Abstract
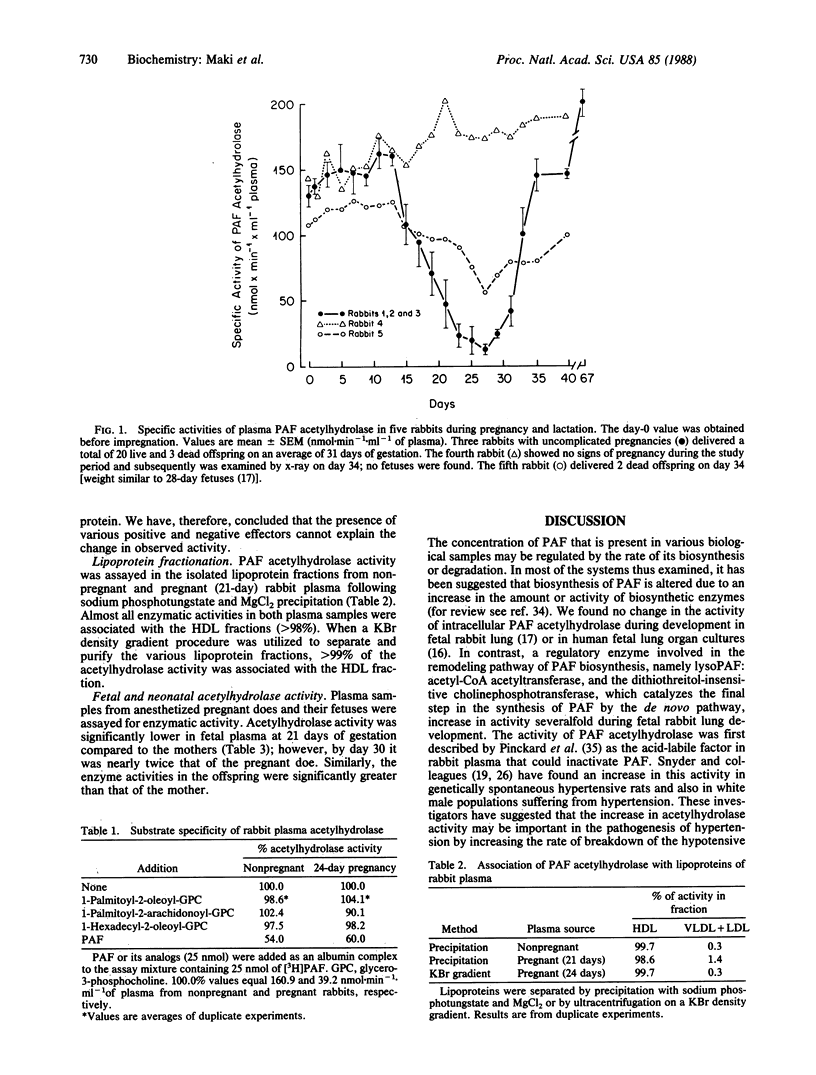
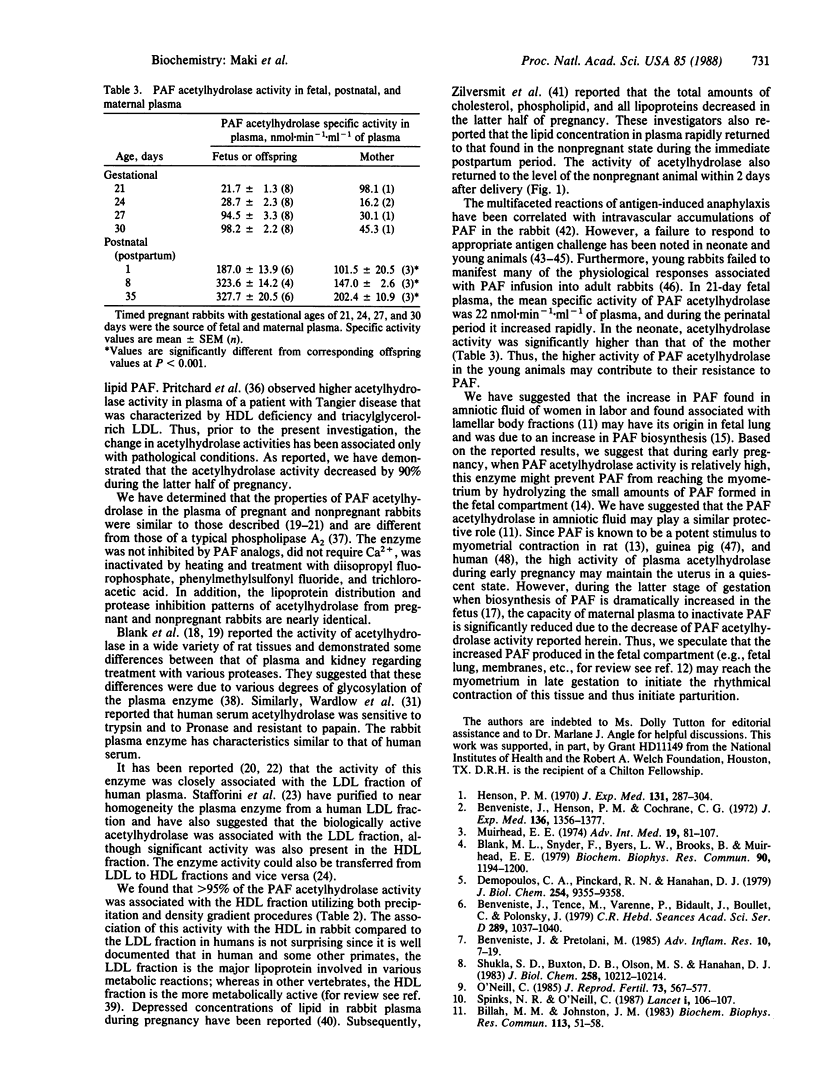
The inactivation of the biologically active ether-containing glycerophospholipid platelet-activating factor (PAF) is catalyzed by the enzyme PAF acetylhydrolase (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine acetylhydrolase, EC 3.1.1.48). The specific activity of acetylhydrolase has been assayed in maternal rabbit plasma prior to and throughout pregnancy and after parturition. The specific activity of acetylhydrolase was 131 +/- 8 nmol.min-1.ml-1 of plasma (mean +/- SEM) in the nonpregnant rabbit. Similar specific activities were found throughout the first half of pregnancy; however, on day 15 the acetylhydrolase activity began to decrease and reached a minimum around day 27. Within 24-48 hr following delivery, the specific activity of the enzyme increased to the levels found in the nonpregnant animals. The specific activity of acetylhydrolase in fetuses (gestational ages of 21-30 days) and neonates increased from 22 nmol.min-1.ml-1 of plasma (21-day-old) to 328 nmol.min-1.ml-1 of plasma (35-day-old rabbits). The decrease in enzymatic activity of the pregnant rabbit plasma cannot be accounted for by the presence of an inhibitor as determined by plasma-mixing experiments with nonpregnant and pregnant (27-day) plasma. The properties of this enzyme in the rabbit were consistent with those reported by others, i.e., substrate specificity, inactivation by heat and various inhibitors, and Ca2+ independency. The activity of PAF acetylhydrolase was associated primarily with the high density lipoprotein fraction in rabbit plasma. The decrease of this enzymatic activity during pregnancy may serve to remove the protective effect on myometrium to allow an increased amount of PAF to come in contact with this tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angle M. J., Pinckard R. N., Showell H. J., McManus L. M. Ontogeny of the responsiveness to intravenous platelet-activating factor. Lab Invest. 1987 Sep;57(3):321–328. [PubMed] [Google Scholar]
- Ban C., Billah M. M., Truong C. T., Johnston J. M. Metabolism of platelet-activating factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) in human fetal membranes and decidua vera. Arch Biochem Biophys. 1986 Apr;246(1):9–18. doi: 10.1016/0003-9861(86)90444-3. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Tencé M., Varenne P., Bidault J., Boullet C., Polonsky J. Semi-synthèse et structure proposée du facteur activant les plaquettes (P.A.F.): PAF-acether, un alkyl ether analogue de la lysophosphatidylcholine. C R Seances Acad Sci D. 1979 Nov 26;289(14):1037–1040. [PubMed] [Google Scholar]
- Billah M. M., Di Renzo G. C., Ban C., Truong C. T., Hoffman D. R., Anceschi M. M., Bleasdale J. E., Johnston J. M. Platelet-activating factor metabolism in human amnion and the responses of this tissue to extracellular platelet-activating factor. Prostaglandins. 1985 Nov;30(5):841–850. doi: 10.1016/0090-6980(85)90012-7. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Johnston J. M. Identification of phospholipid platelet-activating factor (1-0-alkyl-2-acetyl-sn-glycero-3-phosphocholine) in human amniotic fluid and urine. Biochem Biophys Res Commun. 1983 May 31;113(1):51–58. doi: 10.1016/0006-291x(83)90430-8. [DOI] [PubMed] [Google Scholar]
- Binaghi R. A., Oettgen H. F., Benacerraf B. Anaphylactic antibody in the young rat. Int Arch Allergy Appl Immunol. 1966;29(2):105–111. doi: 10.1159/000229692. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Hall M. N., Cress E. A., Snyder F. Inactivation of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine by a plasma acetylhydrolase: higher activities in hypertensive rats. Biochem Biophys Res Commun. 1983 Jun 15;113(2):666–671. doi: 10.1016/0006-291x(83)91778-3. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Lee T., Fitzgerald V., Snyder F. A specific acetylhydrolase for 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (a hypotensive and platelet-activating lipid). J Biol Chem. 1981 Jan 10;256(1):175–178. [PubMed] [Google Scholar]
- Blank M. L., Snyder F., Byers L. W., Brooks B., Muirhead E. E. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1194–1200. doi: 10.1016/0006-291x(79)91163-x. [DOI] [PubMed] [Google Scholar]
- Burstein M., Scholnick H. R. Lipoprotein-polyanion-metal interactions. Adv Lipid Res. 1973;11(0):67–108. [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Farr R. S., Cox C. P., Wardlow M. L., Jorgensen R. Preliminary studies of an acid-labile factor (ALF) in human sera that inactivates platelet-activating factor (PAF). Clin Immunol Immunopathol. 1980 Mar;15(3):318–330. doi: 10.1016/0090-1229(80)90044-6. [DOI] [PubMed] [Google Scholar]
- Farr R. S., Wardlow M. L., Cox C. P., Meng K. E., Greene D. E. Human serum acid-labile factor is an acylhydrolase that inactivates platelet-activating factor. Fed Proc. 1983 Nov;42(14):3120–3122. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargis B. J., Malkiel S. Production of hypersensitivity in the neonatal mouse. J Immunol. 1970 Apr;104(4):942–949. [PubMed] [Google Scholar]
- Henson P. M. Release of vasoactive amines from rabbit platelets induced by sensitized mononuclear leukocytes and antigen. J Exp Med. 1970 Feb;131(2):287–306. doi: 10.1084/jem.131.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D. R., Truong C. T., Johnston J. M. Metabolism and function of platelet-activating factor in fetal rabbit lung development. Biochim Biophys Acta. 1986 Oct 24;879(1):88–96. doi: 10.1016/0005-2760(86)90270-5. [DOI] [PubMed] [Google Scholar]
- Hoffman D. R., Truong C. T., Johnston J. M. The role of platelet-activating factor in human fetal lung maturation. Am J Obstet Gynecol. 1986 Jul;155(1):70–75. doi: 10.1016/0002-9378(86)90081-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee T. C., Malone B., Wasserman S. I., Fitzgerald V., Snyder F. Activities of enzymes that metabolize platelet-activating factor (1-Alkyl-2-acetyl-sn-glycero-3-phosphocholine) in neutrophils and eosinophils from humans and the effect of a calcium ionophore. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1303–1308. doi: 10.1016/0006-291x(82)90928-7. [DOI] [PubMed] [Google Scholar]
- Meng A. L., Pinckard R. N., Halonen M. Ontogeny of the release and reactivity to the mediators of IgE-induced acute allergic reactions in the rabbit: the homologous PCA reaction. J Immunol. 1973 Jun;110(6):1554–1561. [PubMed] [Google Scholar]
- Montrucchio G., Alloatti G., Tetta C., Roffinello C., Emanuelli G., Camussi G. In vitro contractile effect of platelet-activating factor on guinea-pig myometrium. Prostaglandins. 1986 Oct;32(4):539–554. doi: 10.1016/0090-6980(86)90036-5. [DOI] [PubMed] [Google Scholar]
- Muirhead E. E. The role of the renal medulla in hypertension. Adv Intern Med. 1974;19:81–107. [PubMed] [Google Scholar]
- Nishihira J., Ishibashi T., Imai Y., Muramatsu T. Mass spectrometric evidence for the presence of platelet-activating factor (1-0-alkyl-2-acetyl-sn-glycero-3-phosphocholine) in human amniotic fluid during labor. Lipids. 1984 Dec;19(12):907–910. doi: 10.1007/BF02534724. [DOI] [PubMed] [Google Scholar]
- O'Neill C. Examination of the causes of early pregnancy-associated thrombocytopenia in mice. J Reprod Fertil. 1985 Mar;73(2):567–577. doi: 10.1530/jrf.0.0730567. [DOI] [PubMed] [Google Scholar]
- Ostermann G., Kertscher H. P., Winkler L., Schlag B., Rühling K., Till U. The role of lipoproteins in the degradation of platelet-activating factor. Thromb Res. 1986 Nov 1;44(3):303–314. doi: 10.1016/0049-3848(86)90005-8. [DOI] [PubMed] [Google Scholar]
- Pinckard R. N., Farr R. S., Hanahan D. J. Physicochemical and functional identity of rabbit platelet-activating factor (PAF) released in vivo during IgE anaphylaxis with PAF released in vitro from IgE sensitized basophils. J Immunol. 1979 Oct;123(4):1847–1857. [PubMed] [Google Scholar]
- Pritchard P. H., Chonn A., Yeung C. C. The degradation of platelet-activating factor in the plasma of a patient with familial high density lipoprotein deficiency (Tangier disease). Blood. 1985 Dec;66(6):1476–1478. [PubMed] [Google Scholar]
- Sabbah H. N., Khaja F., Brymer J. F., McFarland T. M., Albert D. E., Snyder J. E., Goldstein S., Stein P. D. Noninvasive evaluation of left ventricular performance based on peak aortic blood acceleration measured with a continuous-wave Doppler velocity meter. Circulation. 1986 Aug;74(2):323–329. doi: 10.1161/01.cir.74.2.323. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Buxton D. B., Olson M. S., Hanahan D. J. Acetylglyceryl ether phosphorylcholine. A potent activator of hepatic phosphoinositide metabolism and glycogenolysis. J Biol Chem. 1983 Sep 10;258(17):10212–10214. [PubMed] [Google Scholar]
- Snyder F. Chemical and biochemical aspects of platelet activating factor: a novel class of acetylated ether-linked choline-phospholipids. Med Res Rev. 1985 Jan-Mar;5(1):107–140. doi: 10.1002/med.2610050105. [DOI] [PubMed] [Google Scholar]
- Spinks N. R., O'Neill C. Embryo-deprived platelet-activating factor is essential for establishment of pregnancy in the mouse. Lancet. 1987 Jan 10;1(8524):106–107. doi: 10.1016/s0140-6736(87)91947-7. [DOI] [PubMed] [Google Scholar]
- Stafforini D. M., McIntyre T. M., Carter M. E., Prescott S. M. Human plasma platelet-activating factor acetylhydrolase. Association with lipoprotein particles and role in the degradation of platelet-activating factor. J Biol Chem. 1987 Mar 25;262(9):4215–4222. [PubMed] [Google Scholar]
- Stafforini D. M., Prescott S. M., McIntyre T. M. Human plasma platelet-activating factor acetylhydrolase. Purification and properties. J Biol Chem. 1987 Mar 25;262(9):4223–4230. [PubMed] [Google Scholar]
- Tetta C., Montrucchio G., Alloatti G., Roffinello C., Emanuelli G., Benedetto C., Camussi G., Massobrio M. Platelet-activating factor contracts human myometrium in vitro. Proc Soc Exp Biol Med. 1986 Dec;183(3):376–381. doi: 10.3181/00379727-183-42435. [DOI] [PubMed] [Google Scholar]
- Touqui L., Jacquemin C., Dumarey C., Vargaftig B. B. 1-O-alkyl-2-acyl-sn-glycero-3-phosphorylcholine is the precursor of platelet-activating factor in stimulated rabbit platelets. Evidence for an alkylacetyl-glycerophosphorylcholine cycle. Biochim Biophys Acta. 1985 Jan 9;833(1):111–118. doi: 10.1016/0005-2760(85)90258-9. [DOI] [PubMed] [Google Scholar]
- Wardlow M. L., Cox C. P., Meng K. E., Greene D. E., Farr R. S. Substrate specificity and partial characterization of the PAF-acylhydrolase in human serum that rapidly inactivates platelet-activating factor. J Immunol. 1986 May 1;136(9):3441–3446. [PubMed] [Google Scholar]
- Zilversmit D. B., Hughes L. B., Remington M. Hypolipidemic effect of pregnancy in the rabbit. J Lipid Res. 1972 Nov;13(6):750–756. [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]



