, TX: 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.