Abstract
The role of cAMP in the pulmonary vasculature during the transition from intrauterine to extrauterine life is poorly understood. We hypothesized that cAMP levels are regulated by alterations in phosphodiesterase 3 (PDE3), which hydrolyzes cAMP. PDE3 protein expression and hydrolytic activity were increased in resistance pulmonary arteries (PA) from spontaneously breathing one-day-old (1dSB) lambs relative to equivalent-gestation fetuses. This was accompanied by a decrease in steady-state cAMP. Ventilation with 21% O2 and 100% O2 for 24h disrupted the normal transition, whereas ventilation with 100% O2+inhaled NO (iNO) for 24h restored both PDE3 activity and cAMP to 1dSB levels. Consistent with these findings, relaxation to milrinone, a PDE3 inhibitor, was greater in PA isolated from 1dSB and 100% O2+iNO lambs, relative to fetal, 21% O2, and 100% O2 lambs. In conclusion, PDE3 expression and activity in PA dramatically increase after birth, with a concomitant decrease in steady-state cAMP. Ventilation with either 21% O2 or 100% O2 blunts this PDE3 increase, whereas iNO restores PDE3 activity to levels equivalent to 1dSB lambs. The vasodilatory effects of milrinone were most pronounced in vessels from lambs with the highest PDE3 activity, i.e. 1dSB and 100% O2+iNO lambs. Thus, milrinone may be most beneficial when used in conjunction with iNO.
In utero, pulmonary vascular resistance (PVR) is high as blood shunts through fetal circulatory pathways away from the lungs to the placenta. After birth, PVR rapidly falls to facilitate the intrauterine to extrauterine transition. Multiple mediators have been implicated in the normal transition, including cGMP and cAMP. While regulation of cGMP and its signaling mechanisms have been extensively studied in the neonatal pulmonary vasculature, less is known about the role of cAMP in the pulmonary vascular transition at birth.
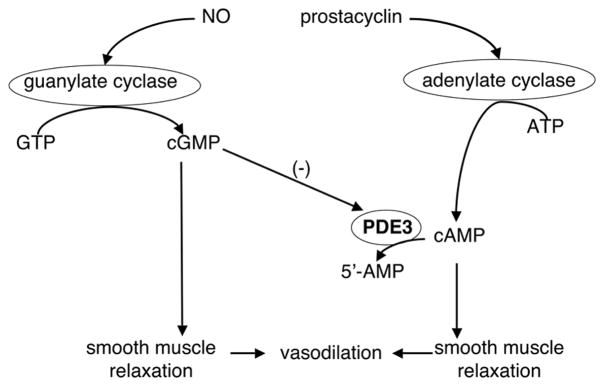
At the time of birth, increased oxygen tension leads to increased cyclooxygenase activity and prostacyclin levels. Prostacyclin activates adenylate cyclases (AC) to synthesize cAMP, and the cyclic nucleotide phosphodiesterases (PDEs) degrade cAMP (Figure 1) (1). AC2 is an isoform identified in pulmonary artery (PA) smooth muscle cells (2) and PDE3 has been implicated in the pulmonary vasculature as one of the predominant isoforms for cAMP degradation. PDE3 has high affinities for both cAMP and cGMP, but the Vmax for cAMP is approximately four- to ten-fold higher than for cGMP (3,4). As PDE3 activity is positively regulated by cAMP and negatively regulated by cGMP (3,5,6), it is referred to as the cGMP-inhibited PDE (Fig. 1).
Figure 1. AC-cAMP-PDE3 pathway.
cAMP is synthesized by AC and degraded by PDE3. cAMP and cGMP compete for the PDE3 active sites, but cGMP is hydrolyzed more slowly than cAMP, resulting in PDE3 inhibition.
The AC-cAMP-PDE3 pathway and its role in pulmonary vascular smooth muscle relaxation have not been well studied perinatally or in infants with impaired transition, such as those with persistent pulmonary hypertension of the newborn (PPHN). PPHN results from multiple causes affecting 2 per 1000 live-born term infants (7). Most therapies for PPHN are supportive, including mechanical ventilation, often with high levels of supplemental oxygen. The only FDA-approved pulmonary vasodilator for neonates with PPHN is inhaled NO (iNO). However, 30–40% of infants do not respond or sustain their response to iNO (8,9), and iNO has not been shown to decrease neurodevelopmental disability in infants with PPHN (9). Thus, additional treatment options are needed.
Milrinone, a PDE3 inhibitor, has an additive pulmonary vasodilator effect when combined with iNO in a rabbit model of experimental pulmonary hypertension (10). Additionally, case reports have described improved oxygenation associated with milrinone use in infants with PPHN (11,12). Although there have been several studies investigating the AC-cAMP-PDE3 pathway using the chronic hypoxia rat model of pulmonary hypertension (5,13,14), no studies to date have been published on the developmental regulation of this pathway. Therefore, using an ovine model, we investigated the hypothesis that the AC-cAMP-PDE3 pathway is developmentally regulated at birth. Because exposure to common treatments for the sick newborn infant, i.e. 100% O2, can cause changes in the pulmonary vasculature of healthy newborn lambs (15), we also determined the impact of mechanical ventilation, supplemental oxygen, and iNO therapy on the regulation of this pathway.
METHODS
Animal Protocols
The Laboratory Animal Care Committee at the State University of New York at Buffalo approved this study and detailed methods were previously published (16). Time-dated pregnant mixed breed Dorsett ewes and newborn lambs were obtained (Swartz family farm; Attica, NY). Five groups were studied: fetus (n=8), spontaneously breathing one-day-old (1dSB) lambs (n=5), lambs ventilated for 24h with 21% O2 (n=6), 100% O2 (n=5), or 100% O2+20ppm iNO (100% O2+iNO, n=5). Lambs were studied at 135d gestation, representing 93% of the 143d term gestation (17). Pregnant ewes were anesthetized with pentothal and halothane; the 135d-gestation fetuses were delivered by Cesarean section and killed by rapid exsanguination through a direct cardiac puncture. 1dSB lambs were healthy newborn lambs that delivered spontaneously and breathed room air; three were born after time-dated pregnancies with gestational ages of 134–138 days, and two additional lambs exhibited hoof maturity equivalent to 135d-gestation lambs. At 24h after birth, these lambs were anesthetized with pentothal and sacrificed as above. Ventilated lambs were delivered by Cesarean section at 135d gestation, intubated, given Infasurf 3mL/kg (ONY Inc, Amherst, NY), and ventilated with 21% O2, 100% O2 or 100% O2+iNO for 24h. Arterial pCO2 was maintained at 35–50 mmHg. Umbilical catheters were inserted, and lambs received IV fluids and sedation with fentanyl and pancuronium bromide. After 24h of ventilation, lambs were anesthetized and sacrificed as above. For animals in each group, heart and lungs were removed en bloc and fifth-generation resistance PA (inner diameter-500 μm) were isolated. Tissue samples were snap-frozen in liquid nitrogen and stored at −80°C.
Western Blot for AC2 and PDE3A
Total protein was isolated from snap-frozen PA tissue from lambs in each study group using the commercially-available PARIS kit (Ambion, Austin, TX) with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitor cocktail (EMD Biosciences, San Diego, CA), as previously described (18). Protein concentration was determined using the Bradford method (19). Western blot was performed as previously described (18). Membranes were incubated with primary antibody at 4°C overnight at the appropriate dilution (1:100 for AC2 [Santa Cruz Biotechnology, Santa Cruz, CA], 1:500 for PDE3A [Santa Cruz], 1:2000 for β-actin [Sigma-Aldrich]) followed by secondary antibody at the appropriate dilution (anti-mouse [1:1000, Pierce, Rockford, IL]; anti-rabbit [1:2000, Pierce]; anti-goat [1:5000, Santa Cruz]). Membranes were washed and bands visualized via chemiluminescence (Pierce) using a Digital Science Image Station (Kodak, Rochester, NY). Expression of AC2 and PDE3A was normalized to β-actin. Results were compared to fetal lambs.
PDE3 Activity Assay
PDE3 activity was measured using a commercially available colorimetric PDE assay kit (Biomol, Plymouth Meeting, PA) as previously described (18). Total protein was prepared as described above. Free phosphate contamination was removed by Centri-Spin 10 columns (Princeton Separations, Adelphia, NJ). The reactions were started in a timed fashion. Each sample was read in duplicate ± milrinone (Biomol, 100 μM) to determine PDE3-specific cAMP-hydrolytic activity. Samples were incubated 30 min at 30°C and stopped in a timed fashion with Biomol Green (Biomol). Samples were incubated on an orbital shaker at room temperature for 30 min. Results were measured using a Labsystems Multiskan EX automated plate reader (Thermo Electron, Milford, MA). The difference between the pmol cAMP hydrolyzed per mg tissue per min with and without milrinone represents the PDE3-specific cAMP-hydrolytic activity.
Immunohistochemistry for AC2 and PDE3A
Lung sections from lambs in each study group were prepared and stained as previously described (18). Briefly, the right lung middle lobe was removed, and OCT compound (VWR Scientific, West Chester, PA) was pushed into the deflated lobe and allowed to solidify on ice for 15–20 min. Blocks were cut into 8–10 μM sections and mounted onto charged slides. Sections were fixed with acetone (Sigma-Aldrich) for 10 min at 4°C, allowed to air-dry, then washed with 1XPBS (Mediatech, Herndon, VA). Sections were stained overnight at 4°C with anti-PDE3A antibody (1:50) or anti-AC2 antibody (1:20). Utilizing an Alexa Fluor 488 donkey anti-goat secondary antibody (1:200, Molecular Probes/Invitrogen, Carlsbad, CA) for PDE3A and a Rhodamine Red Goat anti-rabbit secondary antibody (1:200, Molecular Probes/Invitrogen) for AC2, tissue localization of PDE3A and AC2 was visualized with a Nikon Eclipse TE-300 fluorescent microscope. Fluorescent images were captured using a CoolSnap digital camera with Metamorph imaging software (Molecular Devices, Sunnyvale, CA).
Cyclic AMP Enzyme Immunoassay (EIA)
Snap-frozen sheep PA tissue was prepared, and cyclic nucleotides extracted according to manufacturer’s protocol (Cayman Chemical, Ann Arbor, MI). PA sample cAMP content was measured by EIA in duplicate using a commercially available kit (Cayman Chemical) and Labsystems Multiskan EX automated plate reader (Thermo Electron). Results are shown as pmol cAMP per mg tissue.
Isolated Vessel Studies
Fifth generation intra-lobar PA (~500 μm) were isolated from lambs in each study group, dissected to preserve endothelium integrity, cut into rings 1–2 mm long and 1–2 mg in weight and studied using standard tissue bath techniques as previously described (20). Rings were suspended in water-jacketed chambers filled with aerated (21% O2-6% CO2) Krebs-Ringer solution. A continuous recording of isometric force generation was obtained by tying each vessel ring to a force displacement transducer (model UC2, Statham Instruments, Hato Rey, PR) connected to a recorder (Gould Instrument Systems, Valley View, OH). After the arterial rings were mounted, they equilibrated for 20 min in the bathing solution. A micrometer was used to stretch the tissues repeatedly over 45 min until PA resting tone remained stable at a passive tension of 0.8 g. Preliminary experiments determined that this procedure provided an optimal length for generation of active tone to exogenous norepinephrine (NE). Wet tissue weights were obtained at the end of each experiment, and contraction responses were normalized to tissue weight.
The following pharmacological agents were used: DL-propranolol, NE hydrochloride, and milrinone (Sigma-Aldrich). Isolated PA were pretreated with propranolol (10−6M) to block β-adrenergic receptors. Arteries were contracted with increasing concentrations of NE (10−8 to 3×10−7M). The final concentration of NE provided 40–50% of contraction force (grams of force per grams of tissue weight) generated by 118 mM KCl. Increasing concentrations of milrinone (10−7 to 3×10−6M) were added to the bath and relaxation responses were measured as %NE contraction. One to two PA rings per protocol were studied from each animal.
Statistical Analysis
All data are expressed as means ± SEM, with ‘n’ representing the number of animals and with significance at p<0.05. Data were analyzed using one-way ANOVA with Bonferroni’s post-hoc testing for parametric data and Kruskal-Wallis with Dunn’s post-hoc testing for nonparametric data (Prism, GraphPad Software Inc., San Diego, CA). ANOVA with repeated measures was used for isolated vessel data (StatView, Abacus Concepts, Berkley, CA).
RESULTS
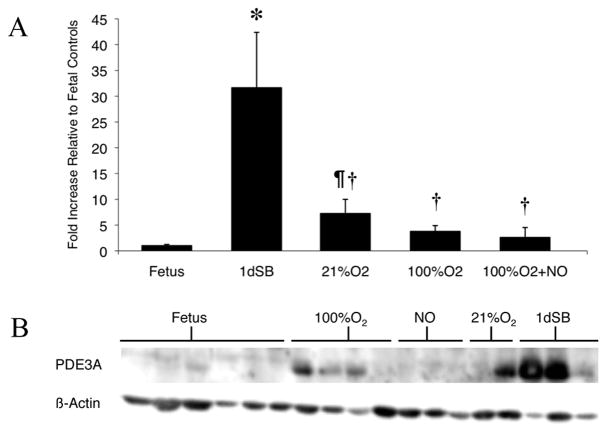
PDE3A protein expression was increased in resistance PA isolated from 1dSB lambs relative to fetal lambs (Figure 2A, B; 31.65±10.74-fold). Expression also increased in 21% O2-ventilated lambsrelative to fetal lambs (Fig. 2A, B; 7.25±2.75-fold); however, this increase was only 23% of the level seen in 1dSB lambs. Lambs ventilated with 100% O2 for 24h displayed decreased PDE3A protein expression to 12% of levels seen in 1dSB lambs (Fig. 2A, B). Lambs ventilated with 100% O2+iNO for 24h had no increase in PDE3A expression compared to fetal lambs, with significant blunting of PDE3A expression when compared to 1dSB lambs.
Figure 2. Increased PDE3A protein expression in resistance PA after birth.
A: PDE3A protein expression was analyzed from PA of fetal lambs, 1dSB lambs, lambs ventilated with 21% O2, lambs ventilated with 100% O2, and lambs ventilated with 100%O2+iNO. Data are shown as means ± SEM relative to fetal lambs. * p<0.001 vs. fetus, ¶ p<0.05 vs. fetus, † p<0.001 vs. 1dSB. B: Representative western blots for PDE3A and β-actin.
PDE3 activity in the PA of the 1dSB lambs was elevated compared to fetal lambs (Figure 3). Lambs ventilated with 21% O2 and 100% O2 had decreased PDE3 activity compared to 1dSB lambs (Fig. 3). Interestingly, the 100% O2+iNO lambs had significantly increased activity relative to fetal lambs, to levels comparable to 1dSB lambs (Fig. 3).
Figure 3. Increased PDE3 activity in resistance PA after birth.
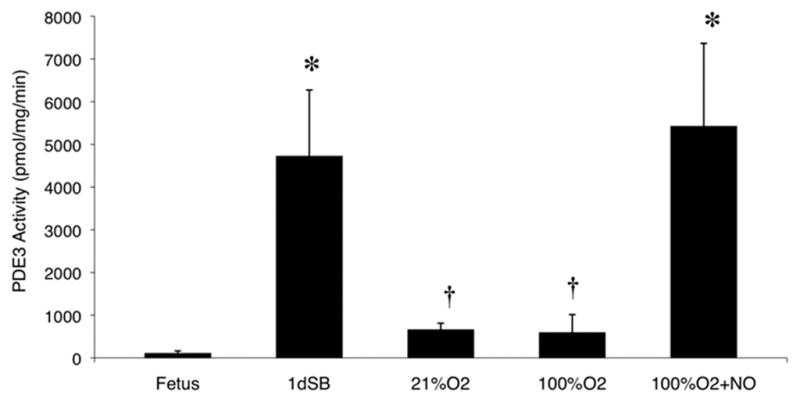
PDE3 activity was measured as the milrinone-inhibitable fraction of total cAMP hydrolysis from PA of fetal lambs, 1dSB lambs, lambs ventilated with 21% O2, lambs ventilated with 100% O2, and lambs ventilated with 100% O2+iNO. Data are shown as means ± SEM (read in duplicate). * p<0.05 vs. fetus, † p<0.05 vs. 1dSB and 100% O2+iNO.
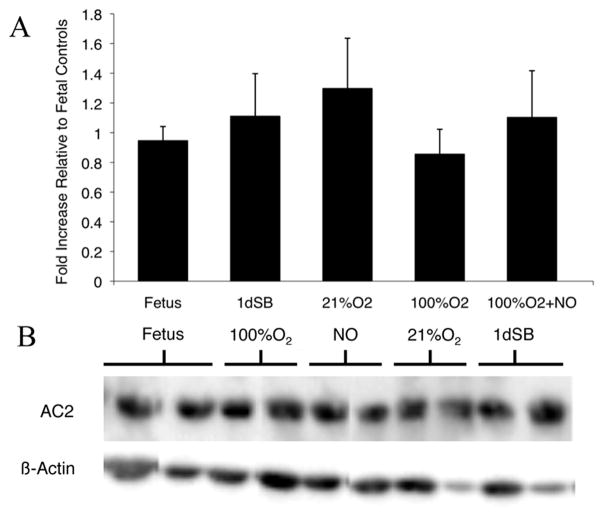
Since cAMP levels are regulated by both AC-mediated production and PDE-mediated degradation, we measured AC2 protein levels in the resistance PA. There was no significant change in AC2 protein expression between the PA of fetal lambs, 1dSB lambs, and those ventilated with 21% O2 or 100% O2±iNO (Figure 4A, B).
Figure 4. No change in AC2 protein expression after birth in resistance PA.
A: AC2 protein expression was analyzed from PA of fetal lambs, 1dSB lambs, lambs ventilated with 21% O2, lambs ventilated with 100% O2, and lambs ventilated with 100% O2+iNO. Data are shown as means ± SEM relative to fetal lambs. B: Representative western blots for AC2 and β-actin.
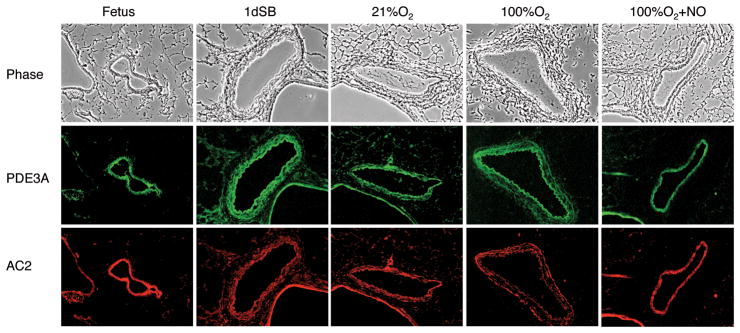
Figure 5 illustrates the localization of the protein expression for PDE3A and AC2 for each study group using immunohistochemistry. PDE3A and AC2 are present in the smooth muscle layer of the PA. Similar to the Western blot results (Fig. 2), PDE3A expression appears highest in the 1dSB lambs and AC2 expression appears uniform across all groups.
Figure 5. PDE3A and AC2 are localized to resistance PA smooth muscle.
The top row shows phase contrast images (10×). The middle and bottom rows show the corresponding sections stained for PDE3A (green) and AC2 (red), respectively. Column 1: fetal lambs, column 2: 1dSB lambs, column 3: lambs ventilated with 21% O2, column 4: lambs ventilated with 100% O2, column 5: lambs ventilated with 100% O2+iNO.
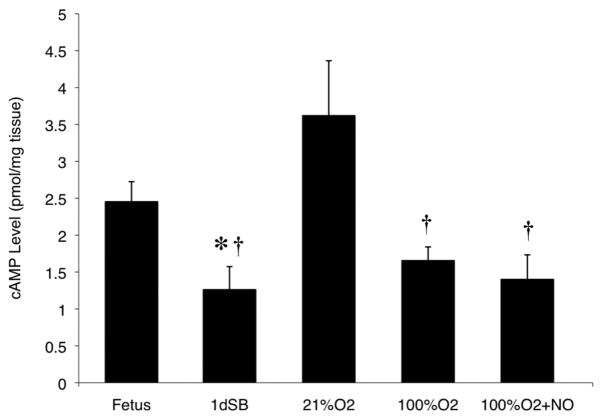
Consistent with the increased PDE3 activity (Fig. 3), steady-state cAMP levels were decreased in resistance PA of 1dSB lambs compared to fetal lambs (Fig. 6). However, in the 21% O2-ventilated lambs, there was reduced PDE3 activity and a significant increase in cAMP compared to 1dSB lambs (Fig. 6). Decreased cAMP levels were seen in lambs ventilated with 100% O2 alone or 100% O2+iNO relative to the 21% O2-ventilated lambs (Fig. 6), but no significant change versus 1dSB lambs.
Figure 6. Decreased cAMP steady-state concentrations after birth in resistance PA.
cAMP concentrations were measured using EIA from PA of fetal lambs, 1dSB lambs, lambs ventilated with 21% O2, lambs ventilated with 100% O2, and lambs ventilated with 100% O2+iNO. Data are shown as means ± SEM (read in duplicate). * p<0.05 vs. fetus, † p<0.01 vs. 21% O2.
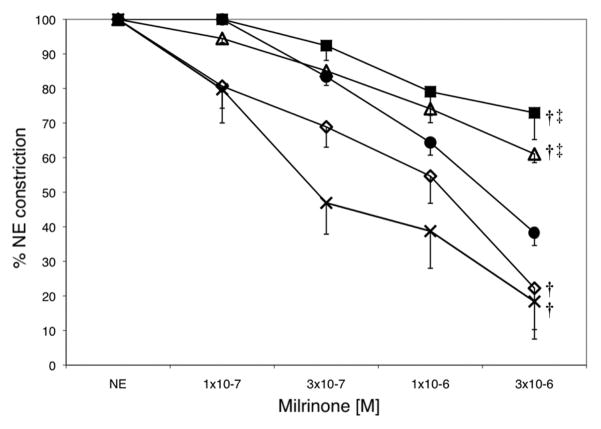
Milrinone relaxed preconstricted PA from fetal lambs in a concentration-dependent manner (Fig. 7). Relative to the fetal lambs, significantly less milrinone-induced relaxation was seen in the PA of lambs ventilated with 21% O2 and 100% O2. The effects of milrinone were significantly enhanced in the PA from 1dSB lambs compared to vessels from fetal lambs and lambs ventilated with either 21% O2 or 100% O2. The 100% O2+iNO lambs displayed milrinone-induced relaxation similar to that seen in PA from 1dSB lambs (Fig. 7).
Figure 7. Milrinone inhibits NE-induced constriction in resistance PA from 1dSB and 100% O2+iNO lambs.
The effect of milrinone was evaluated in isolated PA rings pre-constricted with NE from fetal lambs (filled circles ●, n=10), 1dSB lambs (open diamonds ◇, n=3), lambs ventilated with 21% O2 (open triangles △, n=4), lambs ventilated with 100% O2 (filled squares ■, n=4), and lambs ventilated with 100% O2+iNO (crosshatches ×, n=5). Data are shown as means ± SEM. † p<0.03 vs. fetus, ‡ p<0.02 vs. 1dSB and 100% O2+iNO lambs.
DISCUSSION
Despite promising effects of milrinone in animal models and case studies in promoting pulmonary vascular relaxation for treating pulmonary hypertension (6,10–12,21,22), the AC-cAMP-PDE3 pathway in the pulmonary vasculature has not been well studied in the perinatal period. The ovine pulmonary circulation and its physiologic changes in the fetal to newborn transition period is similar to that of human infants (23); therefore, we used a fetal lamb model to gain a better understanding of this pathway.
In this study, we demonstrate that PA PDE3 expression and activity increased in lambs undergoing normal transition within 24h after birth, corresponding with decreased cAMP. Compared with the normal transition, mechanical ventilation immediately after delivery with either 21% O2 or 100% O2 caused a decrease in PA PDE3 expression and activity, with a resultant loss in vasodilatory response to a PDE3 inhibitor, milrinone. In contrast, following the addition of iNO, a decrease in PDE3A protein expression was observed, but with high PDE3 activity and similar cAMP levels as compared to 1dSB lambs. As a result, milrinone was most effective at inhibiting NE-induced vasoconstriction in the PA of 1dSB and 100% O2+iNO lambs. Taken together, these data support our hypothesis that the AC-cAMP-PDE3 pathway is developmentally regulated within 24h of birth and alterations in this pathway occur with ventilation, oxygen, and iNO exposure. Therefore, we speculate that milrinone use as a pulmonary vasodilator will be most helpful in lambs with elevated PDE3 activity such as those lambs ventilated with 100% O2+iNO.
The increase in PDE3 expression and activity, along with a decrease in cAMP in the 1dSB lambs may contribute to the regulation of basal pulmonary vascular tone after birth. This is in contrast to the increased cGMP levels, the other critical cyclic nucleotide pulmonary vasodilator, which are driven by rising NO levels at the time of birth to promote pulmonary vascular relaxation during the fetal to newborn transition period (24). Our findings indicate that increased PDE3 activity may contribute to the impaired relaxation of the pulmonary vasculature after birth relative to the adult (23), although the regulation of this pathway is still unknown beyond 24h after birth.
Ventilation with both 21% O2 and 100% O2 attenuated this natural rise in PA PDE3 expression and activity (Fig. 2 and 3). However, we noted that cAMP levels were elevated only in the 21% O2-ventilated group (Fig. 6). Physical expansion and mechanical stretch of the lung promotes release of vasoactive substances, thereby increasing pulmonary blood flow and decreasing PVR (23). This effect may be involved in the cAMP increase seen in the 21% O2-ventilated group. This stretch effect of mechanical ventilation appears somewhat different in the context of ventilation with 100% O2, as those animals showed decreased PDE3 expression and activity and impaired responsiveness to milrinone like the 21% O2-ventilated lambs (Figs. 2, 3, and 7), although their cAMP levels were more consistent with the 1dSB lambs (Fig. 6). It has previously been demonstrated that ventilation with 100% O2 increases NE-induced pulmonary vasoconstriction relative to nonventilated lambs, perhaps through oxidative damage of other vasodilatory pathways in the PA (16). The present study provides evidence for deleterious effects of mechanical ventilation with oxygen on the neonatal pulmonary vasculature through disruption of the normal AC-cAMP-PDE3 axis.
There are possible explanations for the relatively low cAMP seen in the 100% O2-ventilated lambs. In the present study, we evaluated the AC-cAMP-PDE3 axis only 24h after birth. It is clear from studies in other vascular tissues that subcellular localization of the components of the AC-cAMP-PDE3 axis, whether cytosolic or membrane-localized, is critical for appropriate function (25). Enzyme complexes may localize substrates to various parts of a cell. Although the substrate measured in the intact PA is relatively unchanged, the particular cellular localization of the substrate may be key to biochemical and physiologic changes. Similarly, our observations represent a single point in time in the immediate postnatal period that may not completely reflect all changes in vascular cAMP after the normal transition.
Ventilation with 100% O2+iNO for 24h had no effect on PA PDE3A expression (Fig. 2), but interestingly, it increased PDE3 activity (Fig. 3) and decreased steady state cAMP to levels similar to 1dSB lambs (Fig. 6). The discordance between PDE3 expression and activity seen in these iNO-treated lambs suggests a dramatic increase in the specific activity of the PDE3 protein. The mechanism for this unexpected effect of iNO is unknown at this time, but we hypothesize that this is due to post-translational modifications, such as nitrosylation or phosphorylation of PDE3. Ventilation of one-month-old lambs with iNO for six hours has been shown to impair endothelial NOS expression and activity in lung tissue, but increase circulating plasma cGMP, which has been described to inhibit PDE3 activity (26). However, these lambs were beyond the neonatal period and it remains unclear whether circulating cGMP necessarily correlates with steady-state levels in resistance PA. One possible explanation is that perhaps cGMP concentrations are much lower relative to cAMP or the subcellular localization of cGMP may be distinct from the subcellular pools of cAMP and PDE3. More consistent with our findings, a decrease in lung tissue cAMP was found in one-month-old lambs ventilated with iNO for 24h (6). In the same study, milrinone prevented rebound pulmonary hypertension upon rapid iNO withdrawal. Although the authors did not directly measure PDE3 expression or activity, their data would support a role for PDE3 in pulmonary vascular tone in those older lambs (6). Thus, our data presented here demonstrate that ventilation with 100% O2+iNO dramatically increases PDE3 activity in neonatal resistance PA despite previous evidence that elevated cGMP levels inhibit PDE3 in other systems (Fig. 1). The mechanism by which iNO increases PDE3 activity in these neonatal lambs is unknown at this time. Although increased PDE3 activity may not intuitively seem favorable, the restoration of activity to levels observed at 24h in healthy spontaneously breathing lambs suggests another potential beneficial effect of iNO in restoring the normal transition.
Interestingly, AC2 expression remained unchanged in 1dSB lambs and after ventilation with 21% O2 and 100% O2±iNO (Fig. 4). Therefore, the changes in vascular cAMP in the perinatal transition period seem to primarily depend on PDE3-mediated hydrolysis. Consistent with this hypothesis, we demonstrated that milrinone inhibited NE-induced vasoconstriction in a concentration-dependent fashion in PA rings from fetal lambs (Fig. 7). The effects of milrinone were significantly less in vessels from the lambs ventilated with 21% O2 and 100% O2 (Fig. 7). This likely reflects the fact that these vessels have very low PDE3 activity (Fig. 3). Furthermore, milrinone appeared to have maximal effects in inhibiting NE-induced vasoconstriction in the 1dSB and 100% O2+iNO vessels, which are those PA with the highest PDE3 activity (Fig. 3).
We acknowledge several limitations to this study. While the perinatal lamb is a robust physiologic model of neonatal pulmonary vascular transition, it is difficult to differentiate the individual effects of mechanical ventilation, hyperoxia, exogenous NO or their combination. Further, we selected lambs that were near-term, and it is possible that further gestational maturation could influence the pathways under investigation. While it appears that iNO rescues normal PDE3 activity, we cannot definitively state that this is solely an effect of iNO or if it requires a combination of iNO with oxygen and/or mechanical ventilation. Further study will be required to delineate the molecular mechanisms for regulation of the AC-cAMP-PDE3 pathway in the neonatal pulmonary vasculature. Finally, we studied the effects of commonly used treatments for neonatal respiratory failure and PPHN, albeit in the healthy lamb. Our findings suggest that milrinone might be an effective adjunctive therapy for iNO. In fact, we recently demonstrated that milrinone relaxed PA isolated from lambs with PPHN (27). However, further study is needed to determine if the combination of milrinone and iNO will be as effective in the remodeled PPHN vasculature.
In conclusion, PDE3 protein expression and activity are increased 24h following birth in ovine PA, with a concomitant decrease in cAMP. This would suggest tight regulation of the AC-cAMP-PDE3 pathway, which may be important in regulating basal pulmonary vascular tone at birth. Impairment of this normal postnatal transition occurs following mechanical ventilation alone and with high levels of supplemental oxygen, while the addition of iNO “normalizes” the PA PDE3 activity to levels seen in nonventilated 24h-old lambs. Although further studies are needed to better elucidate the molecular mechanisms regulating this pathway in the neonatal pulmonary vasculature, these data would suggest that the AC-cAMP-PDE3 pathway might represent an important target with current available therapies, including milrinone, for intervention in infants with a delayed or disrupted pulmonary vascular transition, such as those with PPHN.
Acknowledgments
The authors thank S. Cadichon and P.T. Schumacker for critical reading of this manuscript.
Financial Support: This work was supported by INO Therapeutics Advancing Newborn Medicine Grant for Fellows (BC), Department of Pediatrics, University of Buffalo (SL), and NIH grants HD052902 (KNF), HL086715 (KNF), and HL54705 (RHS).
Abbreviations
- 1dSB
spontaneously breathing one-day-old
- AC
adenylate cyclase
- iNO
inhaled nitric oxide
- NE
norepinephrine hydrochloride
- PA
pulmonary arteries
- PDE
phosphodiesterase
- PPHN
persistent pulmonary hypertension of the newborn
- PVR
pulmonary vascular resistance
References
- 1.Farrow KN, Fliman P, Steinhorn RH. The diseases treated with ECMO: focus on PPHN. Semin Perinatol. 2005;29:8–14. doi: 10.1053/j.semperi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Jourdan KB, Mason NA, Long L, Philips PG, Wilkins MR, Morrell NW. Characterization of adenylyl cyclase isoforms in rat peripheral pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1359–L1369. doi: 10.1152/ajplung.2001.280.6.L1359. [DOI] [PubMed] [Google Scholar]
- 3.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 4.Thompson PE, Manganiello V, Degerman E. Re-Discovering PDE3 Inhibitors - New Opportunities for a Long Neglected Target. Curr Top Med Chem. 2007;7:421–436. doi: 10.2174/156802607779941224. [DOI] [PubMed] [Google Scholar]
- 5.Pauvert O, Salvail D, Rousseau E, Lugnier C, Marthan R, Savineau JP. Characterisation of cyclic nucleotide phosphodiesterase isoforms in the media layer of the main pulmonary artery. Biochem Pharmacol. 2002;63:1763–1772. doi: 10.1016/s0006-2952(02)00919-x. [DOI] [PubMed] [Google Scholar]
- 6.Thelitz S, Oishi P, Sanchez LS, Bekker JM, Ovadia B, Johengen MJ, Black SM, Fineman JR. Phosphodiesterase-3 inhibition prevents the increase in pulmonary vascular resistance following inhaled nitric oxide withdrawal in lambs. Pediatr Crit Care Med. 2004;5:234–239. doi: 10.1097/01.pcc.0000124021.25393.2d. [DOI] [PubMed] [Google Scholar]
- 7.Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, Verter J, Stoll BJ, Lemons JA, Papile L, Shankaran S, Donovan EF, Oh W, Ehrenkranz RA, Fanaroff AA. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 8.Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. The Neonatal Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JD, Fineman JR, Morin FC, 3rd, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, Heymann MA, Zapol WM, Thusu KG, Zellers TM, Wylam ME, Zaslavsky A. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med. 1997;336:605–610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 10.Deb B, Bradford K, Pearl R. Additive effects of inhaled nitric oxide and intravenous milrinone in experimental pulmonary hypertension. Crit Care Med. 2000;28:795–799. doi: 10.1097/00003246-200003000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Bassler D, Choong K, McNamara P, Kirpalani H. Neonatal Persistent Pulmonary Hypertension Treated with Milrinone: Four Case Reports. Biol Neonate. 2006;89:1–5. doi: 10.1159/000088192. [DOI] [PubMed] [Google Scholar]
- 12.McNamara PJ, Laique F, Muang-In S, Whyte HE. Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. J Crit Care. 2006;21:217–222. doi: 10.1016/j.jcrc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Maclean MR, Johnston ED, McCulloch KM, Pooley L, Houslay MD, Sweeney G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther. 1997;283:619–624. [PubMed] [Google Scholar]
- 14.Murray F, MacLean MR, Pyne NJ. Increased expression of the cGMP-inhibited cAMP-specific (PDE3) and cGMP binding cGMP-specific (PDE5) phosphodiesterases in models of pulmonary hypertension. Br J Pharmacol. 2002;137:1187–1194. doi: 10.1038/sj.bjp.0704984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, Wynn KA, Kumar VH, Mathew B, Kirmani K, Morin FC., 3rd Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res. 2007;62:313–318. doi: 10.1203/PDR.0b013e3180db29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakshminrusimha S, Russell J, Wedgwood S, Gugino S, Kazzaz J, Davis J, Steinhorn R. Superoxide Dismutase Improves Oxygenation and Reduces Oxidation in Neonatal Pulmonary Hypertension. Am J Respir Crit Care Med. 2006;174:1370–1377. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith ID. Breed differences in the duration of gestation in sheep. Aust Vet J. 1967;43:63–64. doi: 10.1111/j.1751-0813.1967.tb15065.x. [DOI] [PubMed] [Google Scholar]
- 18.Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res. 2008;102:226–233. doi: 10.1161/CIRCRESAHA.107.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 20.Steinhorn RH, Morin FC, 3rd, Gugino SF, Giese EC, Russell JA. Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol. 1993;264:H2162–H2167. doi: 10.1152/ajpheart.1993.264.6.H2162. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Tolsa JF, Shen H, Raj JU. Effect of selective phosphodiesterase inhibitors on response of ovine pulmonary arteries to prostaglandin E2. J Appl Physiol. 1998;84:13–18. doi: 10.1152/jappl.1998.84.1.13. [DOI] [PubMed] [Google Scholar]
- 22.Hentschel T, Yin N, Riad A, Habbazettl H, Weimann J, Koster A, Tschope C, Kuppe H, Kuebler W. Inhalation of the Phosphodiesterase-3 Inhibitor Milrinone Attenuates Pulmonary Hypertension in a Rat Model of Congestive Heart Failure. Anesthesiology. 2007;106:124–131. doi: 10.1097/00000542-200701000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Fineman JR, Soifer SJ, Heymann MA. Regulation of pulmonary vascular tone in the perinatal period. Annu Rev Physiol. 1995;57:115–134. doi: 10.1146/annurev.ph.57.030195.000555. [DOI] [PubMed] [Google Scholar]
- 24.Shaul PW, Farrar MA, Magness RR. Pulmonary endothelial nitric oxide production is developmentally regulated in the fetus and newborn. Am J Physiol. 1993;265:H1056–H1063. doi: 10.1152/ajpheart.1993.265.4.H1056. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Maurice DH. Expression of cyclic GMP-inhibited phosphodiesterases 3A and 3B (PDE3A and PDE3B) in rat tissues: Differential subcellular localization and regulated expression by cyclic AMP. Br J Pharmacol. 1998;125:1501–1510. doi: 10.1038/sj.bjp.0702227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black SM, Heidersbach RS, McMullan DM, Bekker JM, Johengen MJ, Fineman JR. Inhaled nitric oxide inhibits NOS activity in lambs: potential mechanism for rebound pulmonary hypertension. Am J Physiol. 1999;277:H1849–H1856. doi: 10.1152/ajpheart.1999.277.5.H1849. [DOI] [PubMed] [Google Scholar]
- 27.Lakshminrusimha S, Porta NF, Farrow KN, Chen B, Gugino SF, Kumar VH, Russell JA, Steinhorn RH. Milrinone enhances relaxation to prostacyclin and iloprost in pulmonary arteries isolated from lambs with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med. 2009;10:106–112. doi: 10.1097/PCC.0b013e3181936aee. [DOI] [PMC free article] [PubMed] [Google Scholar]