Abstract
Optimal implementation of adoptive T-cell therapy for cancer will likely require multiple and maintained genetic modifications of the infused T-cells and their progeny so that they home to and recognize tumor cells, overcome tumor immune evasion strategies and remain safe. Retroviral vectors readily transduce T-cells and integrate into the host cell genome, but have a limited capacity for multigene insertion and co-transduction and are prohibitively expensive to produce at clinical grade. Genetic modification of T-cells using transposons as integrating plasmids is an attractive alternative due to the increased simplicity and cost of production. Of available transposons, piggyBac has the higher transposase activity and larger cargo capacity, and we now evaluate piggyBac for potential adoptive therapies with primary T cells. PiggyBac transposons mediated stable gene expression in ~20% of primary T-cells without selection. Treatment and maintenance of T-cells with IL-15 increased stable transgene expression up to ~40% and expression was sustained through multiple logs of expansion for over 9 weeks in culture. We demonstrate simultaneous integration of two independent transposons in 20% of T-cells, a frequency that could be increased to over 85% by selection of a transgenic surface marker (truncated CD19). PiggyBac could also deliver of transposons of up to 13 kb with 10,000 fold expansion of transduced T-cells in culture and finally we demonstrate delivery of a functional suicide gene (iCasp9). PiggyBac transposons may thus be used to express the multiple integrated transgenes that will likely be necessary for the broader success of T-cell therapy.
Keywords: Gene transfer, transposons, T cell immunotherapy, genetically-modified T-cells
INTRODUCTION
Immunotherapy with antigen-specific T-cells can control and eliminate virus infections and Epstein-Barr virus (EBV)-associated lymphoma after hematopoietic stem cell transplantation1–4 and has shown promise for the treatment of lymphoma, melanoma and carcinoma arising in the immunocompetent host.5–8 Nonetheless, the full potential of viral and tumor-specific T-cells will only be reached when they can withstand the multiple immune evasion mechanisms that viruses and malignant cells have developed to inhibit the function and diminish the survival of these cells.9 These active and passive mechanisms include; lack of migration to tumor sites, the secretion of T-cell inhibitory molecules such as transforming growth factor (TGF)-β and indoleamine 2,3-dioxygenase (IDO) by tumors and tumor stroma,10–13 and poor expression of antigen and costimulatory molecules required for T-cell stimulation. Genetic modification of T-cells may allow these evasion mechanisms to be counteracted, and expression of transgenic chemokine receptors,14 dominant negative receptors for inhibitory molecules, shRNAs to inhibit negative signaling molecules and cytokines to maintain T-cell proliferation15;16 may all be of value. Similarly, tumors that downregulate MHC molecules and do not present T-cell epitopes, may be targeted by T-cells expressing artificial chimeric antigen receptors (CARs).17 Since extensive T cell division is an essential part of most successful immune responses, these genetic modifications must be stable and will require vector integration. Hence, there is a concern that such integration will result in genotoxicity due to insertional mutagenesis, particularly if there is a need to express multiple transgenes in a single T-cell as countermeasures to the multiplicity of immune evasion strategies that are displayed by most malignant target cells.
Although T-cells are readily modified by retrovirus and lentivirus vectors, both have limited transgene capacity that allows expression of two or rarely three transgenes.18–20 Sequential transduction with several vectors is possible, but the efficiency of co-transduction is lower, the risk of genotoxicity higher, and the manufacturing and testing expenses prohibitive. In addition, expression of transgenes from retroviral vectors in T-cells is dependent on the activation state of the T-cell, and downregulation swiftly follows in the absence of T-cell activation.21;22 Plasmid based transposons are an alternative means for T-cell modification. The Sleeping Beauty (SB) transposon has been used to express transgenes in T-cells and a clinical trial using T-cells expressing a transgenic CD19-CAR derived from an SB transposon has been approved.23;24Although the costs of manufacturing and testing the encoding plasmids are far lower than for viral vectors, transduction efficiency of SB is such that extensive ex vivo selection and expansion is required. By contrast, the piggyBac transposon system has been shown in several human and mammalian cells to have greater gene transfer efficiency and also a greater coding capacity than SB.25;26;26–31 We now show that piggyBac can stably transduce human T-lymphocytes with multiple genes, and that transduced cells can readily be expanded. We also show that a suicide gene can be co-transfected and expressed in these T-cells, further increasing the safety of our approach.
MATERIALS AND METHODS
Plasmid construction
The piggyBac (PB)-transposase plasmid, pCMV-PB has been described previously.28 To create PB-transposon plasmids, the inverted terminal repeat (IR) elements of PB were cloned into pIRES2-eGFP (Clontech, Mountain View, CA), which is transcriptionally regulated by the cytomegalovirus immediate early gene enhancer/promoter sequence (CMV).28 Figure 1 shows the transposon plasmids used: pIR-eGFP is a PB-transposon plasmid encoding the internal ribosome entry site (IRES) followed by the enhanced green fluorescence protein (eGFP) and a puromycin resistance cassette. pIR-ΔCD19 is a piggyBac -transposon vector encoding IRES and the truncated CD19 (ΔCD19). pIRII-ΔCD19 is identical to pIR-ΔCD19 except that the puromycin resistance gene was deleted. The suicide gene, inducible caspase-9 (iCasp9) in which human caspase 9 deleted for its endogenous dimerizing (CARD) domain was fused to a single FK-binding domain has previously been reported.32;33 Death of iCasp9-expressing cells can be induced with a small chemical inducer of dimerization (CID) (AP20187: ARIAD Pharmaceuticals, Cambridge, MA, kindly provided by Dr Spencer, Baylor College of Medicine)32;33 iCasp9 and ΔCD19 were linked using a 2A-like protease recognition sequence derived from foot-and-mouth disease virus 34;35, and were cloned into a piggyBac-transposon plasmid (pIRII-iCasp9.2A.ΔCD19). pIR-mTorHA-eGFP encodes the DNA for mTor with a hemagglutinin (HA) tag followed by an IRES element, eGFP and the puromycin resistance gene cassette. All plasmid constructs were confirmed by restriction digestion and DNA sequencing. The piggyBac transposase was introduced transiently into T-cells from a separate plasmid to ensure subsequent stability of integrated transposons.
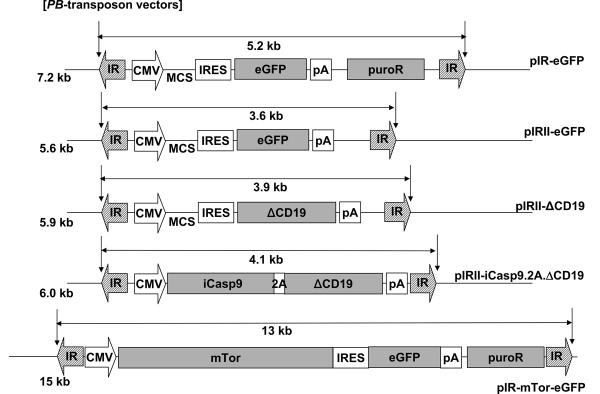
FIGURE 1.
Schema of piggyBac-transposon and transposase plasmids used to transfect PBMCs. The pIR-eGFP, pIRII-eGFP, pIRII-ΔCD19, pIRII-iCasp9.2A.ΔCD19 and pIR-mTorHA-eGFP are piggyBac-transposon plasmids. The pCMV-PB is a piggyBac-transposase plasmid. IR, inverted terminal repeat; MCS, multiple cloning site; CMV, cytomegalovirus immediate early promoter; IRES, internal ribosomal entry site; eGFP, enhanced green fluorescent protein; pA, polyadenylation sequence; ΔCD19, truncated CD19; iCasp9, inducible caspase-9; 2A, 2A self-processing peptide derived from the foot-and-mouth disease virus; puroR, puromycin resistance gene cassette; HA, hemagglutinin tag.
Cell Donors, cell lines and cultures
Peripheral blood mononuclear cells (PBMCs) from healthy volunteers were obtained with informed consent from the Baylor College of Medicine Institutional Review Board. PBMCs were used to generate T-cell blasts or as feeder cells. They were cultured in T-cell media [Advanced RPMI (Gibco-BRL, Gaithersburg, MD) supplemented with 2 mM L-glutamine (GlutaMAX-I, Invitrogen, Carlsbad, CA) and 5% human AB serum]. Artificial K562 cells (aK562), engineered to express CD80, CD86 and 4-1BBL,36 were maintained in RPMI 1640 (Gibco-BRL, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 2mM L-glutamine (GlutaMAX-I, Invitrogen, Carlsbad, CA). aK562 were used as feeder cells to expand piggyBac-transduced T-cells in GP40 culture flasks (Wilson-Wolf Manufacturing Inc., New Brighton, MN). Cytokines were added to the cultures where indicated: IL-2 at 50 units per mL, IL-4 at 1000 units per mL, and IL-7 and IL-15 at 10 ng/mL.
Flow cytometric analysis
We stained the transfected or non-transfected T-cells with phycoerythrin (PE)-, peridinin chlorophyll protein (PerCP)-, or allophycocyanin (APC)-conjugated CD3, CD4, CD8, CD16, CD19, CD28, CD45RO, and CD62L monoclonal antibodies (MAbs). All MAbs were purchased from Becton Dickinson (Franklin Lakes, NJ). Control samples labeled with an appropriate isotype-matched antibody were included in each experiment. Cells were analyzed by a FACSCalibur equipped with the filter set for 4 fluorescence signals using Cell Quest software (Becton Dickinson).
Gene transfer into human T-cells
Five million PBMCs were mixed with piggyBac-transposon plasmids and/or piggyBac-transposase plasmids in the cuvette provided with the kit and then transfected using the Miltenyi Nucleofector Device (program U-014) in combination with the Human T-cell Nucleofector Kit according to the manufacture's instruction (Amaxa, Gaithersburg, MD). The amount of transposon and transposase DNA is shown for each experiment. In some experiments, PBMCs were maintained in cytokines overnight before transfection as described. After transfection, PBMCs were stimulated on non-tissue culture-treated 24 well plates coated with 1 μg/ml OKT3 (Ortho Biotech, Bridgewater, NJ) and 1 μg/ml anti-CD28 (Becton Dickinson) antibodies (CD3/28 MAbs) in the presence of recombinant human interleukin (IL)-2 (50 IU/mL) (Proleukin; Chiron, Emeryville, CA). After 4 days of stimulation, activated T-cells were transferred to tissue-culture treated 24-well plates in T-cell media supplemented with IL-2 (50 IU/mL) or other growth factors as indicated. Cells were restimulated weekly on CD3/28 MAbs-coated plates. For optimized transduction, PBMCs were rested overnight in 10 ng per mL IL-15 then transfected with 5 μg of transposase and 5 μg of transposon DNA, then returned to IL-15 containing medium for 24 hours before activation. On day 1 after nucleofection, transgene-expressing T-cells were considered “transfected”. By day 8, it was assumed that all transgene expressing T-cells had retained integrated piggyBac transposon and so at this point, they were considered “transduced”. Transfected/transduced cells were harvested for counting and FACS analysis as indicated.
Cell selection and expansion
On day 8 after co-transfection of pIRII-eGFP and pIRII-ΔCD19, T-cells were harvested and incubated with CD19-microbeads (Miltenyi Biotec, Bisley, UK) for 15 minutes at 4 °C, then positively selected using Miltenyi Mini-MACS column according to the manufacture's instructions. The selected cells were immediately re-plated in T-cell media with IL-15 on a CD3/CD28 MAbs-coated 24-well plate and cultured for 2 weeks. On day 22 after transfection, transgene expression and cell subset and memory phenotype were determined. For further expansion, 100,000 T-cells were stimulated with CD3/28 MAbs with aK562 or autologous PBMCs feeder cells in GP40 flasks every 2 weeks. IL-15 (10 ng/mL) was added to the media twice weekly.
Statistical analysis
All data are presented as mean ± 1 SD. The Student's t test was used to determine the statistical significance of differences between samples, and P values less than .05 were accepted as indicating a significant difference.
RESULTS
Optimization of piggyBac transduction of primary T-cells
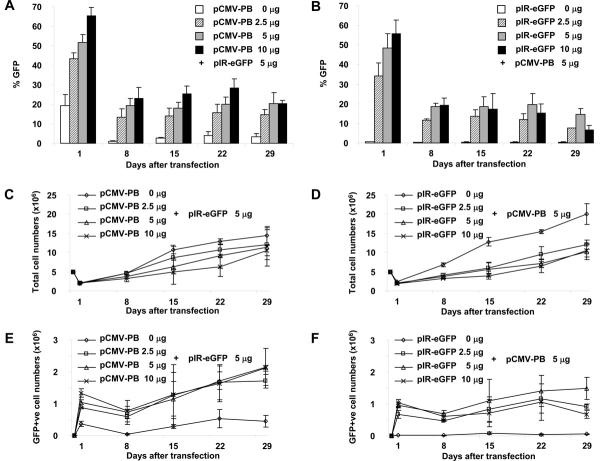
We first evaluated the efficiency and toxicity of introducing the piggyBac transposon and transposase into primary T cells using nucleofection. We found that both the frequency of gene expression and cell viability were greater after transfection of PBMCs than after transfection of CD3/28 MAbs-activated T-cells (not shown). Hence, in all the following experiments, PBMCs were first transfected and then the T cells they contained were activated with plate bound CD3/28 MAbs. To determine the optimal ratio of piggyBac-transposase to transposon DNA, 5 × 106 fresh PBMCs were transfected with 5 μg of piggyBac-transposon plasmid, pIRII-eGFP, and increasing quantities (0, 2.5, 5, or 10 μg) of piggyBac-transposase plasmid, pCMV-PB, or 5 μg of pCMV-PB together with increasing quantities (0, 2.5, 5, or 10 μg) of transposon (pIRII-eGFP). Twenty-four hours after transfection, the cells were counted and activated with CD3/28 MAbs for 7 days. GFP expression was analyzed on day 1 and weekly thereafter. There was significant loss of viability after nucleofection alone; 40% of T cells were dead on day 1 after nucleofection without DNA (not shown). Cell death was increased to about 60% by DNA addition (Figures 2C and 2D). Figures 2A and 2B show that increasing the quantity of both transposon and transposase plasmids increased the frequency of GFP expression at all timepoints, but illustrates that the toxicity also increased with the amount of added DNA (Figures 2C and 2D). The optimal result, a transfection efficiency of 40% to 55% (47.6 ± 7.4%) and viability of ~40% on day 1, was obtained using 5 μg each of transposon and transposase, and these quantities were used in future experiments. By day 8 the frequency of transduced cells had declined by about 50%, but thereafter remained stable for as long as the T-cells were cultured (up to 4 weeks of culture shown in Figures 2E and 2F). The reproducible loss of transgene expression from day 1 to day 8 likely represents transfected cells in which the transposon did not integrate and was therefore lost due to cell division by day 8. Certainly, after day 8 it is possible to use plasmid rescue to confirm integration of piggyBac into the T-cell genome (See Table, Supplemental Digital Content 1, http://links.lww.com/JIT/A7). Since the presence of transposase increased both the frequency and intensity of GFP expression on day 1 (Figures 2A and 2E) the transposase may act as a transactivator of transgene expression.28 While transduced T-cells could be subsequently be maintained in culture over 4 weeks with stable gene expression (Figures 2A, 2B, 2E and 2F), there was little overall expansion in transduced T-cell numbers (Figures 2E and 2F).
FIGURE 2.
Optimization of DNA concentrations in piggyBac transfection of T-cells. To optimize transposase and transposon DNA concentrations, PBMCs were transfected with 5 μg of pIRII-eGFP and 0, 2.5, 5, or 10 μg of pCMV-PB (A, C and E) or 5 μg of pCMV-PB and 0, 2.5, 5, or 10 μg of pIRII-eGFP (B, D and F) respectively. Transfected cells were stimulated with CD3 and CD28 MAbs in the presence of IL-2 and harvested for transgene analysis and counting weekly between days 1 to 29 after transfection (A and B). The growth kinetics of total (C and D) and GFP+ (E and F) T cells are shown. Data represent the mean ± SD of 3 donors.
IL-15 increases the viability of transduced T-cells
Since the above studies showed that the toxicity of DNA transfection diminished the initial survival and subsequent expansion of transgene-expressing T cells, we sought to improve the viability of transfected cells. We first determined if incubation of the PBMCs in media containing anti-apoptotic cytokines37 improved cell survival during transfection. We added IL-4, IL-7 and IL-15 to PBMCs both prior to and after transfection, and Figure 3 shows that IL-7 and IL-15 increased both the frequency and number of GFP-expressing cells on day 1 and at all time points thereafter (Figures 3E). Although we still observed a 60% initial loss in viability due to the transfection and a 50% reduction in transgene expression between days 1 and 8, both the frequency of GFP+ T-cells and their subsequent expansion was increased by the addition of cytokines. In three experiments, the increase in the frequency of transduced T cells was 1.27 ± 0.07 fold for IL-7 and 1.43 ± 0.16 fold for IL-15 on day 1, and 1.24 ± 0.28 fold for IL-7 and 1.41 ± 0.28 fold for IL-15 on day 8. Similarly, the increase in transduced cell numbers was1.55 ± 0.16 fold for IL-7 and 2.01 ± 0.62 fold for IL-15 on day 8. Hence the major benefit of these cytokines was to increase the subsequent expansion of transduced T cells. Overall there were a 2.83 fold higher number of viable transduced cells on day 8 in the presence of IL15 than in control cultures lacking this cytokine. Since results with IL-15 were consistently although not significantly slightly better than for IL-7 and since the IL-7 receptor, but not the IL-15 receptor is downregulated with time after T cell activation, IL-15 alone was used in all subsequent experiments at all time points. Indeed, when culture was continued in IL-15, the superiority over IL-2 became more apparent, with stable expression of GFP in 40% of T-cells and up to 30-fold expansion of transduced T-cells by day 29 of culture (Figures 3D and 3E). Hence, we used IL-15 in all subsequent experiments.
FIGURE 3.
Improved viability of transduced T-cells using cytokines. Untreated or cytokine-pre-treated PBMCs were transfected with pIRII-eGFP (5 μg) and pCMV-PB (5 μg), then stimulated with CD3/28 MAbs in the presence of the same cytokine as used for pre-treatment or IL-2 (control). Cells were counted and analyzed for GFP expression by flow cytometry on day 1 and day 8 and weekly thereafter. Effects of cytokines on the frequency (A), and number (B) of transgene expressing cells and total cells (C) on day 1 and day 8 after transfection are shown. Longer term proliferation of the transduced T-cells PBMCs in the presence of IL-15 or IL-2 (Control) is shown in D and E. Data represent the mean ± SD of 3 donors.
Increasing transgene numbers
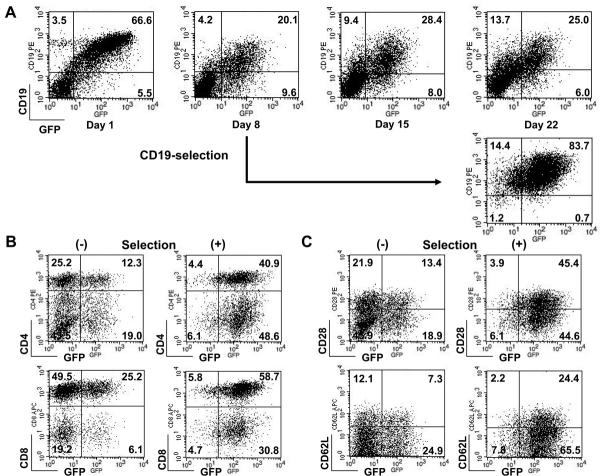
When PBMCs from four different donors were treated with IL-15 and co-transfected with the two separate transposons pIRII-eGFP (a 3.6 kb transposon in a 5.6 kb plasmid and pIRII-ΔCD19 (a 3.9 kb transposon in a 5.4 kb plasmid) together with the transposase plasmid pCMV-PB (in a 5.4 kb plasmid) the frequency of cells co-expressing GFP and ΔCD19 on day 1 ranged from 30.4% to 66.6% (mean 46.4%), while an additional 5.5% to 13.2% (mean 10.2%) expressed only GFP and 1.4% to 5.8% (mean 4.8%) expressed only ΔCD19. By day 8, these frequencies had decreased, so that from 5.6% to 22% (mean 14.4%) of T-cells expressed both plasmids, with means of 8.6% and 3.7% of T-cells singly expressing GFP and ΔCD19 respectively. Figure 4A shows a representative experiment in which the level of transduction stabilized between 20–28% from days 8 to 22. Co-transfection with these two small transposons did not significantly decrease cell viability over that obtained with a single small transposon (49 ± 8% vs 42 ± 2%). Therefore, piggyBac can stably transfer genes from separate transposon plasmids for multigene delivery.
FIGURE 4.
Expression and selection of 2 transgenes from 2 plasmids. PBMCs were cotransfected with pIRII-eGFP (5 μg), pIRII-ΔCD19 (5 μg), and pCMV-PB (5 μg). The co-transfected cells were stimulated with CD3/28 MAbs in the presence of IL-15. A, The cells were analyzed for GFP and CD19 expression by flow cytometry on day 1 and weekly thereafter. B, On day 8 a fraction of the T cells were selected for expression of CD19 using magnetic beads, and then immediately stimulated on CD3/28 MAbs-coated plates in the presence of IL-15 for 2 weeks. The cells were analyzed for GFP and CD19 expression on day 22. At this time the T-cell phenotype of selected and unselected cells were compared using the subset markers, CD4 and CD8, and memory markers, CD28 and CD62L, as shown in C and D.
Selection and expansion of transduced T-cells
To obtain large numbers of transduced cells with a high frequency of transgene expression, we co-transfected IL-15-cultured PBMCs with two transposons expressing GFP and ΔCD19 respectively. On day 8, transduced cells were selected based on their expression of CD19 using magnetic beads conjugated to CD19 antibodies, then restimulated on CD3/28 MAbs-coated plates in the presence of IL-15. On day 22, over 80% of cells expressed both transgenes, with the remaining cells expressing only ΔCD19 (Figure 4B). Phenotypic analysis demonstrated variable but apparently non-substantive differences in T-cell subsets and memory markers between cells that were selected and expanded versus cells that were expanded without selection (a mean of 43 ± 4% vs 18 ± 16% of CD4+ T-cells, a mean of 61 ± 7% vs 86 ± 11% of CD8+ T-cells, a mean of 55 ± 10% vs 35 ± 8% of CD28+ T-cells and a mean of 34 ± 14% vs 34 ± 12% of CD62L+ T-cells) (Figures 4C and D). However, for all donors, both CD4+ and CD8+ and memory T-cells were represented in transgene-positive populations.
Enhanced expansion of piggyBac-transduced T-cells using feeder cells
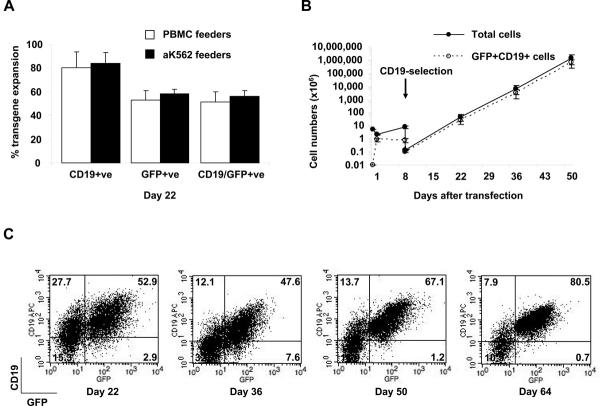
Although piggyBac-transduced T-cells could be expanded by culture in IL-15, their rate of expansion remained lower than for non-transfected T-cells. For many T-cell therapy applications, large T cell numbers are required, and over 1010 T-cells have been given to human subjects in several clinical trials.1;38–40 To rapidly expand transgene-expressing T-cells, we compared the efficacy of two different irradiated feeder cell types; autologous PBMCs and aK562 cells modified to express the costimulatory molecules CD80, CD86, CD83 and 4-1BB ligand.36 Fifteen million PBMCs cultured overnight with IL-15 were co-transfected with pIRII-eGFP, pIRII-ΔCD19, and pCMV-PB. 6.0 to 6.2 × 106 (mean 6.3 × 106) transfected cells were recovered on day 1 and by day eight, 17.1 to 27.0 × 106 (mean 21.9 × 106) cells were recovered, of which 6% to 17% remained GFP+ CD19+ double-positive . ΔCD19-expressing cells were selected on day 8 using anti-CD19-microbeads and 4.3 × 105 cells were recovered. 1 × 105 cells were activated with CD3/28 MAbs and co-cultured with either 30 Gyirradiated autologous PBMCs or with 80-Gy irradiated aK562 at a responder: feeder ratio of 1:20. Cultures were fed twice weekly with IL-15. Two weeks later, cells were counted and analyzed for GFP and ΔCD19 expression. Similar frequencies of GFP-expressing T-cells were obtained by culture on PBMCs and aK562 feeders (Figure 5A), while a range of 165 to 422 fold expansion (mean 296 fold) of GFP+CD19+ transduced T-calls was achieved after culture on aK562 cells, compared with 16 to 400 fold expansion (mean 155) on PBMCs feeders. After restimulation with CD3/28 MAbs every two weeks, cells could be further expanded on aK562 feeder cells at a similar rate for up to 50 days to produce an extrapolated expansion of up to 8 logs (calculated from small aliquots of T cells maintained in culture) (see Figure 5B) with over 80% of T-cells expressing both transgenes by day 64 (see Figure 5C).
FIGURE 5.
Expansion of selected transgenic T-cells. PBMCs were co-transfected with pIRII-eGFP, pIRII-ΔCD19 and pCMV-PB and then selected for CD19 expression on day 8. The selected cells were immediately transferred into 30 mL of T-cell media with IL-15 in a GP40 flask which had been pre-coated with CD3/28 MAbs together with 30 Gy-irradiated autologous PBMCs or 80-Gy irradiated aK562 at a responder: feeder cell ratio of 1:20 for 2 weeks. A, T-cells were counted and analyzed for GFP and CD19 expression on day 22 after co-transfection. B and C, A fraction of the transduced T-cells were expanded on aK562 cells in GP40 flasks for up to 64 days of total culture. The potential total number of cells on day 50 was extrapolated for part (B). The stability of dual transgene expression over the 64 days in culture with IL-15 and irradiated aK562 feeder cells is shown in C.
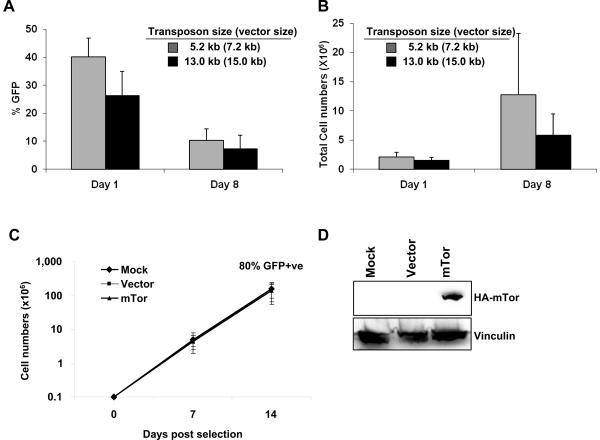
T-cells can be transduced with large transposons and expanded
Expression of a large single or multiple small transgenes in T-cells could be achieved using a single large transposon. To determine how the size of the transposon plasmid affected transfection efficiency and cell expansion, we tested GFP expression from increasingly large transposons. We found that increasing transposon size from 5.2 kb in a 7.2 kb plasmid (pIR-eGFP) to 13 kb in a 15 kb plasmid (pIR-mTorHA-eGFP) consistently decreased the percentage of GFP+ cells at day 1 (range 32% to 47% and 15% to 37% respectively). By day 8, the percentage of transduced cells was similar between the two vectors (range 5% to 16% for both vectors, see Figure 6A), but the expansion of cells transfected with the larger vector consistently declined over the first 8 days of culture (Figure 6B), likely reflecting the toxicity of the large plasmid. However, after sorting for GFP expression on day 8, all transfected cells expanded similarly and rapidly (up to 3 logs in 14 days) and maintained GFP expression (85% for pIR-eGFP and 81% for pIR-mTorHA-eGFP on day 14 after sorting) (Figure 6C). They expressed transgenic mTor protein as shown by western blotting in Figure 6D, indicating that large transposons can be integrated into T cells and do not hinder T-cell expansion once they are stably integrated.
FIGURE 6.
PiggyBac can stably transfer large genes. IL-15-treated PBMCs (from 4 healthy donors) were co-transfected with 5.7 μg pCMV-PB and 5 μg pIR-eGFP (5.7 kb transposon in a 7.2 kb vector), plus 5.6 μg pUC19 (as filler DNA) or 10.6 μg pIR-mTorHA-eGFP (13 kb transposon in a 15 kb vector) and stimulated with OKT3 (50 ng/mL) on feeder cells in the presence of IL-15 (10 ng/mL). The percentage of transfected (GFP+) cells (A) and total cell number (B) was determined at 1 and 8 days post-transfection by FACS analysis and counting by trypan blue exclusion, respectively. Transfected cells were enriched in each culture to >80% after sorting for GFP+ cells. Sorted GFP+ cells from the pIR-eGFP and pIR-mTorHA-eGFP transfections expanded similarly and rapidly after stimulation with OKT3 on feeder cells and maintained GFP expression (shown in parentheses above each data point) (C). Mock transfected cells were subjected to the transfection procedure without DNA and were not sorted. Expansion data is representative of 3 healthy donors. D is a western blot showing the expression of mTorHA in transduced T cells using an antibody to the HA tag.
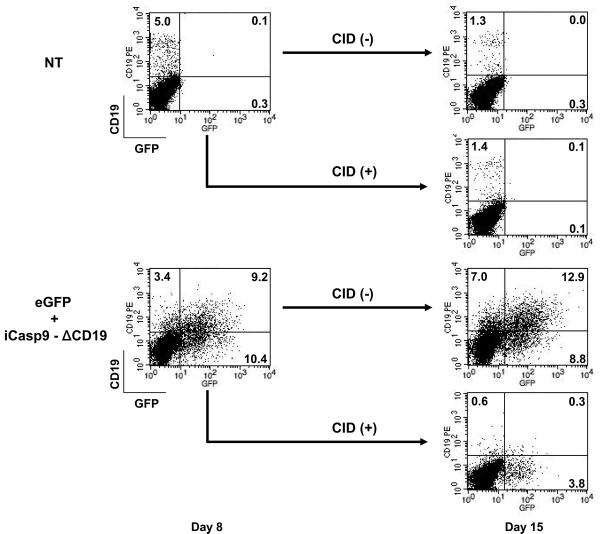
Selection of T-cells expressing a functional suicide gene
Since any transduced T cell has the potential for long term survival and expansion in vivo, and hence for sustained and progressive adverse effects, we evaluated the function of the inducible caspase 9, (iCasp9) suicide gene when it was inserted in the piggyBac transposon together with a selectable marker, ΔCD19.32;41 IL-15-treated PBMCs were co-transfected with three transgenes on two transposons, pIRII-iCasp9.2A.ΔCD19 (5μg), pIRII-eGFP (5μg), and pCMV-PB (5μg). Non-transfected PBMCs were used as controls. After 7 days stimulation with CD3/28 MAbs in the presence of IL-15, the CID was added at 50 nM. After one week, we analyzed the cells for their expression of ΔCD19, which was expressed from the same transposon as iCasp9. Cells expressing eGFP alone from the second transposon were unaffected by the dimerizer drug and survived in the culture, but the frequency of CD19+ T-cells in the population that received pIRII-iCasp9.2A.ΔCD19 was reduced from 20% to less than 1% (Figure 7). This was below the percentage of CD19+ cells in the control eGFP alone-transduced population and likely represented contamination from normal CD19+ B cells rather than a surviving transduced population.
FIGURE 7.
Safety switch: Function of suicide transgene. IL-15-treated PBMCs were co-transfected with a suicide gene-encoding plasmid, pIRII-iCasp9.2A.ΔCD19 (5 μg), pIRII-eGFP (5 μg), and pCMV-PB (5 μg) and then stimulated with CD3/28 MAbs in the presence of IL-15. On day 8 the chemical inducer of dimerization (CID) AP20187 was added to the cultures at a concentration of 50 nM. After one week of treatment with CID, we analyzed for GFP and CD19 expression by flow cytometry on day 15.
DISCUSSION
Our results show effective non-viral genetic modification of primary human T lymphocytes using the piggyBac transposon system. Stable reporter gene expression was initially around 20%, but after culture optimization and addition of IL-15, this level increased to ~40%, even without selection. We showed efficient multigene delivery using two separate transposons and stable co-expression of two separate genes in up to 28% of T-cells. After magnetic bead selection, we obtained stable expression of genes from two separate transposons at >80% efficiency in cells which could be rapidly expanded in culture, for over 50 days whilst maintaining stable transgene expression. We also demonstrate the feasibility and functionality of piggyBac-mediated delivery of an inducible suicide gene into human T-cells.
The major difficulty we faced was the toxicity of the transduction procedure. Nucleofection itself is toxic to T cells and the addition of DNA further decreased cell survival to about 40% by 24 hours after transfection. More importantly it also reduced the subsequent expansion rate of both transduced and non-transduced T cells. Increasing the amount of DNA and the size of the transposon plasmid further increased this toxicity. Other methods of gene transfer produce significantly less toxicity than nucleofection. Lentivirus and retrovirus vectors have high transduction efficiency in human T cells and low toxicity,42 and have been used successfully in T-cell gene transfer studies, both for therapeutic transgenes and for the transmission of marker genes.43;44 However lentiviruses and retroviruses have a smaller cargo capacity and are prohibitively expensive to manufacture at clinical grade. Lipofection approaches have less toxicity than electroporation, but have poor efficacy with suspension cells and efficient lipofection of primary human T-cells has not been reported.45
Given the potential advantages of transposons, we sought means of improving culture conditions, to compensate for the toxicity. Although the addition of survival-promoting cytokines such as IL-15 or IL-7 did not appear to decrease the initial adverse effects of nucleofection, these agents increased both the frequency of gene expression and the ability of the transduced T cells to expand. These cytokines are known to induce the expression of anti-apoptotic molecules like bcl2 that promote T cell survival.46;47 T-cell expansion could be improved further by the use of feeder cells and both autologous PBMCs and K562 cells modified to express the costimulatory molecules, CD80, CD86 and 4-1BB ligand proved highly effective.36 Autologous PBMCs are the simplest source of feeder cells, but PBMCs cannot be standardized, large cell numbers may be required, making scalability difficult, and cancer patient PBMCs may contain negative regulatory components. aK562 cells are HLA class I and class II-negative and therefore do not induce alloreactivity or skew the antigen specificity of the T-cell population.48 aK562 reproducibly enhanced growth rates to a greater level than PBMCs. This difference is likely because aK562 cells express costimulatory molecules at a higher level than PBMCs, in which these molecules are largely limited to monocytes and B cell subpopulations. Gene-modified K562 cells have already been used as tumor vaccines in clinical trials49 and so the use of this cell line for the expansion of clinical grade T-cells is feasible. Using a combination of piggyBac transduction and aK562 feeder cells and a proposed starting blood volume of ~50 mL, initial yields of about 2 × 106 selected, stably transduced T-cells could be expanded by 4 to 5 logs to over 1010 transduced T-cells in four to five weeks and to 1012 in six to seven weeks. These numbers would be more than adequate for any current T-cell transfer clinical study.3;4;43;50
The piggyBac strategy was effective even for large transgenes like mTor (Figure 6). This is in contrast to our previous failed attempts to transfer mTor into human T-cells using lenti- or retrovirus vectors (unpublished observations). We are currently redesigning our transposons to eliminate unnecessary DNA sequences and to include the piggyBac enzyme within the plasmid, but outside of the transposon sequences so that the transposase is not integrated. Other modifications of plasmids that may reduce size and toxicity include DNA condensation, the use of minicircles in which the DNA backbone is eliminated and reduction of CpG sequences that may activate toll-like receptors.51
Transposons dramatically increase the rate of integration of transfected DNA into cells compared to that of standard plasmids. Previous reports have evaluated the SB transposon for the gene modification of human T-cells. Huang et al.52 described 3–10% stable reporter gene expression in T-cells and subsequently 1–3% expression of chimeric antigen receptors (CARs) in T-cells that could subsequently be selected for expansion by flow cytometry.53 Singh et al also used SB to express CD19-CAR, but did not evaluate gene expression until day 28 after CD19 antigen-driven expansion, at which time CD19-CAR was expressed in over 25% of T-cells.23 Further antigen driven expansion in the presence of IL-15 led to CD19-CAR expression in 99% of T-cells by day 70, with retention of memory markers on subpopulations of T-cells. Our own data show stable transfection of T cells without cell selection and transgene expression remains stable for at least 9 weeks, regardless of the T cell activation state. This is in contrast with the downregulation of transgene expression from retrovirus vectors that occurs in human T cells in the absence of antigenic stimulation.21;22
The capacity to select T-cells carrying suicide genes increases the safety of gene modified T-cells. If transgenes are to be expressed from multiple transposons, the suicide gene must be expressed in all transduced cells. This requires first, that a significant number of cells are transfected with the transposase plasmid as well as with multiple transposon plasmids and second, that the transposase can integrate two or more transposons. Our data confirm that piggyBac can stably lead to expression of multiple transposons in each T cell, data which replicate our earlier observations in human embryonic kidney cells,28 and that are also consistent with their recent use to generate inducible pluripotent stems cells from normal skin fibroblasts by transfer of canonical ES cell transcriptional regulators.25;28;30
The potential for piggyBac to stably transduce T cells with multiple genes is important for successful cancer therapy. Although adoptively transferred T-cells have regularly proved able to control virus infections after stem cell transplantation and to produce complete remissions in EBV-associated lymphoma and nasopharyngeal carcinoma as well as in melanoma, extension to other cancers has proved more difficult, due to their multiplicity of immune evasion strategies. The ability to introduce multiple countermeasures into the transferred T cells and their progeny may therefore broaden the applicability of adoptive T cell therapies to many additional tumors. Adoption of piggyBac transposons for this purpose should be facilitated by their lower costs and high efficiency.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Malcolm K Brenner for careful reading of the manuscript.
Sources of Support. This work was supported in parts by a Specialized Centers for Cell-based Therapy (SCCT) grant from NIH-NHLBI 1 U54 HL1081007 and an NIH-NCI lymphoma SPORE P50 CA126752. MHW is supported by a career development award from the Department of Veterans Affairs.
Footnotes
Financial Disclosure: All authors have declared there are no conflicts of interest in regards to this work.
Reference List
- 1.Riddell SR, Walter BA, Gilbert MJ, Greenberg PD. Selective reconstitution of CD8+ cytotoxic T lymphocyte responses in immunodeficient bone marrow transplant recipients by the adoptive transfer of T cells clones. Bone Marrow Transpl. 1994;14:S78–S84. [PubMed] [Google Scholar]
- 2.Rooney CM, Smith CA, Ng C, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 3.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat.Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 4.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp.Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straathof KC, Bollard CM, Popat U, et al. Treatment of Nasopharyngeal Carcinoma with Epstein-Barr Virus-specific T Lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 6.Bollard C, Gottschalk S, Buza E, et al. The Use of Autologous LMP2-Specific Cytotoxic T Lymphocytes for the Treatment of Relapsed EBV +ve Hodgkin Disease and Non-Hodgkin Lymphoma [abstract] Molecular Therapy. 2005 [Google Scholar]
- 7.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following nonmyeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J.Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer Regression in Patients After Transfer of Genetically Engineered Lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leen AM, Rooney CM, Foster AE. Improving T Cell Therapy for Cancer. Annu.Rev.Immunol. 2006 doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 10.Newcom SR, Gu L. Transforming growth factor beta 1 messenger RNA in Reed-Sternberg cells in nodular sclerosing Hodgkin's disease. J Clin Pathol. 1995;48:160–163. doi: 10.1136/jcp.48.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders [In Process Citation] Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 12.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat.Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 13.Mellor A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem.Biophys.Res.Commun. 2005;338:20–24. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]
- 14.Kershaw MH, Wang G, Westwood JA, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2 3. Hum.Gene Ther. 2002;13:1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 15.Bollard CM, Rossig C, Calonge MJ, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 16.Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc.Natl.Acad.Sci.U.S.A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintarelli C, Vera JF, Savoldo B, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan RA, Couture L, Elroy-Stein O, et al. Retroviral vectors containing putative internal ribosome entry sites: development of a polycistronic gene transfer system and applications to human gene therapy. Nucleic Acids Res. 1992;20:1293–1299. doi: 10.1093/nar/20.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen XY, Mandelbaum S, Li ZH, et al. Tricistronic viral vectors co-expressing interleukin-12 (1L-12) and CD80 (B7-1) for the immunotherapy of cancer: preclinical studies in myeloma. Cancer Gene Ther. 2001;8:361–370. doi: 10.1038/sj.cgt.7700321. [DOI] [PubMed] [Google Scholar]
- 21.Wagner HJ, Bollard CM, Vigouroux S, et al. A strategy for treatment of Epstein-Barr virus-positive Hodgkin's disease by targeting interleukin 12 to the tumor environment using tumor antigen-specific T cells. Cancer Gene Ther. 2004;11:81–91. doi: 10.1038/sj.cgt.7700664. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein MP, Salem ML, Kadima AN, et al. Loss of T cell-mediated antitumor immunity after construct-specific downregulation of retrovirally encoded T-cell receptor expression in vivo. Cancer Gene Ther. 2009;16:171–183. doi: 10.1038/cgt.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh H, Manuri PR, Olivares S, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Guo H, Kang J, et al. Sleeping Beauty transposon-mediated engineering of human primary T cells for therapy of CD19+ lymphoid malignancies. Mol.Ther. 2008;16:580–589. doi: 10.1038/sj.mt.6300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser MJ, Ciszczon T, Elick T, Bauser C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol.Biol. 1996;5:141–151. doi: 10.1111/j.1365-2583.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 26.Ding S, Wu X, Li G, et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Wu SC, Meir YJ, Coates CJ, et al. piggyBac is a flexible and highly active transposon as compared to sleeping beauty, Tol2, and Mos1 in mammalian cells. Proc.Natl.Acad.Sci.U.S.A. 2006;103:15008–15013. doi: 10.1073/pnas.0606979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson MH, Coates CJ, George AL., Jr. PiggyBac transposon-mediated gene transfer in human cells. Mol.Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 29.Karsi A, Moav B, Hackett P, Liu Z. Effects of insert size on transposition efficiency of the sleeping beauty transposon in mouse cells. Mar.Biotechnol.(NY) 2001;3:241–245. doi: 10.1007/s101260000072. [DOI] [PubMed] [Google Scholar]
- 30.Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009 doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaji K, Norrby K, Paca A, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009 doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan L, Freeman KW, Khan T, Pham E, Spencer DM. Improved artificial death switches based on caspases and FADD. Hum.Gene Ther. 1999;10:2273–2285. doi: 10.1089/10430349950016924. [DOI] [PubMed] [Google Scholar]
- 33.Straathof KC, Pule MA, Yotnda P, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnelly ML, Hughes LE, Luke G, et al. The `cleavage' activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring `2A-like' sequences. J.Gen.Virol. 2001;82:1027–1041. doi: 10.1099/0022-1317-82-5-1027. [DOI] [PubMed] [Google Scholar]
- 35.Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol.Ther. 2007;15:981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc.Natl.Acad.Sci.U.S.A. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boeckh M, Manley P, Greenberg P, Kirby K, Riddell S. A phase II study of cellular adoptive immunotherapy with donor-derived CMV-specific T- cell clones as prophylaxis for CMV disease in allogeneic HCT recipients [abstract] Bone Marrow Transpl. 2007;39(Sullp 1):S2. [Google Scholar]
- 40.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008 doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Straathof KC, Spencer DM, Sutton RE, Rooney CM. Suicide genes as safety switches in T lymphocytes. Cytotherapy. 2003;5:227–230. doi: 10.1080/14653240310001497. [DOI] [PubMed] [Google Scholar]
- 42.Rossig C, Bollard CM, Nuchtern JG, Merchant DA, Brenner MK. Targeting of G(D2)-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes 5. Int.J Cancer. 2001;94:228–236. doi: 10.1002/ijc.1457. [DOI] [PubMed] [Google Scholar]
- 43.Rooney CM, Smith CA, Ng CYC, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 44.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in neuroblastoma patients. Nat Med. 2008 doi: 10.1038/nm.1882. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puls R, Minchin R. Gene transfer and expression of a non-viral polycation-based vector in CD4+ cells. Gene Ther. 1999;6:1774–1778. doi: 10.1038/sj.gt.3301022. [DOI] [PubMed] [Google Scholar]
- 46.Li XC, Demirci G, Ferrari-Lacraz S, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat.Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 47.Crawley AM, Katz T, Parato K, Angel JB. IL-2 receptor gamma chain cytokines differentially regulate human CD8+CD127+ and CD8+C. Int.Immunol. 2009;21:29–42. doi: 10.1093/intimm/dxn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amrolia PJ, Muccioli-Casadei G, Huls H, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nemunaitis J, Jahan T, Ross H, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006;13:555–562. doi: 10.1038/sj.cgt.7700922. [DOI] [PubMed] [Google Scholar]
- 50.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gill DR, Pringle IA, Hyde SC. Progress and Prospects: The design and production of plasmid vectors. Gene Ther. 2009 doi: 10.1038/gt.2008.183. [DOI] [PubMed] [Google Scholar]
- 52.Huang X, Wilber AC, Bao L, et al. Stable gene transfer and expression in human primary T cells by the Sleeping Beauty transposon system. Blood. 2006;107:483–491. doi: 10.1182/blood-2005-05-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang X, Wilber A, McIvor RS, Zhou X. DNA transposons for modification of human primary T lymphocytes. Methods Mol.Biol. 2009;506:115–126. doi: 10.1007/978-1-59745-409-4_9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.