SUMMARY
Axon regeneration failure accounts for permanent functional deficits following CNS injury in adult mammals. However, the underlying mechanisms remain elusive. In analyzing axon regeneration in different mutant mouse lines, we discovered that deletion of suppressor of cytokine signaling 3 (SOCS3), in adult retinal ganglion cells (RGCs), promotes robust regeneration of injured optic nerve axons. This regeneration-promoting effect is efficiently blocked in SOCS3-gp130 double knockout mice, suggesting that SOCS3 deletion promotes axon regeneration via a gp130-dependent pathway. Consistently, a transient up-regulation of ciliary neurotrophic factor (CNTF) was observed within the retina following optic nerve injury. Intravitreal application of CNTF further enhances axon regeneration from SOCS3-deleted RGCs. Together, our results suggest that compromised responsiveness to injury-induced growth factors in mature neurons contributes significantly to regeneration failure. Thus, developing strategies to modulate negative signaling regulators may be an efficient strategy of promoting axon regeneration after CNS injury.
INTRODUCTION
A salient feature of axon regeneration in mammals is the drastic difference between the peripheral nervous system (PNS) versus the central nervous system (CNS). In contrast to robust axon regeneration in the PNS neurons, regenerative growth in the adult CNS is minimal. Previous studies have been aimed at characterizing environmental inhibitory molecules in the adult CNS (reviewed by (Harel and Strittmatter, 2006; Schwab and Bartholdi, 1996; Yiu and He, 2006). While several myelin associated molecules and chondroitin sulfate proteoglycans (CSPGs) in the glial scar have been implicated as inhibitors of axon regeneration (Filbin, 2006; Fitch and Silver, 2008), blockade of these inhibitory signals results in limited axon regeneration (Case and Tessier-Lavigne, 2005).
An alternate explanation is that the adult PNS and CNS neurons differ in intrinsic growth ability (Goldberg and Barres, 2000; Zhou and Snider, 2006). Current hypotheses suggest that adult CNS neurons lose axonal growth ability. Several potential players have been implicated, such as development-dependent decline of neuronal cAMP levels (Cai et al., 2001) or Bcl-2 (Cho et al., 2005). Goldberg et al (2002) found a dramatic decrease in axonal growth rates of dissociated RGCs at the neonatal stage and suggested that this may be triggered by signals from amacrine cells. However, the molecular nature of such signals still remains unclear. Furthermore, other mechanisms might be involved in the transition from the rapid growth mode of immature neurons to the poor growth mode of mature CNS neurons.
In an effort to identify molecular pathways that limit the intrinsic regenerative capacity of adult RGCs, we discovered that deletion of phosphatase and tensin homolog (PTEN) or tuberous sclerosis protein 1 (TSC1), two negative regulators of the mammalian target of rapamycin (mTOR) pathway, in adult RGCs promotes robust axon regeneration following optic nerve injury (Park et al., 2008). We also found that axotomy triggers a dramatic down-regulation of mTOR activity in adult RGCs. Given the well-established role of mTOR in regulating cap-dependent protein translation (Ma and Blenis, 2009), our results indicate that the diminished ability to synthesize new proteins contributes significantly to the failure of axon regeneration in adult RGCs. However, despite increased mTOR activity, PTEN or TSC1 deleted RGCs initiate axon regrowth only after axonal injury, suggesting that injury-induced signals may be required to activate the axonal regeneration program.
What injury-triggered signals can promote axon regeneration? Studies in the PNS implicate several mechanisms, including injury-induced cytokines and growth factors (Zigmond et al., 1996), local protein synthesis and increased axonal transport of signaling components (Hanz et al., 2003). For example, sciatic nerve lesion promotes expression of interleukin 6 (IL6) in axotomized sensory neurons and/or Schwann cells in the lesion sites, subsequently activating the JAK/STAT pathway in injured sensory neurons (Cafferty et al., 2001; Cao et al., 2006). Activation of this pathway correlates with enhanced axonal regenerative responses in DRG neurons (Miao et al., 2006). While IL6 can increase axonal growth in vitro and in vivo (Cafferty et al., 2001; Cao et al., 2006), IL6 knockout mice show normal axon regeneration (Cao et al., 2006). Thus, how this and other related pathways are involved in regulating axon regeneration in the PNS remains unclear. A further question is whether these injury signals exist in the CNS. In this study, we present genetic results demonstrating a role of the gp130-dependent pathway in promoting axon regeneration in adult RGCs.
RESULTS
SOCS3 deletion promotes optic nerve regeneration in adult mice
To identify molecular pathways critical to axon regeneration, we applied optic nerve crush injury to assess axon regeneration in conditional floxed mice (Park et al., 2008). Intravitreal application of recombinant adeno-associated viruses expressing Cre (AAV-Cre) results in Cre-dependent reporter expression in more than 90% of RGCs and thus was used to delete floxed genes in mature RGCs in vivo. Regeneration of injured optic nerve axons was traced by injecting cholera toxin beta subunit (CTB), an anterograde tracer, into the vitreous of the retina. Neuronal survival was assessed by immunostaining the whole-mount sections with an anti-β-III tubulin antibody.
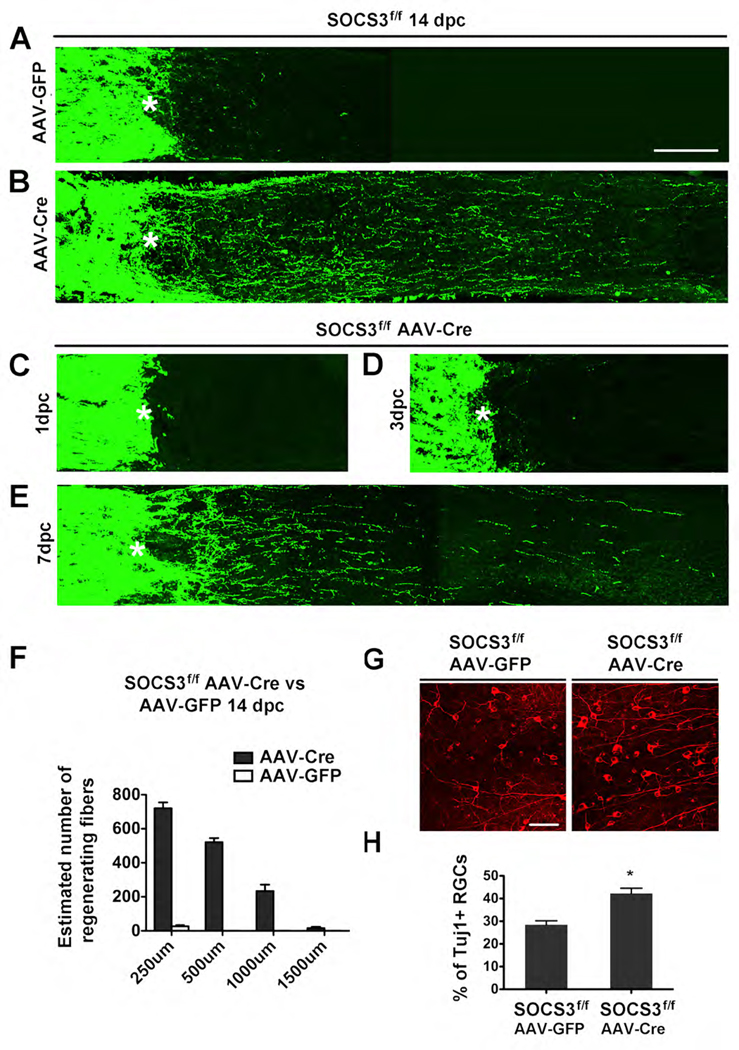
We observed significant axon regeneration in SOCS3 conditional knockout mice. In AAV-GFP (control) treated SOCS3f/f mice (Mori et al., 2004), a few axonal sprouts could be found close to the crush site, but none extended distally beyond 500 µm (Figures 1A and 1F). However, AAV-Cre treated SOCS3f/f mice exhibited significant enhancement in the number of regenerating axons beyond the crush site (Figure 1B and 1F). Cell viability analysis revealed significant increase of neuronal survival following optic nerve crush in SOCS3 deleted mice (Figure 1G–1H). A similar regeneration phenotype was seen in the SOCS3f/f mice crossed with a Thy1-Cre line, where Cre expression starts from embryonic stages (Dewachter et al., 2002) (data not shown). Taken together, our results suggest that SOCS3 deletion in RGCs promotes both neuronal survival and axon regeneration following optic crush injury.
Figure 1. SOCS3 deletion promotes RGC axon regeneration.
(A–E) Confocal images of optic nerves showing CTB-labeled axons around the lesion sites at 14 days (A and B), 1 day (C), 3 day (D), 7 days (E) post crush injury (dpc) from SOCS3f/f mice injected with AAV-GFP (A) or AAV-Cre (B–E). *: crush site. Scale bar: 100 µm.
(F) Quantification of regenerating axons at different distances distal to the lesion sites at 14 days after crush injury. At least 5 different sections (every 4th section) from each animal were quantified. At 14 dpc, there were significant differences between control and SOCS3-deleted mice groups (ANOVA with Bonferroni’s post-test, p < 0.05 for each distance, 8 animals in each group).
(G) Fluorescent photomicrographs of retinal whole-mounts showing surviving TUJ1+ RGCs at 14 days after injury. Scale bar: 50 µm.
(H) Quantification of RGC survival at 14 dpc, expressed as a percentage of the total number of TUJ1+ RGCs in the contralateral (intact) retina. (n = 8 for each group). For each retina, 15–20 fields were chosen from different parts of the retina. The total viable RGC number was obtained by multiplying the average number per field of TUJ1+ cells in the ganglion cell layer by the retinal area. *: p < 0.01, Dunnett’s test.
To examine the temporal effects of SOCS3 deletion, we assessed axon regeneration in SOCS3 conditional knockout mice at different post-injury time points. At 1 day post crush (1 dpc), CTB labeled axons stopped at the crush site, with no labeled fibers found distal to the lesion site (Figure 1C). At 3 dpc, a few short sprouts could be seen close to the lesion site (Figure 1D). However, at 7 dpc, more regenerating axons were observed beyond the crush site (Figure 1E). The AAV-GFP injected control animals showed no axons beyond the lesion site at any time point (data not shown). This differs from the observation of numerous axonal sprouts seen at 3 dpc in PTEN deleted mice (Park et al., 2008), suggesting that the majority of axon regeneration in SOCS3 deleted RGCs starts between 3–7 days post-injury. Glial scar responses were similar in both control and SOCS3 deleted mice (Figure S1), suggesting that SOCS3 deletion in RGCs did not affect glial responses in the lesion site.
Alteration of mTOR activity in SOCS3-deleted RGCs after injury
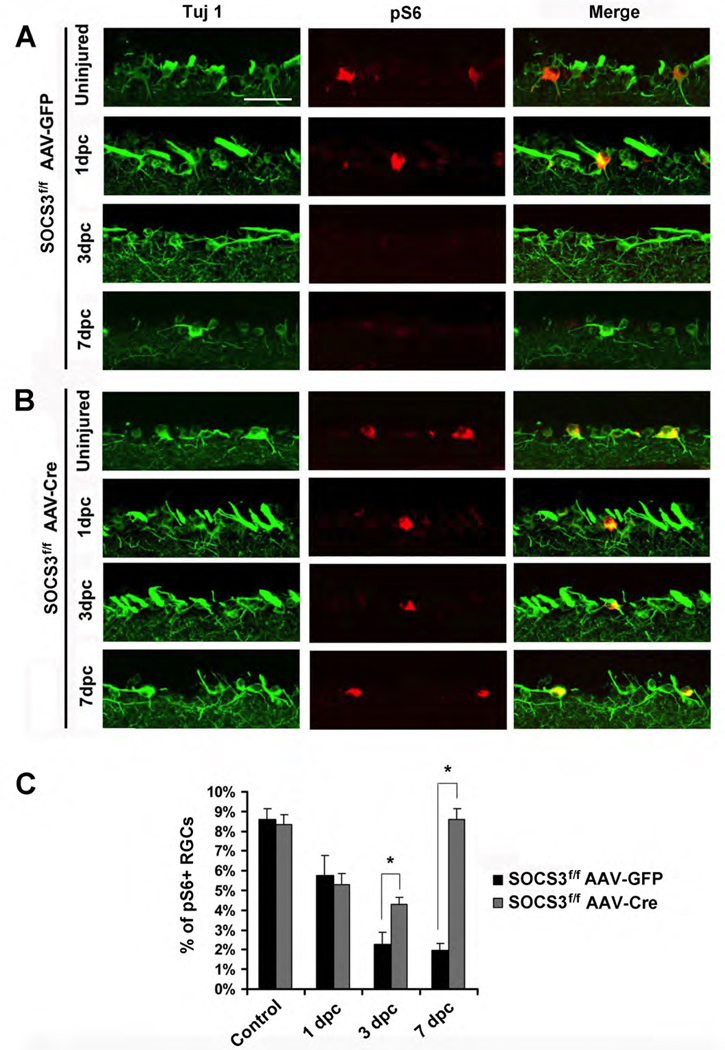
Our previous studies suggested that mTOR activity is an important indicator of axon regenerative ability in RGCs (Park et al., 2008). We therefore assessed the regulation of mTOR activity in SOCS3-deleted RGCs following optic nerve injury by immunohistochemical staining of anti-phospho-S6 (p-S6) antibody, a marker of the mTOR activity (Park et al., 2008). In un-injured SOCS3f/f mice injected with either AAV-GFP (Figures 2A and 2C) or AAV-Cre (Figures 2B and 2C), similar percentages (~8–10%) of RGCs showed detectable p-S6 signals, implying that SOCS3 deletion does not affect the basal levels of mTOR activity of RGCs. As previously reported (Park et al., 2008), p-S6 levels are significantly reduced following optic nerve crush injury. In SOCS3f/f mice, p-S6 signal is reduced at 1 dpc in both AAV-GFP and AAV-Cre treated mice (Figure 2), suggesting that SOCS3 deletion does not alter axotomy-triggered mTOR down-regulation in the early post-injury stage.
Figure 2. Phospho-S6 levels in RGCs of SOCS3f/f mice with AAV-GFP or AAV-Cre after optic nerve injury.
(A, B) Immunofluorescence analysis with anti-p-S6 or TUJ-1 antibodies of the retinal sections from SOCS3f/f mice injected with AAV-GFP (A) or AAV-Cre (B) at different time points post-crush. Scale bar, 50 µm.
(C) Quantification of p-S6+ RGCs. Data is presented as mean percentages of p-S6+and TUJ1+ cells among total TUJ1+ cells in the ganglion cell layer of each retina. Cell counts were performed on at least 4 non-consecutive sections for each animal, from four or five mice per group. *: p<0.01 by Dunnett’s test.
Significant differences in these groups appear at later time points. In the control group (SOCS3f/f with AAV-GFP), the percentages of p-S6-positive RGCs continue to decrease at 7 dpc (Figures 2A and 2C). However, SOCS3 deleted mice (SOCS3f/f with AAV-Cre) showed a significant recovery in the percentage of p-S6-positive RGCs starting at 3 dpc (Figures 2B and 2C). By 7 dpc, coinciding with the appearance of larger numbers of regenerating axons (Figures 1E and 1G), these SOCS3 deleted mice had percentages (approximately 8%) of p-S6 positive RGCs approaching levels observed before injury (Figures 2B and 2C). Thus, despite an initial injury-induced mTOR down-regulation, SOCS3-deleted RGCs recover mTOR activity at late time points. Since SOCS3 is a negative regulator of signaling pathways in response to cytokines and perhaps other growth factors (Croker et al., 2008), SOCS3 deletion may allow RGCs to respond to injury-triggered factors, resulting in restoration of mTOR activity.
gp130 deletion abolishes axon regeneration-promoting effect of SOCS3 knock-out
SOCS3 was initially identified as a negative regulator of the JAK/STAT signaling pathway triggered by gp130 ligands such as interleukin-6, CNTF, and cardiotrophin-1 (Croker et al., 2008, Pennica et al., 1996). Most adult RGCs express both SOCS3 and gp130 (Figure S2). We attempted to examine the activation of gp130-dependent pathways by immunohistochemical methods but failed to detect tyrosine phosphorylated STAT3 with available antibodies. This might be due to low abundance of tyrosine phosphorylated STAT3 protein within adult RGCs, as the same antibodies could detect specific signals in axotomized DRG neurons (data not shown).
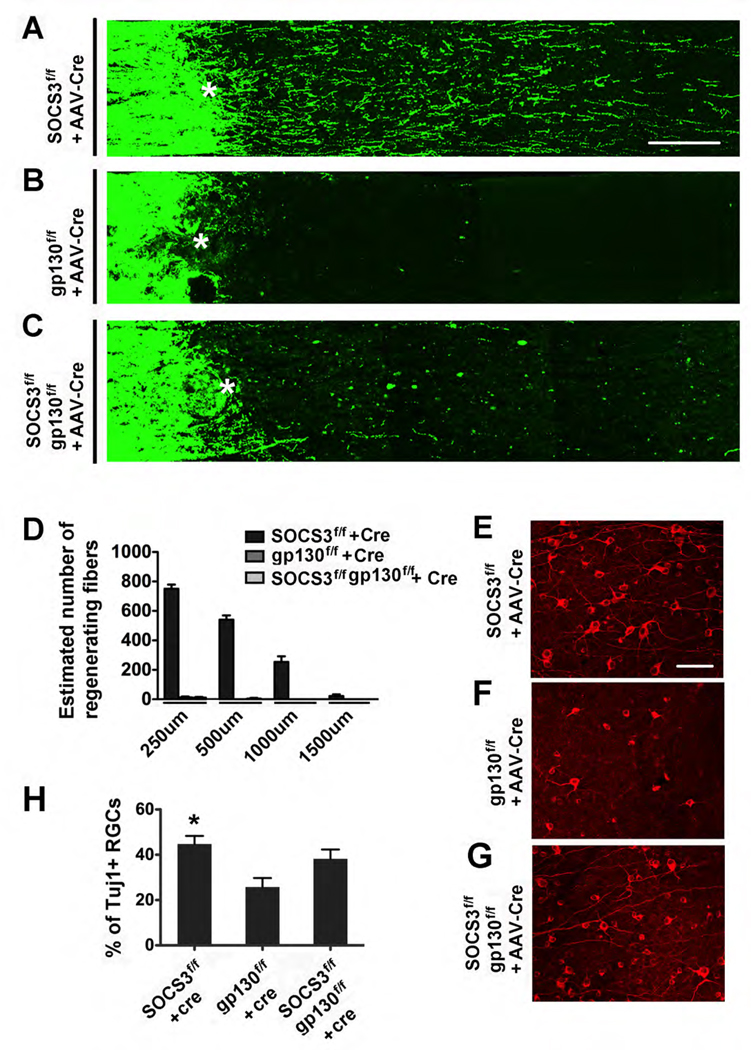
To directly test the contribution of gp130-dependent pathways to the effects of SOCS3 deletion on axon regeneration, we crossed gp130f/f mice (Betz et al., 1998) and SOCS3f/f mice to generate mutants carrying two floxed genes. The double mutants received intravitreal viral injection to induce the deletion of both genes in RGCs. As expected, SOCS3 deletion resulted in increased neuronal survival and axon regeneration (Figures 3A, 3D, 3E and 3H). However, gp130 knockout did not alter the survival of injured RGCs or regeneration of injured optic nerve axons (Figures 3B, 3D, 3F and 3H). Interestingly, double knockout of SOCS3 and gp130 revealed a significant loss of the axon regeneration observed in SOCS3-deleted mice (Figures 3C and 3D), suggesting that axon regeneration induced by SOCS3 deletion is largely dependent upon gp130-mediated signaling.
Figure 3. gp130 co-deletion abolishes axon regeneration effects of SOCS3 knockout.
(A–C) Confocal images of optic nerves showing CTB-labeled axons around the lesion sites at 14 days post crush injury (dpc) from SOCS3f/f mice (A), gp130f/f mice (B), or SOCS3f/f and gp130f/f mice (C) injected with AAV-Cre. *: crush site. Scale bar: 100 µm.
(D) Quantification of regenerating axons at different distances distal to the lesion sites at 14 days after crush injury. At least 5 different sections (every 4th section) from each animal were quantified. There were significant differences between the SOCS3f/f group and the other two groups (ANOVA with Bonferroni’s post-tes,t p < 0.05 for each distance, 8 animals used in each groups).
(E–G) Fluorescent photomicrographs of retinal whole-mounts showing surviving TUJ1+ RGCs at 14 days after injury in AAV-Cre-injected SOCS3f/f (E), gp130f/f (F) or SOCS3f/f and gp130f/f (G) mice. Scale bar: 50 µm.
(H) Quantification of RGC survival at 14 dpc expressed as a percentage of the total number of TUJ1+ RGCs in the contralateral (intact) retina (n = 8 for each group). For each retina, 15–20 fields were chosen from different parts of the retina. The total viable RGC number was obtained by multiplying the average number per field of TUJ1+ cells in the ganglion cell layer by the retinal area. There is a significant difference between the SOCS3f/f group and gp130f/f group, but not between SOCS3f/f group and SOCS3f/f/gp130f/f group (p < 0.05, ANOVA with post-hoc Tukey’s test).
Surprisingly, neuronal survival in the double mutants is higher than controls (SOCS3f/f mice with AAV-GFP) or gp130f/f with AAV-Cre (Figures 3G and 3H), suggesting a contribution of gp130-independent pathways to the effects of SOCS3 deletion on neuronal survival. Thus, our results indicate that while gp130-dependent pathways are required for axonal regeneration, both gp130-dependent and gp130-independent pathways might be involved in neuronal survival effects of SOCS3 deletion.
Transient up-regulation of CNTF in retina after optic nerve injury
We next investigated the possible identity of gp130-dependent ligands that could be responsible for promoting axon regeneration following injury in SOCS3 deleted RGCs. These ligands could be derived from either glial cells in the lesion site and/or other cells within the retina. To assess these possibilities, we used an explant cultures. AAV-Cre or AAV-GFP was injected to the vitreous body of SOCS3f/f mice at the age of 4 weeks and the retina was dissected 14 days post-injection. In the explants from the SOCS3f/f mice with AAV-GFP, only a few short sprouts grew out (Figure S3A and S3C). However, in the explants derived from SOCS3f/f mice with AAV-Cre, a dramatic increase in the number of neurites extending from the explants was observed (Figure S2B and S2C). These SOCS3-deleted retinal explants show a dramatic increase in the number of neuritis in the absence of the injured optic nerve tissues, supporting the possibility that retina-derived ligands act on the gp130 and its co-receptors on RGCs.
Previous studies suggest a possible up-regulation of CNTF following injury (Muller et al., 2007; Park et al., 2009). We therefore examined by in situ hybridization the levels of CNTF, IL-6, and cardiotrophin-1 following optic nerve injury. We observed a modest but consistent increase of CNTF mRNA in the ganglion cell layer of the mice at 6 hr after optic nerve injury (Figure S4B), while the expression levels of IL6 and CT-1 are not altered (Figure S4E, S4F and data not shown). However, we cannot distinguish the identity of the retinal cell types expressing CNTF. The up-regulation of CNTF expression appears to be transient in nature, since it returns to control levels after 24 hours post injury (Figure S4D). In line with this, a mild increase of SOCS3 mRNA signal was also transiently increased (data not shown). These results suggest that injury-induced up-regulation of CNTF and perhaps other gp130-dependent cytokines may account for the effects of SOCS3 deletion on axon regeneration.
CNTF enhances the axon regeneration promoting effect of SOCS3 knockout
Our results suggest that SOCS deletion allows mature RGCs to gain responsiveness to injury-induced cytokines. If this is the case, exogenously provided CNTF should further enhance axon regeneration from SOCS3-deleted RGCs. To test this, we intravitreously injected 1 µl CNTF immediately before injury and 3 days after injury. PBS injected control animals (SOCS3f/f with AAV-GFP) showed no sign of axon regeneration (Figures 4A, 4E, and 4F), while CNTF showed modest axon regeneration in these control mice (Figures 4B, 4E, and 4F), consistent with previous reports (Muller et al., 2009, Leaver et al., 2006). SOCS3 knockout mice, treated with PBS, showed a similar regeneration effect to that observed in non-injected animals (Figures 4C, 4E and 4F). In contrast, CNTF injection resulted in a dramatic increase of axon regeneration in SOCS3 deleted mice (Figures 4D, 4E and 4F). The numbers and lengths of regenerating axons are comparable to that seen after PTEN deletion (Park et al., 2008). Thus, exogenous CNTF further enhances axon regeneration from SOCS-deleted RGCs following optic nerve injury.
Figure 4. Enhancement of axon regeneration in SOCS3-deleted mice by CNTF.
(A–D) Confocal images of optic nerves showing CTB-labeled axons around the lesion ites at 14 days post crush injury (dpc) from SOCS3f/f mice with AAV-GFP (A, B) or AV-Cre (C, D) and subsequent injection of PBS (A, C) or CNTF (B, D). *: crush site. Scale bar: 100 µm.
(E, F) Quantification of regenerating axons at different distances (250–1000 µm in E, and 1500–2500 µm in F) distal to the lesion sites at 14 days after crush injury. At least 5 different sections (every 4th section) from each animal were quantified. There were significant differences between the SOCS3f/f with CNTF group and the other three groups (ANOVA with Bonferroni’s post-test, p < 0.05 for each distance, 3 animals in each group).
DISCUSSION
In this study, we show that SOCS3 deletion in RGCs promotes dramatic axon regeneration following optic nerve injury in a gp130-dependent manner. Importantly, exogenously delivered CNTF could further enhance the extent of axon regeneration in SOCS3 deleted mice. Thus, reduced responsiveness to injury-induced growth promoting factors may be an important limiting factor for successful axon regeneration in mature CNS neurons. These findings also provide a plausible explanation for previous observations that exogenously delivered cytokines have limited effects on promoting survival and regeneration following optic nerve injury (Cui et al., 1999; Watanabe et al., 2003) or spinal cord injury (Lacroix et al., 2002).
In the adult CNS, the receptors and signaling molecules that mediate the activity of CNTF and other cytokines are highly expressed in glial cells (Schobitz et al., 1992). Upon injury, up-regulated cytokines act as pro-inflammatory agents as a part of a defense mechanism, but excessive or prolonged activation could be detrimental to the CNS. SOCS3 family members, which are rapidly up-regulated by cytokines (Croker et al., 2008), are crucial in executing tight control of the strength and duration of these cytokine-triggered signaling pathways. Thus, it is possible that limited regenerative responses of RGCs to injury-induced CNTF could be, at least partially, attributed to this characteristic tight regulatory mechanism of the JAK/STAT pathway.
Previous studies in culture showed that a specific JAK2 inhibitor does not affect the axonal growth of embryonic DRG neurons but could efficiently block axonal elongation of adult sensory neurons after a conditioning lesion (Liu and Snider, 2001, Neumann and Woolf, 1999). Similarly, motoneuron-specific deletion of STAT3 has no significant effect on the survival of embryonic motoneurons, but results in increased death of motoneurons after facial nerve injury (Schweizer et al., 2002). Together with our findings, these studies suggest that gp130-dependent signaling may be critical for injury-induced responses such as neuronal survival and axon regeneration. In this regard, lens injury has been shown to promote axon regeneration after optic nerve injury (Fischer et al., 2000; Leon et al., 2000) and a combinatorial treatment of lens injury and cAMP agonists results in robust axon regeneration (Muller et al., 2007). However, the contribution of JAK/STAT pathway to these effects remains to be determined (Cui et al., 2008).
How are cytokine-triggered pathways involved in controlling neuronal survival and axon regeneration? In SOCS3 deleted RGCs, mTOR is initially down-regulated but recovers at later post-injury points, in contrast to the rapid and persistent suppression in injured RGCs in the control mice (Park et al., 2008). These mTOR alterations correlate with the extent and time course of axon regeneration observed in both PTEN and SOCS-deleted RGCs. These results support a model in which mTOR determines the competence of regeneration in RGCs, while SOCS3-regulated pathways act as an injury induced signal that turns on the regenerative program in injured RGCs. The dynamics of mTOR activity implies that augmented and prolonged signaling events as a result of SOCS3 deletion could allow injured neurons to restore mTOR activity and regain axonal growth ability. Previous studies suggest the involvement of several signaling pathways, such as PI3K/akt, MAPK/ ERK, or JAK/STAT, in CNTF-triggered effects on neuronal survival after injury (Park et al., 2004). Thus, cytokine-triggered pathways may directly initiate an axon regrowth program via these signaling pathways. On the other hand, gp130-dependent signaling is required for axotomy-triggered expression of neuropeptides such as pituitary adenylate cyclase activating polypeptide (Habecker et al., 2009). Because these neuropeptides have been shown to promote axon growth, they might indirectly contribute to the survival and regeneration effects observed in SOCS3-deleted mice.
Interestingly, while gp130 deletion abolishes the axon regeneration promoting effect of SOCS3 knockout, a partial neuronal survival effect is preserved in SOCS3 and gp130 double mutants. In this regard, it has been previously shown that in cultured non-neuronal cells, tyrosine phosphorylation of SOCS3 could be induced by gp130-independent growth factors such as interleukin-2 and erythropoietin. In addition, tyrosine-phosphorylated SOCS3 not only inhibits STAT activation but also binds to p120 RasGAP and activates Ras (Cacalano et al., 2001). Given the established role of Ras in neuronal survival (Reichardt, 2006), it will be interesting to determine whether this or other mechanisms mediate the gp130-independent survival effect of SOCS3 deletion.
Together with our previous finding of robust axon regeneration in RGCs with deletion of PTEN or TSC1 (Park et al., 2008), our current results reveal a critical limiting mechanism of CNS axon regeneration: reduced or lost neuronal responses to growth-promoting factors. Thus, targeting negative regulators of signaling pathways of cytokine and growth factors to enhance neuronal responses to growth-promoting factors may represent a potential therapeutic strategy for promoting axon regeneration following CNS injury in the adult. Future studies will be geared towards determining whether these regenerating axons are able to reconnect with their targets and assessing whether other types of CNS neurons are sensitive to the manipulations of gp130-dependent or independent signaling pathways.
EXPERIMENTAL PROCEDURES
Mice, intravitreal injection and optic nerve injury
All experimental procedures were performed in compliance with animal protocols approved by the IACUC at Children's Hospital, Boston. C57BL6/J mice or various floxed mice (aged p21) including SOCS3f/f and/ or gp130f/f were injected intravitreally with 1 µl volume of AAV-GFP or AAV-Cre (titers at 0.5–1.0 × 1012) or CNTF (Peprotech, 1 ug/ml). Mice were anaesthetized with ketamine and xylazine. For each intravitreal injection, the micropipette was inserted in peripheral retina, just behind the ora serrata, and was angled to avoid damage to the lens. Two weeks after injection, the left ON was exposed intraorbitally and crushed with jeweler’s forceps (Dumont #5; Roboz) for 5 seconds approximately 1 mm behind the optic disc. To preserve the retinal blood supply, care was taken not to damage the underlying ophthalmic artery. Mice received a subcutaneous injection of buprenorphine as post-operative analgesic. Eye ointment containing atropine sulphate was applied preoperatively to protect the cornea during surgery. Complete optic nerve transection was verified by demonstrating that both retrograde and anterograde tracers fail to reach the retina or the superior colliculus, respectively, after lesion (Park et al., 2008). Preparation of AAVs was described in Park et al., 2008.
RGC axon anterograde labeling
For anterograde labeling of RGC axons, l µl of cholera toxin β subunit (CTB) (2 µg/µl, Invitrogen) was injected into the vitreous with a Hamilton syringe. Animals were given a lethal overdose of anesthesia and perfused with 4% PFA. For the animals with an optic nerve injury, eyes with the nerve segment still attached were dissected out and post-fixed in the same fixative overnight at 4°C. Tissues were cryoprotected through increasing concentrations of optimal cutting temperature compound (Tissue Tek). Eyes were snap-frozen in dry ice and serial cross-sections (16 µm) were cut and stored at −20°C. Optic nerves were cut longitudinally (8 µm).
Regenerating RGC axons in injured optic nerves distal to the crush site were quantified as described previously (Leon et al., 2000, Park et al., 2008). The number of CTB labeled axons was estimated by counting the number of CTB- labeled fibers extending different distances from the end of the crush site in 5 sections (every 4th section) per animal. The cross-sectional width of the nerve was measured at the point at which the counts were taken and was used to calculate the number of axons per millimeter of nerve width. The number of axons per millimeter was averaged over all sections. Σad, the total number of axons extending distance d in a nerve having a radius of r, was estimated by summing over all sections having a thickness t (8 µm): Σad = πr2 × [average axons/mm]/t
Retina explant culture
4 week-old SOCS3f/f mice were intravitreally injected with AAV-Cre as the treated group, or AAV-GFP as the control group (n=4 each group). Retinas were isolated after 14 days. Retinas were then cut into 8 pieces radially and cultured in laminin-poly-D-lysine coated dishes. Culture medium was Neurobasal-A with 5% B27 supplement (GIBCO), penicillin/streptomycin (GIBCO), and glutamine (GIBCO). Explants were maintained for 4 days and then fixed with 4% PFA. Outgrowing neurites were then stained with anti-TUJ1 antibody. For each animal, fluorescent images of 3 or 4 pieces of the explants were taken (100x, Nikon). With Metamorph software (Molecular Devices), the edge of the explant was outlined and areas at 0–40 um, 40–80 um, and 80–120 um distal to the edge were defined all the way around the explant. The numbers of neurites were counted in the three zones and averaged over the 3–4 explants for each animal. The differences between the two groups were analyzed using t-test.
Details for the immunohistochemistry and in situ hybridization experiments are provided in the Supplemental Materials.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. K. Rajewsky for providing gp130f/f mice, A. Yoshimura for providing SOCS3f/f mice. This study was supported by grants from the Canadian Institutes of Health Research (to PDS), Craig Nelson Foundation (to KKP), NINDS and Adelson Medical Research Foundation (to ZH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Betz UA, Bloch W, van den Broek M, Yoshida K, Taga T, Kishimoto T, Addicks K, Rajewsky K, Muller W. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J Exp Med. 1998;188:1955–1965. doi: 10.1084/jem.188.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacalano NA, Sanden D, Johnston JA. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat Cell Biol. 2001;3:460–465. doi: 10.1038/35074525. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Gardiner NJ, Gavazzi I, Powell J, McMahon SB, Heath JK, Munson J, Cohen J, Thompson SW. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J Neurosci. 2001;21:7161–7170. doi: 10.1523/JNEUROSCI.21-18-07161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, Filbin MT. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci. 2006;26:5565–5573. doi: 10.1523/JNEUROSCI.0815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LC, Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr Biol. 2005;15:R749–R753. doi: 10.1016/j.cub.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Cho KS, Yang L, Lu B, Feng Ma H, Huang X, Pekny M, Chen DF. Re-establishing the regenerative potential of central nervous system axons in postnatal mice. J Cell Sci. 2005;118:863–872. doi: 10.1242/jcs.01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker BA, Mielke LA, Wormald S, Metcalf D, Kiu H, Alexander WS, Hilton DJ, Roberts AW. Socs3 maintains the specificity of biological responses to cytokine signals during granulocyte and macrophage differentiation. Exp Hematol. 2008;36:786–798. doi: 10.1016/j.exphem.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Benowitz L, Yin Y. Does CNTF mediate the effect of intraocular inflammation on optic nerve regeneration? Brain. 2008;131:e96. doi: 10.1093/brain/awn027. author reply e97. [DOI] [PubMed] [Google Scholar]
- Cui Q, Lu Q, So KF, Yip HK. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci. 1999;40:760–766. [PubMed] [Google Scholar]
- Dewachter I, Reverse D, Caluwaerts N, Ris L, Kuiperi C, Van den Haute C, Spittaels K, Umans L, Serneels L, Thiry E, et al. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. J Neurosci. 2002;22:3445–3453. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006;361:1565–1574. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Pavlidis M, Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci. 2000;41:3943–3954. [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Barres BA. The relationship between neuronal survival and regeneration. Annu Rev Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Sachs HH, Rohrer H, Zigmond RE. The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev Neurobiol. 2009;69:392–400. doi: 10.1002/dneu.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix S, Chang L, Rose-John S, Tuszynski MH. Delivery of hyper-interleukin-6 to the injured spinal cord increases neutrophil and macrophage infiltration and inhibits axonal growth. J Comp Neurol. 2002;454:213–228. doi: 10.1002/cne.10407. [DOI] [PubMed] [Google Scholar]
- Leaver SG, Cui Q, Bernard O, Harvey AR. Cooperative effects of bcl-2 and AAV-mediated expression of CNTF on retinal ganglion cell survival and axonal regeneration in adult transgenic mice. Eur J Neurosci. 2006;24:3323–3332. doi: 10.1111/j.1460-9568.2006.05230.x. [DOI] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Snider WD. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J Neurosci. 2001;21:RC164. doi: 10.1523/JNEUROSCI.21-17-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Miao T, Wu D, Zhang Y, Bo X, Subang MC, Wang P, Richardson PM. Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J Neurosci. 2006;26:9512–9519. doi: 10.1523/JNEUROSCI.2160-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- Muller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130:3308–3320. doi: 10.1093/brain/awm257. [DOI] [PubMed] [Google Scholar]
- Muller A, Hauk TG, Leibinger M, Marienfeld R, Fischer D. Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol Cell Neurosci. 2009;41:233–246. doi: 10.1016/j.mcn.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Park K, Luo JM, Hisheh S, Harvey AR, Cui Q. Cellular mechanisms associated with spontaneous and ciliary neurotrophic factor-cAMP-induced survival and axonal regeneration of adult retinal ganglion cells. J Neurosci. 2004;24:10806–10815. doi: 10.1523/JNEUROSCI.3532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Hu Y, Muhling J, Pollett MA, Dallimore EJ, Turnley AM, Cui Q, Harvey AR. Cytokine-induced SOCS expression is inhibited by cAMP analogue: impact on regeneration in injured retina. Mol Cell Neurosci. 2009;41:313–324. doi: 10.1016/j.mcn.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D, Arce V, Swanson TA, Vejsada R, Pollock RA, Armanini M, Dudley K, Phillips HS, Rosenthal A, Kato AC, Henderson CE. Cardiotrophin-1, a cytokine present in embryonic muscle, supports long-term survival of spinal motoneurons. Neuron. 1996;17:63–74. doi: 10.1016/s0896-6273(00)80281-0. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobitz B, Holsboer F, Kikkert R, Sutanto W, De Kloet ER. Peripheral and central regulation of IL-6 gene expression in endotoxin-treated rats. Endocr Regul. 1992;26:103–109. [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Schweizer U, Gunnersen J, Karch C, Wiese S, Holtmann B, Takeda K, Akira S, Sendtner M. Conditional gene ablation of Stat3 reveals differential signaling requirements for survival of motoneurons during development and after nerve injury in the adult. J Cell Biol. 2002;156:287–297. doi: 10.1083/jcb.200107009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003;116:733–742. doi: 10.1016/s0306-4522(02)00562-6. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Snider WD. Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci. 2006;361:1575–1592. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE, Hyatt-Sachs H, Mohney RP, Schreiber RC, Shadiack AM, Sun Y, Vaccariello SA. Changes in neuropeptide phenotype after axotomy of adult peripheral neurons and the role of leukemia inhibitory factor. Perspect Dev Neurobiol. 1996;4:75–90. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.