Abstract
During the development of the inner ear, the Notch cell signaling pathway is responsible for the specification of the pro-sensory domain and influences cell fate decisions. It is assumed that Notch signaling ends during maturity and cannot be reinitiated to alter the fate of new or existing cells in the organ of Corti. This is in contrast to non-mammalian species which reinitiate Delta1-Notch1 signaling in response to trauma in the auditory epithelium, resulting in hair cell regeneration through transdifferentiation and/or mitosis. We report immunohistochemical data and Western protein analysis showing that in the aminoglycoside-damaged guinea pig organ of Corti, there is an increase in proteins involved in Notch activation occurring within 24 hours of a chemical hair cell lesion. The signaling response is characterized by the increased presence of Jagged1 ligand in pillar and Deiters cells, Notch1 signal in surviving supporting cell nuclei, and the absence of Jagged2 and Delta-like1. The pro-sensory bHLH protein Atoh1 was absent at all time points following an ototoxic lesion, while the repressor bHLH transcription factors Hes1 and Hes5 were detected in surviving supporting cell nuclei in the former inner and outer hair cell areas, respectively. Notch pathway proteins peaked at 2 weeks, decreased at 1 month, and nearly disappeared by 2 months. These results indicate that the mammalian auditory epithelium retains the ability to regulate Notch signaling and Notch-dependent Hes activity in response to cellular trauma and that the signaling is transient. Additionally, since Hes activity antagonizes the transcription of prosensory Atoh1, the presence of Hes after a lesion may prohibit the occurrence of transdifferentiation in the surviving supporting cells.
Introduction
Auditory hair cells are inner ear sensory mechanoreceptors which transduce acoustic input into neural signals that elicit hearing. Hair cells are vulnerable to damage from acoustic over-stimulation, ototoxic drugs such as aminoglycosides, or aging, while their non-sensory supporting cell counterparts are substantially more resistant to these factors. Non-mammalian vertebrates can replace lost hair cells through direct and indirect transdifferentiation of surviving supporting cells (Adler and Raphael, 1996; Baird et al., 1996; Corwin and Cotanche, 1988; Roberson et al., 1996; Ryals and Rubel, 1988; Taylor and Forge, 2005). Mammals, including humans, do not spontaneously replace auditory hair cells when lost (Bermingham-McDonogh and Rubel, 2003; Hawkins and Johnsson, 1976; Roberson and Rubel, 1994; Rubel et al., 1995). However, proof of concept exists showing that in mammals surviving supporting cells can be forced to transdifferentiate into new hair cells given the right stimulus: namely, over-expression of the pro-hair cell gene Atoh1, which is normally only present during fetal development (Izumikawa et al., 2005; Kawamoto et al., 2003; Shou et al., 2003; Zheng and Gao, 2000). This suggests that although the mammalian cochlear sensory epithelium has lost the ability to spontaneously initiate the events needed to replace hair cells, the molecular activity required for inducing a hair cell fate is still present and functional in mature supporting cells. Furthermore, it suggests that unlike many other mature cell types, mammalian supporting cell fate may be altered upon providing appropriate signals.
The Notch pathway has been identified as one of the cell signaling pathways critical for both the initial specification of the prosensory domain as well as the subsequent cell fate decisions of sensory progenitors (Daudet et al., 2007; Kelley, 1997; Lewis et al., 1998a). During embryogenesis, Notch signaling directs the formation of a structured sensory epithelium consisting of hair cells surrounded by supporting cells––all progeny of presumably identical sensory progenitors. Fate heterogeneity is accomplished via lateral inhibition, a mechanism where a differentiating cell transmits inhibitory signals to its neighbors to prevent them from differentiating into the same cell type (Bray, 2006; Lai, 2004). In the cochlea, cells differentiating to a hair cell fate produce ligands that inhibit their neighbors from also developing as hair cells; instead they become supporting cells.
In inner ear development, Notch signaling directly influences the expression of the gene Atoh1 by regulating the expression of Hes genes (Hes1 and Hes5) which antagonize Atoh1 expression (Daudet and Lewis, 2005; Daudet et al., 2007; Kiernan et al., 2006; Kiernan et al., 2005; Kiernan et al., 2001; Lanford et al., 1999; Woods et al., 2004). The Notch molecules reported during the development of the mammalian inner ear include the Notch1 transmembrane receptor and the transmembrane ligands Delta-like1 (Dll1), Jagged1, and Jagged2 (Ehebauer et al., 2006; Lanford et al., 1999; Lewis et al., 1998b; Weir et al., 2000). During signaling, the binding of the Notch1 receptor with a ligand in a neighboring cell initiates two sequential cleavage events in the receptor, one by TACE and another by γ-secretase. This latter cleavage event liberates the Notch intracellular domain (NICD) which then travels to the nucleus to form a transcriptional complex with the proteins CSL (CBF1/RBP-J in mammals, Suppressor of Hairless in Drosophila and Xenopus, and Lag-1 in C. elegans) and Mastermind. This complex positively affects the gene transcription of repressor basic helix-loop-helix (bHLH) gene subtypes, namely Hes1 and Hes5. In the absence of NICD, the CSL-Mastermind complex acts as a repressor to Hes gene transcription, freeing the transcription of prosensory bHLH genes like Atoh1, whose accumulation promotes a hair cell fate (Kelley, 2002; Kelley, 2003; Kiernan et al., 2005; Woods et al., 2004).
While the Notch pathway has been implicated in hair cell regeneration following trauma to the basilar papilla in avians (Stone and Rubel, 1999), the status of Notch molecules in the damaged mammalian auditory epithelium is not well characterized. Additionally, it is important to determine whether and how the activity of Notch-dependent proteins (such as Hes) changes following a lesion, as is the case in some other regenerative tissues. Interfering with the developmental expression of Hes1 in the cochlea results in over-abundance of inner hair cells, while knocking out Hes5 results in over-abundance of outer hair cells (Zheng et al., 2000; Zine et al., 2001). Presumably, the elimination of the repressive (and locale-specific) signals provided by Hes activity allows for the over-expression of Atoh1, directing more cells than is normal to a hair cell fate. This repressive activity is crucial for producing a heterogeneous mosaic of hair cells and supporting cells during mammalian ear development, but continued Hes activity in adults may continue to promote maintenance of the supporting cell phenotype in maturity and pose a barrier to supporting cell-hair cell transdifferentiation following a lesion. Studies suggest that interfering with Notch signaling via γ-secretase inhibition or Notch-RBP/J inhibition results in the appearance of hair cell-like cells in the damaged mature cochlea or overpopulation of hair cell-like cells in the developing cochlea (Hori et al., 2007; Takebayashi et al., 2007; Yamamoto et al., 2006). This suggests that there is a constitutive Notch-dependent signal promoting the maintenance of supporting cell fate, which can be altered by a γ-secretase blocker to relieve repression. These studies provide the impetus to examine whether Notch-dependent Hes activity is present in the adult mammalian organ of Corti and accounts for failure to regenerate new hair cells after a lesion. Furthermore, it is important to determine whether Notch signaling is up-regulated after a lesion as the timing for that up-regulation may suggest intervals for effective manipulation of those signals.
We report immunohistochemistry and Western blot protein analysis of Notch molecules and downstream bHLH proteins in the normal and lesioned guinea pig organ of Corti at acute and chronic time points. The mature organ of Corti exhibited low level cytoplasmic Notch1 signal in the Deiters cells, very low Jagged1 signal in the pillar cell bodies, and low level nuclear Hes1 signal in the supporting cells in the inner sulcus. By 24 hours following ototoxic drug delivery, we observed a large increase of nuclear Notch1 signal in the Deiters cells, high Jagged1 signal in the inner and outer pillar cells as well as nuclear Hes1 and Hes5 labeling in the surviving supporting cells in the former inner and outer hair cell areas, respectively. The increase in Notch proteins appears to peak at 14 days post-ototoxicity, before declining below baseline levels by 60 days.
Materials and Methods
Animals
All animal experiments were approved by the University of Michigan Institutional Committee on Care and Use of Animals (UCUCA) and were performed using acceptable veterinary standards. We used 88 pigmented adult male guinea pigs (Elm Hill Breeding Laboratory) with a normal Preyer’s reflex and weighing between 250–400g at the beginning of the experiments. Animals were housed at the Animal Care facility at the University of Michigan. Seventy-four animals were systemically deafened with kanamycin and ethacrynic acid (with ten animals being replaced as they either died or had hair cells remaining). An additional ten animals were used as normal controls. Four animals received sham saline injections to provide a control for the deafening surgery (no difference was observed between saline and un-injected controls). Prior to all surgical procedures, guinea pigs were anesthetized with a subcutaneous injection of xylazine (10 mg/kg) and ketamine HCl (40 mg/kg).
Deafening surgery and progression of hair cell loss
Seventy-four adult guinea pigs were bilaterally deafened via the combination of a single subcutaneous dose of kanamycin (500 mg/kg) followed two hours later by an intravenous dose of ethacrynic acid (50 mg/kg) in the jugular vein. The pairing of these two drugs systemically is designed to consistently eliminate all outer hair cells and most or all inner hair cells while initially sparing supporting cells, in the lower three turns of the cochlea (Izumikawa et al., 2005; West et al., 1973). Hair cell elimination begins to be evident at about 24 hours following drug administration, with a few missing hair cells in the organ of Corti. A complete lesion of all hair cells in the lower three turns is consistently achieved by 3 days following this ototoxicity protocol, therefore figures of lesioned tissue from animals sacrificed at 24 hours following drug administration will contain some residual hair cells. All cochlear tissue was from the second turn. Animals were sacrificed and prepared for analysis (immunocytochemistry or protein extraction from dissected cochlear lysate) at the following time points: 1 hour (n=5), 4 hours (n=5), 24 hours (n=8), 3 days (n=8), 5 days (n=8), 7 days (n=8), 2 weeks (n=8), 1 month (n=8), or 2 months (n=6).
Immunocytochemistry
Animals were deeply anesthetized with xylazine and ketamine as above, decapitated, and the temporal bones were removed. The apical tip of the otic capsule was removed, the oval window opened by dislodging the stapedial footplate, and the round window opened with forceps. Paraformaldehyde (PFA) 4% in phosphate buffered saline (PBS) was locally perfused through the cochlea. The temporal bone with cochlea in situ was allowed to fix for two hours, at which time the bony capsule was dissected away and the modiolus with surrounding cochlear duct removed. These cochleae were stripped of the stria vacularis, tectorial membrane, and Reissner’s membrane to fully expose the organ of Corti. Specimens were washed three times for ten minutes in PBS and permeabilized using 0.1% Triton X-100 in PBS for ten minutes. Tissue was then blocked using 10% normal goat serum (Jackson Immunoresearch, West Grove, PA) in PBS for thirty minutes at room temperature. Following blocking, specimens were incubated overnight at 4°C in a 1:100 primary antibody solution in PBS. Primary antibodies utilized were raised in rabbit against the C-terminus of Notch1 (Abcam, ab27526, lot# 181697; whole receptor = ~280 kDa, cleaved portion NICD = 120 kDa), Jagged1 (Santa Cruz, H-114, sc8303, lot # E0604; 150 kDa), Jagged2 (Santa Cruz, H-143, sc5604, lot# K1904; 150 kDa), Dll1 (Santa Cruz, T-20, sc9932, lot# C1505; 75 kDa), Atoh1/Math1 (Abcam, ab22270-100, lot# 151411; 38 kDa), Hes1 (Chemicon, Ab5702, lot# LV1438380; ~30 kDa), and Hes5 (Chemicon, Ab5708, lot# 0511016244; ~20 kDa). The following morning, samples were washed three times in PBS and incubated in a Rhodamine (TRITC)-conjugated AffiniPure goat anti-rabbit IgG secondary antibody (Jackson ImmunoResearch, 111-025-144) for thirty minutes at room temperature. All samples were additionally incubated with Alexa Fluor 488 conjugated phalloidin (Molecular Probes Invitrogen, Carlsbad, CA) for twenty minutes. If visualization of the nucleus was desired, samples were incubated in the DNA-binding fluorescent stain 4′, 6-diamidino-2-phenylindole (DAPI) diluted 1:600, for three minutes. The cochleae were then washed three times for five minutes in PBS, dissected into four pieces (apex, 3rd turn, 2nd turn, base) and whole mounted onto slides with Gel/Mount mounting media (Biomeda, Foster City, CA). The second and third turns were used for analysis.
Because the Notch1 antibody used recognizes the c-terminal fragment of the Notch1 receptor, it labels membrane-bound and cytoplasmic epitopes when un-cleaved, and the nuclear domain (NICD) when cleaved/activated. To rule out background fluorescence, we incubated normal and lesioned (1 day post drug administration) guinea pig organ of Corti in just primary or just secondary antibody. We observed no signal in controls incubated in primary alone. A very low level of uniform background immunofluorescence was seen in controls incubated in just secondary antibody, which did not correspond to the location, intensity, and pattern of fluorescence reported here.
Confocal Microscopy
Immunohistochemically-processed cochlear whole mounts were examined with a Zeiss LSM 510-META Laser Scanning Confocal Microscope mounted on a Zeiss Axiovert 100M inverted microscope, equipped with four lasers: a Coherent Enterprise laser for UV (351,364 nm), an Argon laser for FITC/GFP (458, 488, 514 nm), a Helium Neon 1 laser for Rhodamine, Texas Red, and Cy3 (543 nm), and a Helium Neon 2 laser for Cy5 (633 nm). Wavelengths utilized in this study include 364 nm (for DAPI), 488 nm (for Alexa 488-conjugated phalloidin), and 543 nm (for Rhodamine). Scanning was performed with all lasers simultaneously in the multi-track setting and META-analysis provided fine separation of wavelengths and the ability to track all dyes in the sample simultaneously. The Linear Unmixing function was used to separate mixed signals pixel by pixel, using the entire emission spectrum of each dye in the examined specimen and eliminating broadband autofluorescence. Scans were all recorded in 1μm single images at 40x or 60x magnification with zoom. Confocal images were cropped, labeled, and spaced in Adobe Photoshop.
Western Protein Analysis of Dissected Cochlear Lysates
Cochlear lysates of control and hair cell-lesioned guinea pigs were examined for the presence of Notch proteins before and after a lesion at varying time-points. Cochleae were dissected from the temporal bone and the auditory nerve and as much bone and tissue as possible was removed from the organ of Corti with a fine needle under a microscope. Remaining tissue was incubated in cold T-Per Lysis Buffer (Pierce Biotechnology, Thermo Fischer, Rockford, IL) for 30 minutes then homogenized with a small plastic pestle. The homogenate was kept on ice a further 30 minutes then spun in a centrifuge until a visible pellet formed. The supernatant was retained and the amounts of protein standardized (30 μg). Separation of proteins was achieved using Nu-Page SDS-PAGE with X-Cell SureLock system and Novex 4–12% Bris-Tris gels with MOPS buffers (Invitrogen, Carlsbad, CA). Separated proteins were transferred to PVDF membrane by electrophoresis. Afterwards, the membrane was blocked with 5% dry milk in PBS for an hour and then incubated in 1% milk in PBS plus the primary antibody (1:5000) overnight at 4°C. Following three brief washes in TBS-T, the membranes were incubated for 30 minutes in a complementary HRP-conjugated secondary antibody solution (1:8000) in 5% dry milk. The membranes were again washed in TBS-T three times for 10 minutes each. Developing fluid (Pierce Biotechnology, Thermo Fischer, Rockford, IL) was applied to the membrane, and film was quickly placed on the membrane in the dark and developed. Average film development time was one minute in a sealed cassette. Film was then processed in the dark and the film was digitally scanned. The scan was inverted and sharpened in Adobe Photoshop. Cell lysates RAW 264.7 and ECV304 (Santa Cruz Biotechnology, Santa Cruz, CA) were used as positive controls of the proteins Jagged1 and Dll1 respectively, and both contained the Notch1 receptor. Mouse embryo tissue extract (from embryonic day17–19) were used as positive controls for Hes1 and Hes5. Our blot indicated that the RAW 264.7 lysate was positive for both Jagged1 and Dll1 while ECV304 produced a positive band only in the case of Dll1. A lane with buffer and no protein served as a negative control. In addition to the predicted bands (for mouse) referenced above, the Hes5 blot had a faint non-specific band at ~40 kDa, and the Notch1 blot had a faint nonspecific band at ~250 kDa. The Hes1 blot was clean of non-specific bands. GAPDH, a common cellular housekeeping protein (37 kDa), was used as a loading control to quantify relative protein amount using “ImageJ” analysis software.
Results
Notch molecules and Hes labeling in the normal organ of Corti
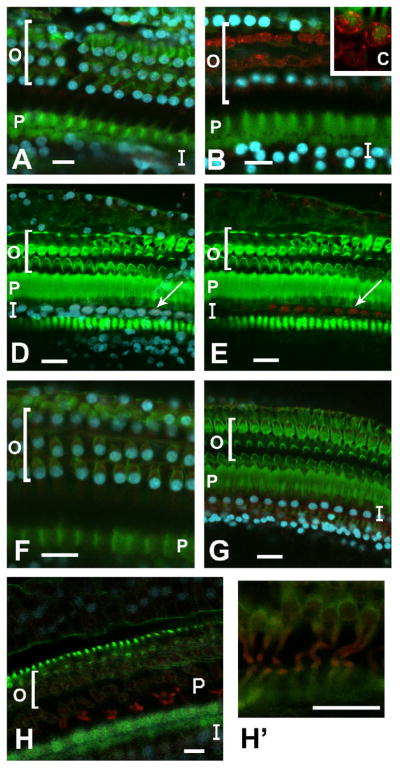
To determine baseline levels and locales of Notch1, Jagged1, Jagged2, Dll1, Atoh1, Hes1, and Hes5, we performed immunohistochemistry to detect these molecules as well as the nuclear stain DAPI in the normal guinea pig organ of Corti. We did not observe Notch1 signal in the nuclei of the normal cells in the organ of Corti (Fig. 1A), but more apical to the nuclei we did observe punctate membranous and cytoplasmic Notch1 signal in Deiters cells (Fig. 1B). Higher magnification of Notch1-positive Deiters cells also revealed non-nuclear cytoplasmic Notch1 signal which appeared to outline the cell body (Fig. 1C). Hes1 signal was faintly observed only in some of the nuclei of the supporting cells flanking the inner hair cell area, as illustrated by DAPI co-label (Fig. 1D; Fig. E shows same field without DAPI). Hes5 signal was not observed in the nuclei of any sensory or nonsensory cells in the outer (Fig. 1F) or inner (Fig. 1G) hair cell area. However, diffuse low-level cytoplasmic Hes5 label was observed in some cells in the inner hair cell area. The ligand Jagged1 was weakly expressed in the outer pillar cells (Fig. 1H, 1H’). Higher magnification of the Jagged1-positive outer pillar cells suggested that only the middle region of the cell body was positive for Jagged1 (Fig. 1H’). The ligands for Dll1 or Jagged2 were not detected (data not shown).
Figure 1. Confocal images of whole mounts of the normal guinea pig organ of Corti stained with fluorescent phalloidin (green), DAPI (blue), and antibodies to Notch signaling molecules (red: A–C, Notch1; D–E, Hes1; F–G, Hes5; H, Jagged1).
A, B and inset C: The region of the organ of Corti that contains nuclei is largely negative for the Notch1 receptor (A), punctate Notch1 positive areas are evident in non-nuclear locations such as the cell membrane and cytoplasm of Deiters cells more apical to the cell nuclei (B, and higher magnification inset C). D and E: Hes1 is found to be co-labeled in some supporting cell nuclei (D) in the inner hair cell area. An arrow labels one of several Hes1 positive nuclei (D is Hes1, phalloidin and DAPI, and E is Hes1 and phalloidin). F and G: Nuclei in the outer hair cell region (F) and inner hair cell region (G) are all negative for Hes5 although some cytoplasmic Hes5 signal was observed. H and H’: Low-level Jagged1 signal was seen in the pillar cells (H, shown focused on the area of positive outer pillar cell bodies in higher magnification in H’). Labels: ‘O’ refers to the outer hair cell area; ‘I’ refers to the inner hair cell area; ‘P’ refers to the apical surface of the inner and outer pillar cells. The lateral edge of the organ of Corti is oriented towards the top of each panel, and the medial edge is oriented towards the bottom. Scale bars represent 2μm.
Notch molecules and Hes labeling is up-regulated in the traumatized organ of Corti
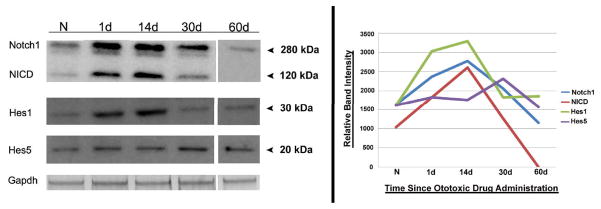
Expression of the whole Notch1 receptor, the cleaved Notch1 receptor (NICD), Jagged1, Hes1 and Hes5 all appeared to be significantly increased in response to ototoxicity, with striking increases apparent by 24 hours following the ototoxic drug administration. The nuclei of many remaining supporting cells in the organ of Corti were now positive for Notch1 signal, indicating the intracellular portion of the receptor which had traveled to the nucleus following its cleavage (Fig. 2A, 2A’ and 2A’’). Some non-nuclear, cytoplasmic or membranous Notch1 signal was also evident in many of the remaining supporting cells. Many cell nuclei in the outer hair cell area were labeled with Hes5 (Fig. 2B, B’ and B’’). Very few nuclei of cells in the inner hair cell area or the inner sulcus were positive for Hes5, instead many nuclei in these areas were positive for Hes1 (Fig. 2C and 2C’). The nuclei of cells immediately adjacent to the inner hair cells all appeared to be more brightly positive for Hes1 signal than cell nuclei positioned more medially towards the inner sulcus (less bright nuclei shown by right arrows in 2C and 2C’). Cytoplasmic Hes1 labeling was strongly observed in these cells, yet nuclear label was significantly brighter. Hes1 signal was also observed in most Hensen cell nuclei and in the cytoplasm (Fig. 2D and D’). Jagged1 signal was positive in the inner (Fig. 2E) and outer (Fig. 2E’) pillar cell bodies and in the Deiters cells. Western blot analysis of protein from guinea pig dissected traumatized cochlear lysates detected the significantly increased presence of Hes1, Hes5, NICD, and Notch1 as compared with normal baseline levels of these proteins (Fig. 3).
Figure 2. Confocal images of guinea pig organ of Corti whole mounts one day following ototoxic drug administration, stained with fluorescent phalloidin (green), DAPI (blue), and antibodies to Notch signaling molecules (red: A–A’’, Notch1; B–B’’, Hes5; C–D’, Hes1; E–E’, Jagged1).
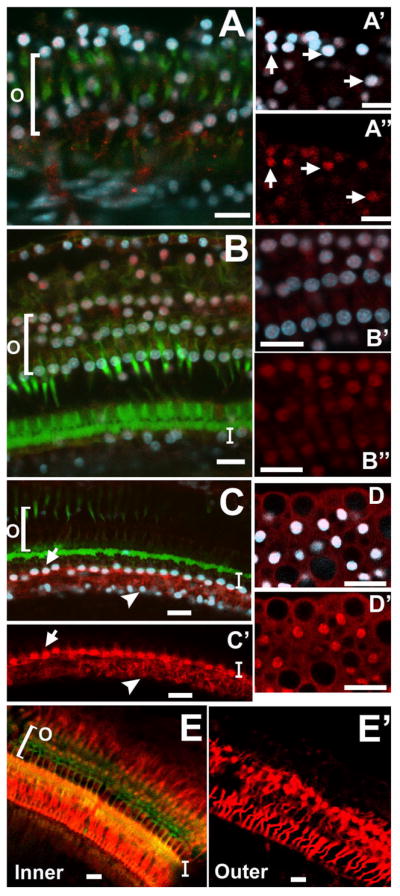
A, A’, and A’’: One day following drug administration, many DAPI-labeled nuclei (blue, in A and A’) were also positive for Notch1 (red in A’ and A’’, arrows point to examples of double-labeling). A’ and A’’ are higher magnification of the Deiters cell nuclei. Focus is at the level in the tissue where the nuclei of the remaining supporting cells were visible. Some diffuse membrane- or cytoplasmically-located Notch1 signal was still evident. B, B’, and B’’: Many DAPI-labeled nuclei in the remaining supporting cells in the outer hair cell area were co-labeled with Hes5 (B, higher magnification of DAPI and Hes5 signal in B’ and Hes5 signal alone in B’’). C, C’, D, D’: Many DAPI-labeled nuclei (blue in C and D) in the supporting cells of the inner hair cell area (C, C’) as well as the Henson cells (D, D’) were co-labeled with Hes1 signal. Cytoplasmic Hes1 signal was also observed in these same cell types to a lesser degree. E, E’: Both inner (E) and outer (E’, at a lower focal plane with the red channel alone) pillar cell bodies, and the Deiters cells were very brightly positive for Jagged1. Labels: ‘O’ refers to the outer hair cell area; ‘I’ refers to the inner hair cell area; ‘P’ refers to the apical surface of the inner and outer pillar cells. The lateral edge of the organ of Corti is oriented towards the top of each panel, and the medial edge is oriented towards the bottom. Scale bars represent 2μm.
Figure 3. Western blot analysis and quantification graph of Notch1, NICD, Hes1, and Hes5 in normal and drug-damaged organ of Corti.
Notch1, NICD, Hes1, and Hes5 all appeared to rise after a lesion, with the first three peaking at 2 weeks and Hes5 appearing to peak at one month following ototoxicity. The Notch1 blot exhibited bands at ~280 kDa corresponding to the whole receptor, ~120 kDa corresponding to the cleaved portion (NICD), and one faint non-specific band at ~250kDa. The Hes1 blot exhibited the predicted band at ~30 kDa and no nonspecific bands. The blot to Hes5 exhibited the predicted band at ~20 kDa and one faint nonspecific band at ~40 kDa. GAPDH was used as a loading control for relative quantification of protein amounts with ImageJ (graph at right). N = normal, 1d = 1 day following the lesion, 14d = 14 days after the lesion, 30d = thirty days after the lesion, 60d = 60 days after the lesion.
The traumatized organ of Corti remained positive for Notch1, Jagged1, Hes1, and Hes5 for at least one month following ototoxic drug administration and the presence of these molecules reached baseline levels by two months. According to Western blot analysis of protein from traumatized cochlear lysates at several time points following ototoxicity, the highest levels of Notch1, NICD, and Hes1 was at 14 days following ototoxic drug administration (Fig. 3). Immunohistochemistry to Notch1 at two weeks revealed widespread nuclear Notch1 signal in most of the remaining supporting cells of the traumatized organ of Corti (Fig. 4A and B). Higher magnification revealed less punctate cytoplasmic Notch1 signal than at one day following exposure, with the majority of Notch1 localized to nuclei (Fig. 4B and B’). The inner and outer pillar cells of the 14-day post traumatized organ of Corti were still brightly positive for Jagged1 signal (Fig. 4C). Hes1 signal was localized in supporting cell nuclei medial to the former inner hair cell area and nuclei in the inner sulcus, with high concentrations of Hes1 protein visible in scars where inner hair cells were absent (arrows in Fig. 4D and Fig. 4D’). Some cytoplasmic Hes1 signal was also visible in this area. Hensen cell nuclei as well as cytoplasm were still positive for Hes1 (data not shown), similar to their appearance at 24 hours following ototoxicity (Fig. 2D and 2D’). Hes5 signal was observed in the DAPI-stained nuclei of the remaining supporting cells in the outer hair cell area (Figs. 4E and 4E’), but not in the nuclei of the remaining cells in the inner hair cell area. Some cytoplasmic Hes5 signal was observed in these cells at low levels. At no observed time point following ototoxicity did the signal of Hes1 and Hes5 appear to overlap.
Figure 4. Confocal images of guinea pig organ of Corti whole mounts two weeks following ototoxic drug administration, stained with fluorescent phalloidin (green), DAPI (blue), and antibodies to Notch signaling molecules (red: A–B, Notch1; C, Jagged1; D–D’, Hes1; E–E’, Hes5).
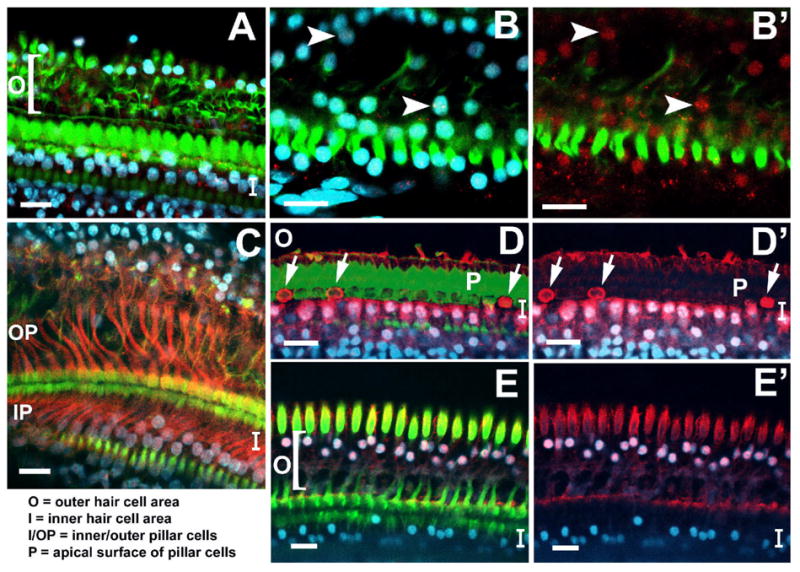
A, B: Nearly all DAPI-labeled nuclei (blue) of remaining supporting cells were also positive for Notch1 (red). In B’, DAPI signal is omitted to more clearly show Notch1. Arrows in B and B’ point to two double-labeled nuclei. Very little diffuse membrane- or cytoplasmically-located Notch1 signal was evident at this time point. C: All the inner and outer pillar cells and most of the Deiters cells were still brightly positive for Jagged1 signal (red). D, D’: Many supporting cell nuclei medial to the inner hair cell area (I) were co-labeled with DAPI and Hes1 (red). Additionally, there were high concentrations of non-nuclear Hes1 protein in some scars where inner hair cells had died (arrows in D, D’ which shows DAPI and Hes1 only). E, E’: Supporting cell nuclei in the outer hair cell area were co-labeled with DAPI and Hes5 signal, while all nuclei in the inner hair cell area were negative for Hes5. Labels: ‘O’ refers to the outer hair cell area; ‘I’ refers to the inner hair cell area; ‘P’ refers to the apical surface of the inner and outer pillar cells with ‘IP’ and ‘OP’ referring to inner and outer pillar cells, respectively. The lateral edge of the organ of Corti is oriented towards the top of each panel, and the medial edge is oriented towards the bottom. Scale bars represent 2μm.
Western blot analysis of protein from traumatized cochlear lysates detected the increased presence of Hes1, Hes5, NICD, and Notch1 at 2 weeks and one month following ototoxicity, as compared with normal baseline levels of these proteins. However, at one month, with the exception of Hes5 which could not definitively be noted to change, there was a decrease in these protein amounts as compared to 24 hours and 14 days following drug administration. This decrease in protein amount at 30 days was reflected in decreased immunohistochemical signal as well (not shown). At two months following ototoxicity, the blot indicated that levels of all observed proteins were at or below normal baseline levels (Fig. 3, graph at right illustrates trends). No perceptible immunohistochemical signal was observed at 2 months following ototoxicity for any of the proteins mentioned (Supplemental Figure 1 shows Notch1 and Jagged1, others not shown). Immunohistochemical analysis to Dll1, Atoh1, and Jagged2 were all negative at all time points observed (not shown).
Summary and Discussion
Summary of results
We have shown that in the guinea pig organ of Corti, Notch signaling is active at low levels in normal animals and dramatically increases in response to trauma induced by the delivery of ototoxic chemicals. Baseline presence of Notch molecules consists of non-nuclear Notch1, low level Jagged1 in pillar cells, and low level nuclear Hes1 in supporting cells medial to the inner hair cells. Post-lesion signaling, which is characterized by an increase in Jagged1 and nuclear Notch1 signal, is coupled with increases in Notch-dependent proteins Hes1 and Hes5 in supporting cell nuclei. Hes1 and Hes5 immunolabel following a lesion appeared to be location-specific and non-overlapping, with Hes1 located in the nuclei and cytoplasm of remaining supporting cells in the inner hair cell area and Hes5 located in the nuclei and cytoplasm of supporting cells in the outer hair cell area. The intensity of the signaling response varies over time: Notch1, Jagged1, Hes1, and Hes5 appeared to peak at between 14 and 30 days following the lesion (Fig. 3) and were at or below baseline levels by 2 months (Supplemental Figure 1).
Initiation of Notch signaling following trauma
After cell fates are determined during development, Notch signaling is static in tissues which do not have active cell turnover (Djalilian et al., 2008; Krawczyk et al., 2008; Shimojo et al., 2008). However, in some tissues trauma triggers Notch signaling as part of the process of cellular replacement, transdifferentiation, or repair. Post-developmental initiation of Notch signaling has been reported to accompany changes in cell fate in mouse endothelial cells (Lindner et al., 2001), mammalian muscle precursor cells (Conboy et al., 2003), mammalian dental pulp cells (Lovschall et al., 2005; Mitsiadis et al., 1999), intestinal crypt cells in the fruit fly, zebrafish, rodents, and humans (van Es and Clevers, 2005), adult neural stem cells in the mammalian subventricular zone (Givogri et al., 2006), Barrett’s metaplasia in humans (Liu et al., 2007), as well as the avian basilar papilla during trauma and hair cell regeneration (Stone and Rubel, 1999). One factor in post-traumatic Notch up-regulation may be the severity of the lesion and cell damage in the epithelium. This would help explain why the few studies reporting on the status of the organ of Corti following a lesion have produced varied results(Hori et al., 2007; Oesterle et al., 2008). In one recent study, both Sox2 and Jagged1 were reportedly unchanged following a partial lesion of the organ of Corti in mouse (Oesterle et al., 2008). This particular study utilized a single high-dose injection of kanamycin (1000 mg/kg) followed 30 minutes later by a dose of furosemide (400 mg/kg) which was reported to lesion outer hair cells almost exclusively, sparing inner hair cells. Our lesion model in guinea pigs achieves a more complete lesion, yielding a nearly complete loss of outer hair cells and inner hair cells (Abrashkin et al., 2006; Izumikawa et al., 2005; Raphael and Altschuler, 1991; Webster and Webster, 1981; West et al., 1973).
It is possible that in addition to loss of hair cells, indirect injury to supporting cells leads to a drastic up-regulation of Notch signaling in the auditory epithelium. This notion is supported by the observation that in the mouse, where the organ of Corti is more resistant to ototoxic drug regimens, the loss of outer hair cells was not accompanied by a substantial increase in Notch family molecules (Oesterle et al., 2008). In contrast, using our insult in the guinea pig ear, both outer and inner hair cells are eliminated. In addition, supporting cells are also likely affected, leading to a drastic increase in Notch signaling in these cells. The change in two Notch molecules in the guinea pig was also noted in another study (Hori et al., 2007).
Hes labeling is increased in surviving supporting cell nuclei
Notch-dependent Hes1 and Hes5 were observed to increase in the nuclei of supporting cells in response to ototoxicity, hypothetically as a result of increased Notch signaling in the damaged organ of Corti. During development of the organ of Corti, Hes gene expression has a deleterious effect on prosensory fate (hair cell fate) via the antagonism of Atoh1 (Zheng et al., 2000). It is probable that Hes expression persists at low levels in the organ of Corti in order to maintain non-sensory fate in mature supporting cells. Following an aminoglycoside-induced hair cell lesion, the cellular and structural integrity of the organ of Corti is compromised and the epithelium undergoes reorganization, perhaps to preserve any remaining function. We hypothesize that the increased Notch signaling is due to the loss of hair cells and the changes in supporting cells, and leads to increased Hes labeling. It is unclear at present whether the changes in supporting cells that up-regulate Notch are direct toxicity from the drugs, or a response to the loss of the neighboring hair cells, or both.
Hes activity may serve to discourage or prevent transdifferentiation of supporting cells to hair cells. Transdifferentiation in the absence of mitosis would rapidly result in supporting cell depletion in and the eventual destruction of the remaining portions of the organ of Corti. It is possible that transdifferentiation does not occur spontaneously to prevent this eventuality.
We observed Hes1 and Hes5 in mostly non-overlapping areas of the organ of Corti, with Hes1 restricted to the former inner hair cell area and the Hensen cells, and Hes5 located in the former outer hair cell area with diffuse labeling in some Hensen cells. It is interesting to note that this post-lesion localization corresponds to the developmental presence of these molecules. In the developing organ of Corti, Hes1 becomes restricted to the supporting cells of the inner hair cell area and Hes5 is likewise in the supporting cells of the outer hair cell area (Zine et al., 2000). This suggests that mature supporting cells may react differently to post-lesion Notch signaling depending on their prior developmental history and their location in the mature organ of Corti. It further suggests that positional information that impacts cell fate and protein production in response to signaling events is retained in the mature organ of Corti.
We show that the Notch1 receptor and Jagged1 ligand are increased in surviving supporting cells in response to cellular trauma and hair cell loss. We hypothesize that the remaining supporting cells engage in Notch1-Jagged1 signaling, resulting in NICD cleavage from the Notch1 receptor and its translocation to the nucleus. We believe that the increases seen in Hes1 and Hes5 in the damaged organ of Corti are due to promotion of their transcription in response to nuclear NICD in supporting cells. This in turn results in accumulations of Hes protein in the supporting cells, creating an antagonistic environment for direct supporting cell to hair cell transdifferentiation to occur. This is supported by the proposed role of Jagged1 in prosensory cell developmental, as illustrated by the application of antisense to Jagged1 to cultured cochlea at embryonic day 18 (Zine et al., 2000). This treatment resulted in an indiscriminate overpopulation of hair cells (2 rows of inner hair cells and several extra rows of outer hair cells) and the disruption of stereocilia polarity. This contributes to the notion that Jagged1 could be a promiscuous inhibitory signal for hair cell fate at the crucial stage in prosensory cell development (around E18), acting to restrict the overpopulation of hair cells and maintain the proper balance of cell types. We would further posit that similar phenotypes to the Jagged1 antisense can be attributed to developmental Notch reduction (and thereby, Hes reduction) in the developing cochlea. Hes1−/− mice exhibited reduced IHC population while Hes5−/− mice exhibited reduced OHC population (Zine et al., 2000). One way of looking at this could be that the Jagged1 signal at this stage might prompt locale-specific Hes expression (1 or 5, depending on the area) and that the global reduction of all Jagged1 signal results in the indiscriminate overpopulation of both hair cell types.
The pattern of Notch activation we report is fundamentally different than what has been reported in avian hair cell regeneration following trauma, which is characterized by Delta1-Notch1 signaling and the presence of Atoh1 in areas of regeneration (Cafaro et al., 2007; Stone and Rubel, 1999). We did not observe the Delta1 homolog Dll1, or Jagged2, or Atoh1 at any time point following a lesion in mammals, although at 24 hours following the lesion.
Clinical Implications and Future Directions
In light of our data reporting Hes labeling in the lesioned organ of Corti, it is reasonable to assume that forced mammalian hair cell regeneration via transdifferentiation may be accomplished by Hes repression. However, it may also be necessary to increase Atoh1 levels to change the fate of the remaining cells in the auditory epithelium. Future studies should explore whether Notch inhibition following a lesion has a direct effect on decreasing Hes activity in the organ of Corti. Because Notch signaling and Hes activity peak at around 14 days following a lesion, it would likely be beneficial to deliver therapies which relied on Notch interference before this time point. It is also important to explore the possibility of enhancing hair cell regeneration via simultaneous delivery of prosensory gene therapy and inhibitors of Notch signaling, as well as determining whether over-expression of Atoh1 in supporting cells is overriding the repressive effect Hes may have on transdifferentiation. Additionally, future work may address whether it may be possible to selectively induce regeneration of an inner or outer hair cell by eliminating the expression of solely one of the Hes genes (for example, through siRNA). Our data indicated that following a lesion, Hes5 was predominantly located in the nuclei of supporting cells in the outer hair cell area, and Hes1 was located in nuclei of cells in the inner hair cell area. If Hes1 and Hes5 partially recapitulate their developmental role of Atoh1 antagonism in locale-specific ways following a lesion, specifically blocking Hes1 after a lesion may increase the likelihood of hair cell regeneration of supporting cells to inner hair cells and blocking Hes5 may provide a similar effect for supporting cell to outer hair transdifferentiation. Finally, future work should focus on additional pathways, either independent of or complicit with Notch signaling, which could also discourage the spontaneous transdifferentiation of supporting cells in the damaged mammalian organ of Corti.
Conclusions
We demonstrate that mammals preserve the ability to reinitiate Notch signaling following hair cell loss. A severe lesion to the organ of Corti leads to up-regulation of Jagged1-Notch1 signaling and the presence of the bHLH proteins Hes1 and Hes5. The changes in Notch signaling following a lesion to the mammalian auditory epithelium are inherently different from the response preceding hair cell regeneration in birds, where Delta1-Notch1 signaling is increased after a lesion, followed by the production of prosensory bHLH protein Atoh1 (Cafaro et al., 2007; Stone and Rubel, 1999). Our data present a potential mechanism by which mammalian supporting cell transdifferentiation may be repressed by Notch-dependent Hes activity after hair cell loss, and outlines a window of time in which Notch signals might be optimally manipulated for inducing therapeutic transdifferentiation.
Supplementary Material
Supplemental Figure 1: Grey scale confocal images of guinea pig organ of Corti whole mounts stained for fluorescent phalloidin (A and B) and Notch1 (A’) or Jagged1 (B’). Two months following ototoxic drug administration, immunohistochemistry to Notch1 and Jagged1 was negative. Actin labeling in the upper panels illustrates a mature scar in the organ of Corti, devoid of inner and outer hair cells. Actin junctions are compressed between supporting cells and the areas where hair cells once were. The lateral edge of the organ of Corti is oriented towards the top of each panel, and the medial edge is oriented towards the bottom. Scale bars = 2 μm.
Acknowledgments
This work was supported by the R. Jamison and Betty Williams Professorship, the A. Alfred Taubman Medical Research Institute, a gift from Berte and Alan Hirschfield and NIH-NIDCD Grants R01-DC01634, T32-DC00011, and P30-DC05188.
References
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL, Beyer LA, Gong TW, Raphael Y. The fate of outer hair cells after acoustic or ototoxic insults. Hear Res. 2006 doi: 10.1016/j.heares.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear [published erratum appears in Neurosci Lett 1996 May 24;210(1):73] Neurosci Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann N Y Acad Sci. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119–26. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236:156–70. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–7. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–4. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–51. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–78. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Djalilian AR, Namavari A, Ito A, Balali S, Afshar A, Lavker RM, Yue BY. Down-regulation of Notch signaling during corneal epithelial proliferation. Mol Vis. 2008;14:1041–9. [PMC free article] [PubMed] [Google Scholar]
- Ehebauer M, Hayward P, Martinez-Arias A. Notch signaling pathway. Sci STKE. 2006:cm7. doi: 10.1126/stke.3642006cm7. [DOI] [PubMed] [Google Scholar]
- Givogri MI, de Planell M, Galbiati F, Superchi D, Gritti A, Vescovi A, de Vellis J, Bongarzone ER. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Jr, Johnsson LG. Patterns of sensorineural degeneration in human ears exposed to noise. In: Henderson D, et al., editors. Effects of noise on hearing. New York: Raven Press; 1976. pp. 91–110.pp. 270 [Review] [27 refs] [Google Scholar]
- Hori R, Nakagawa T, Sakamoto T, Matsuoka Y, Takebayashi S, Ito J. Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport. 2007;18:1911–4. doi: 10.1097/WNR.0b013e3282f213e0. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Cellular commitment and differentiation in the cochlea: potential advances using gene transfer. [Review] [80 refs] Audiol Neurootol. 1997;2:50–60. doi: 10.1159/000259229. [DOI] [PubMed] [Google Scholar]
- Kelley MW. Determination and commitment of mechanosensory hair cells. ScientificWorldJournal. 2002;2:1079–94. doi: 10.1100/tsw.2002.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Cell adhesion molecules during inner ear and hair cell development, including notch and its ligands. Curr Top Dev Biol. 2003;57:321–56. doi: 10.1016/s0070-2153(03)57011-9. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–62. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Ahituv N, Fuchs H, Balling R, Avraham KB, Steel KP, Hrabe de Angelis M. The Notch ligand Jagged1 is required for inner ear sensory development. Proc Natl Acad Sci U S A. 2001;98:3873–8. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk CM, Sun J, Pearce EJ. Th2 differentiation is unaffected by Jagged2 expression on dendritic cells. J Immunol. 2008;180:7931–7. doi: 10.4049/jimmunol.180.12.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–73. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea [see comments] Nat Genet. 1999;21:289–92. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lewis AK, Frantz GD, Carpenter DA, de Sauvage FJ, Gao WQ. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech Dev. 1998a;78:159–63. doi: 10.1016/s0925-4773(98)00165-8. [DOI] [PubMed] [Google Scholar]
- Lewis AK, Frantz GD, Carpenter DA, de Sauvage FJ, Gao WQ. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech Dev. 1998b;78:159–63. doi: 10.1016/s0925-4773(98)00165-8. [DOI] [PubMed] [Google Scholar]
- Lindner V, Booth C, Prudovsky I, Small D, Maciag T, Liaw L. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. Am J Pathol. 2001;159:875–83. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang X, So CK, Wang S, Wang P, Yan L, Myers R, Chen Z, Patterson AP, Yang CS, Chen X. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis. 2007;28:488–96. doi: 10.1093/carcin/bgl176. [DOI] [PubMed] [Google Scholar]
- Lovschall H, Tummers M, Thesleff I, Fuchtbauer EM, Poulsen K. Activation of the Notch signaling pathway in response to pulp capping of rat molars. Eur J Oral Sci. 2005;113:312–7. doi: 10.1111/j.1600-0722.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Fried K, Goridis C. Reactivation of Delta-Notch signaling after injury: complementary expression patterns of ligand and receptor in dental pulp. Exp Cell Res. 1999;246:312–8. doi: 10.1006/excr.1998.4285. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Scar formation after drug-induced cochlear insult. Hear Res. 1991;51:173–83. doi: 10.1016/0378-5955(91)90034-7. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Rubel EW. Cell division in the gerbil cochlea after acoustic trauma. Am J Otol. 1994;15:28–34. [PubMed] [Google Scholar]
- Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Auditory Neuroscience. 1996;2:195–205. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Dew LA, Roberson DW. Mammalian vestibular hair cell regeneration. Science. 1995;267:701–7. doi: 10.1126/science.7839150. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–6. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–79. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Development. 1999;126:961–73. doi: 10.1242/dev.126.5.961. [DOI] [PubMed] [Google Scholar]
- Takebayashi S, Yamamoto N, Yabe D, Fukuda H, Kojima K, Ito J, Honjo T. Multiple roles of Notch signaling in cochlear development. Dev Biol. 2007;307:165–78. doi: 10.1016/j.ydbio.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Taylor R, Forge A. Developmental biology. Life after deaf for hair cells? Science. 2005;307:1056–8. doi: 10.1126/science.1109680. [DOI] [PubMed] [Google Scholar]
- van Es JH, Clevers H. Notch and Wnt inhibitors as potential new drugs for intestinal neoplastic disease. Trends Mol Med. 2005;11:496–502. doi: 10.1016/j.molmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Webster M, Webster DB. Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res. 1981;212:17–30. doi: 10.1016/0006-8993(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Weir J, Rivolta MN, Holley MC. Notch signaling and the emergence of auditory hair cells. Arch Otolaryngol Head Neck Surg. 2000;126:1244–8. doi: 10.1001/archotol.126.10.1244. [DOI] [PubMed] [Google Scholar]
- West BA, Brummett RE, Himes DL. Interaction of kanamycin and ethacrynic acid. Severe cochlear damage in guinea pigs. Arch Otolaryngol. 1973;98:32–7. doi: 10.1001/archotol.1973.00780020036009. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–8. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tanigaki K, Tsuji M, Yabe D, Ito J, Honjo T. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med. 2006;84:37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–6. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127:4551–60. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- Zine A, Van De Water TR, de Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127:3373–83. doi: 10.1242/dev.127.15.3373. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–20. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Grey scale confocal images of guinea pig organ of Corti whole mounts stained for fluorescent phalloidin (A and B) and Notch1 (A’) or Jagged1 (B’). Two months following ototoxic drug administration, immunohistochemistry to Notch1 and Jagged1 was negative. Actin labeling in the upper panels illustrates a mature scar in the organ of Corti, devoid of inner and outer hair cells. Actin junctions are compressed between supporting cells and the areas where hair cells once were. The lateral edge of the organ of Corti is oriented towards the top of each panel, and the medial edge is oriented towards the bottom. Scale bars = 2 μm.