Abstract
Lung cancer has a strong etiological association with cigarette smoking. Nicotine, a major component in tobacco smoke, functions as a survival agonist that inhibits apoptosis following various stresses. However, the mechanism of action remains elusive. Mcl-1, a major antiapoptotic protein of the Bcl2 family, is extensively expressed in both small cell (SCLC) and non-small cell lung cancer (NSCLC) cells, suggesting that Mcl-1 may be a therapeutic target of patients with lung cancer. Here we found that nicotine induces Mcl-1 phosphorylation through activation of ERK1/2 in association with increased chemoresistance of human lung cancer cells. Since nicotine stimulates Mcl-1 phosphorylation and survival in cells expressing WT but has no such effects in cells expressing T163A Mcl-1 mutant, this indicates that nicotine induces Mcl-1 phosphorylation exclusively at the T 163 site and that phosphorylation of Mcl-1 at T163 is required for nicotine-induced survival. Mechanistically, nicotine-induced Mcl-1 phosphorylation significantly enhances the half-life of Mcl-1, which renders Mcl-1 a long-term survival activity. Specific depletion of Mcl-1 by RNA interferenceblocks nicotine-stimulated survival and enhances apoptotic cell death. Thus, nicotine-enhanced survival of lung cancer cells may occur through activation of Mcl-1 by phosphorylation at T163 site, which may contribute to development of human lung cancer and/or chemoresistance.
Introduction
Lung cancer is the main cause of cancer deaths in both sexes with an annual mortality rate of 91% (1). Cigarette smoking is by far the most important risk factor in the development of lung cancer. For example, cigarette smokers have a 20-fold higher relative risk of developing lung cancer compared with nonsmokers (1). Ninety percent of all lung cancers are caused by cigarette smoke including second hand smoke (2). Cigarette smoke contains about 4,000 chemicals, 55 of which have been evaluated as carcinogens (3). Nicotine is a major component in tobacco that exists at high concentrations (~90–1000nM) in the blood of smokers (4). Nicotine functions as a survival agonist to inhibit apoptosis induced by diverse stimuli, including chemotherapeutic drugs (5). However, the intracellular signal transduction mechanism(s) involved in nicotine suppression of apoptosis remains enigmatic.
Bcl-2 family members are key regulators of apoptotic cell death and deregulation of these proteins could be oncogenic (6–7). There are at least 20 members in the Bcl2 family, all of which share at least one BH (Bcl-2 homology) domain (8). Recent studies suggest that prognosis of lung cancer is closely linked to the Bcl-2 family members (9–11). Our previous studies have demonstrated that nicotine induces Bcl2 phosphorylation at serine (S) 70 in association with prolonged cell survival (12). We recently discovered that nicotine can also stimulate phosphorylation and inactivation of the proapoptotic proteins (i.e. Bad and Bax) (13–14). However, whether other Bcl-2 family member(s), for example, Mcl-1, is involved in nicotine signal transduction pathways remains unclear. Mcl-1 is a major antiapoptotic member of the Bcl2 family which is extensively expressed in various human lung cancer cells (15). Mcl-1 is one unique member of the Bcl2 family because of its short half-life (30 min ~3h in various cell types) and short-term pro-survival function, which probably relates to the presence of a long proline-, glutamic acid-, serine-, and threonine-rich (PEST) region upstream of the BH domain (16–19). Thus, the mechanism(s) to prolong the half-life of Mcl-1 protein is critical for its long-term survival function. Mcl-1 protein can be phosphorylated at multiple sites that distinctly regulate Mcl-1 protein turnover. For example, ERK1/2-mediated T163 site phosphorylation enhances Mcl-1’s the half-life and antiapoptotic function (20–21). In contrast, S159 phosphorylation by GSK-3β facilitates Mcl-1 ubiquitination and degradation to reduce its survival activity (22). Additionally, the Cdk1/2-mediated S64 site phosphorylation increases the antiapoptotic function but has no effect on its half-life (16). Since nicotine can activate ERK1/2 (1, 12), which is a physiological T163 site kinase of Mcl-1 (20). In this study, we have demonstrated that nicotine induces Mcl-1 phosphorylation at T163 through activation of ERK1/2, which leads to prolonged half-life of Mcl-1 with enhanced antiapoptotic function in human lung cancer cells.
Results
Nicotine Induces Mcl-1 Phosphorylation in Association with Prolonged Survival of Human Lung Cancer Cells
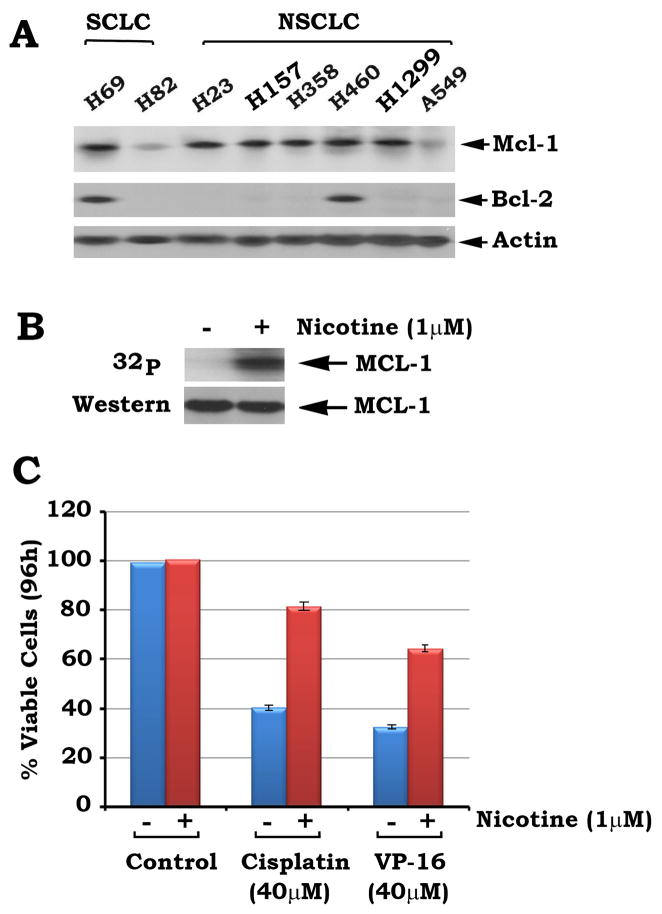
Mcl-1 is a major antiapoptotic member of Bcl2 family, Intriguingly, Mcl-1 is more widely expressed in human lung cancer cells, including SCLC and NSCLC cells, than Bcl2 (Fig. 1A). This suggests that Mcl-1 may play a more extensive role in survival and chemoresistance of human lung cancer cells, especially in those cells that express low or undetectable levels of endogenous Bcl2. To test whether nicotine stimulates Mcl-1 phosphorylation, H1299 cells that express high levels of endogenous Mcl-1 but does not express Bcl2 were metabolically labeled with 32P-orthophosphoric acid and treated with nicotine (1μM) for 30 min. Results indicate that nicotine potently stimulates phosphorylation of endogenous Mcl-1 in human lung cancer H1299 cells (Fig. 1B). Cisplatin and etoposide (VP-16) are currently the most useful clinical drugs for treatment of patients with lung cancer (23). To test the effect of nicotine-induced Mcl-1 phosphorylation on apoptosis, H1299 cells were treated with cisplatin or VP-16 in the absence or presence of nicotine for 96h. Results indicate that cisplatin or VP-16 induces more than 60% of cells undergoing apoptosis and nicotine significantly prolongs cell survival following treatment with cisplatin or VP-16 (Fig. 1C). These findings reveal that nicotine-prolonged cell survival is closely associated with Mcl-1 phosphorylation. Data represent the mean ± S.D. of three determinations. Other lung cancer cell lines (i.e. H69 or H157) were also tested and similar results were obtained (data not shown).
FIGURE 1. Nicotine induces Mcl-1 phosphorylation in association with increased chemoresistance of lung cancer cells.
A, Expression of Mcl-1 or Bcl2 in various lung cancer cell lines was analyzed by Western blot. B, H1299 cells were metabolically labeled with 32P-orthophosphoric acid for 90 min and treated with nicotine for 30 min. Phosphorylation of Mcl-1 was determined by autoradiography. C, H1299 cells were treated with cisplatin (40 μM) or VP-16 (40 μM) in the presence or absence of nicotine (1μM) for 96h. Cell viability was determined by analyzing annexin-V binding on FACS. Data represent the mean ± S.D. of three separate determinations.
Nicotine Induces Activation of ERK1/2 Which Co-Localizes with Mcl-1, and Active ERK1 and ERK2 Directly Phosphorylate Mcl-1 In Vitro
It has been reported that ERK-mediated phosphorylation of Mcl-1 at T163 can positively regulate its antiapoptotic activity (20). To test whether nicotine-stimulated Mcl-1 phosphorylation occurs through ERK1/2, H1299 cells were treated with increasing concentrations of nicotine for 30 min. Phosphorylation of ERK1/2 was analyzed by Western blot using a phospho-specific ERK antibody as previously described (12). Results reveal that nicotine induces phosphorylation and activation of ERK1/2 in a dose-dependent manner (Fig. 2A). Co-immunofluorescent staining using p-ERK and Mcl-1 antibodies shows that treatment of cells with nicotine significantly enhances the phosphorylated form of ERK1/2 (i.e. pERK) to co-localize with Mcl-1 (Fig. 2B). Intriguingly, nicotine not only enhances pERK (green) but also Mcl-1 (red), suggesting that pERK may phosphorylate Mcl-1 and stabilize/enhance Mcl-1 levels since the active ERK1/2 can directly phosphorylate Mcl-1 in vitro (Fig. 2BC). Thus, nicotine-induced phosphorylation of Mcl-1 may occur through activation of ERK1/2.
FIGURE 2. Nicotine induces phosphorylation of ERK1/2 which co-localizes with Mcl-1 in cytoplasm and ERK1/2 directly phosphorylates Mcl-1 in vitro.
A, H1299 cells were treated with increasing concentrations of nicotine for 30 min. Western blot analysis was performed to detect the phosphorylated ERK1/2 (p-ERK1/2) using a phospho-specific ERK antibody. B, H1299 cells were treated with nicotine (1μM) for 60 min. Cells were fixed, incubatedwith a mouse pERK and a rabbit Mcl-1 primaryantibody and stained with Alexa 488 (green) conjugatedanti-mouse or Alexa 594 (red)-conjugated anti-rabbit secondary antibodies. Individual green- and red-stained images derived from the samefield were merged using Openlab 3.1.5 software. C, Mcl-1 was immunoprecipitated from H1299 cells and incubated with purified ERK1 or ERK2 in an in vitro kinase assay. Phosphorylation of Mcl-1 was analyzed by autoradiography.
Nicotine Stimulates Mcl-1 Phosphorylation at T163 Site, Which Is Required for Nicotine-Induced Survival of Lung Cancer Cells
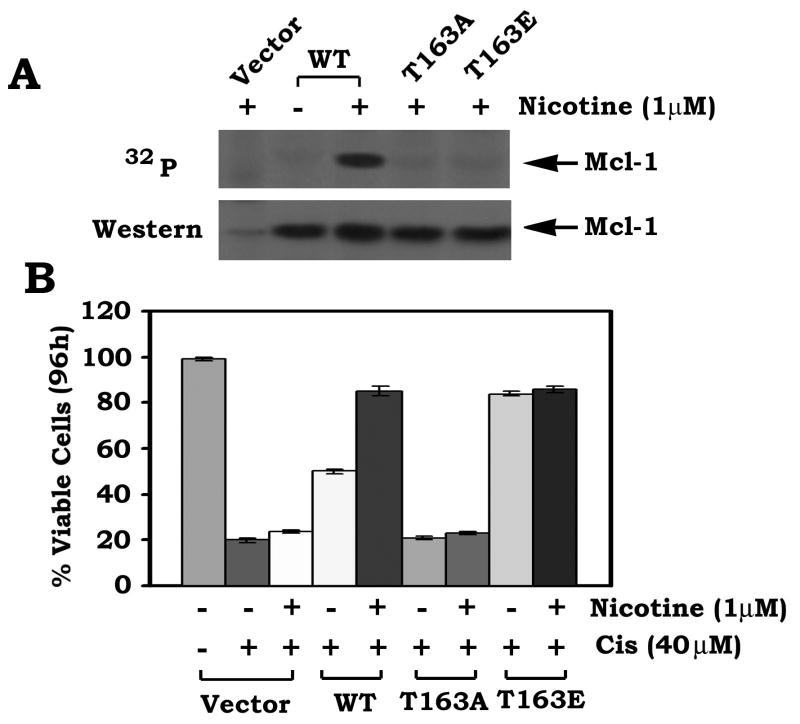
MAP kinases ERK1 and ERK2 are the proline (Pro)-directed kinases that can phosphorylate substrate (s) at serine (S) or threonine (T) residues immediately followed by Pro (20). Interestingly, T163 site in the PEST region of Mcl-1 represents a complete consensus MAP kinase phosphorylation sequence (PXT163P). Previous report demonstrated that ERK1/2-mediated Mcl-1 phosphorylation occurs at T163 site, which enhances Mcl-1 stability and antiapoptotic activity (20). Since ERK1/2 functions as nicotine-activated Mcl-1 kinase (Figs 1 and 2), nicotine-induced Mcl-1 phosphorylation may occur at T163 site (a proline-directed phosphorylation site). To test this, we have created the non-phosphorylatable T163A and the phosphomimetic T163E Mcl-1 mutants. WT and each of the Mcl-1 mutants were overexpressed in H82 cells that express relatively low levels of endogenous Mcl-1. Because nicotine can induce phosphorylation of WT but not T163A Mcl-1 mutant, this indicates that nicotine stimulates Mcl-1 phosphorylation exclusively at T163 (Fig. 3A). Intriguingly, nicotine enhances survival of cells expressing WT but not the cells expressing the T163A Mcl-1 mutant (Fig. 3B). Nicotine has no additional survival activity in cells expressing T163E (Fig. 3B). These findings provide genetic evidence that phosphorylation of Mcl-1 at T163 is required for nicotine-induced survival.
FIGURE 3. Nicotine induces Mcl-1 phosphorylation at T163 site, which is essential for nicotine-stimulated survival.
A, H82 cells expressing WT, T163A or T163E Mcl-1 mutant or vector-only control cells were metabolically labeled with 32P-orthophosphoric acid and treated with nicotine for 30 min. Mcl-1 was immunoprecipitated using a Mcl-1 antibody. Phosphorylation of Mcl-1 was determined by autoradiography. B, H82 cells expressing WT, T163A or T163E Mcl-1 mutant were treated with cisplatin (40μM) in the presence or absence of nicotine (1μM) for 96h. Cell viability was determined by analyzing annexin-V binding on FACS. Data represent the mean ± S.D. of three separate determinations.
The MEK/ERK Inhibitor PD98059 Suppresses Nicotine-Induced Mcl-1 Phosphorylation and Survival
Our data strongly suggest that ERK1 and 2 function as nicotine-activated Mcl-1 kinase to induce Mcl-1 phosphorylation at T163 (Figs 1–2). To further test whether inhibition of MAPK/ERK1/2 affects nicotine-induced Mcl-1 phosphorylation, H1299 cells expressing high levels of endogenous Mcl-1 were metabolically labeled with 32P-orthophosphoric acid and treated with nicotine in the absence or presence of increasing concentrations of PD98059 (1–10uM). Results indicate that PD98059 suppresses nicotine-stimulated Mcl-1 phosphorylation in a dose-dependent manner (Fig. 4A). Functionally, PD98059 significantly reduces nicotine-induced survival following treatment with the clinically relevant chemotherapeutic drug, cisplatin (Fig. 4B), suggesting that inhibition of MEK/ERK1/2 activity in human lung cancer cells may be critical for the suppression of nicotine-induced Mcl-1 phosphorylation and chemoresistance.
FIGURE 4. The MEK/ERK specific inhibitor PD98059 blocks nicotine-induced Mcl-1 phosphorylation and enhances apoptosis of human lung cancer cells.
A, H1299 cells were metabolically labeled with 32P-orthophosphoric acid for 90 min and treated with nicotine in the absence or presence of increasing concentrations of PD98059 for 30 min. Phosphorylation of Mcl-1 was determined by autoradiography. B, H1299 cells were treated with cisplatin (40μM) and nicotine (1μM) in the presence or absence of increasing concentrations of PD98059 for 48h. Cell viability was determined by analyzing annexin-V binding on FACS. Data represent the mean ± S.D. of three separate determinations.
The β-adrenergic Receptor Specific Inhibitor Propranolol Potently Blocks Nicotine-Induced Mcl-1 Phosphorylation and Enhances Apoptosis
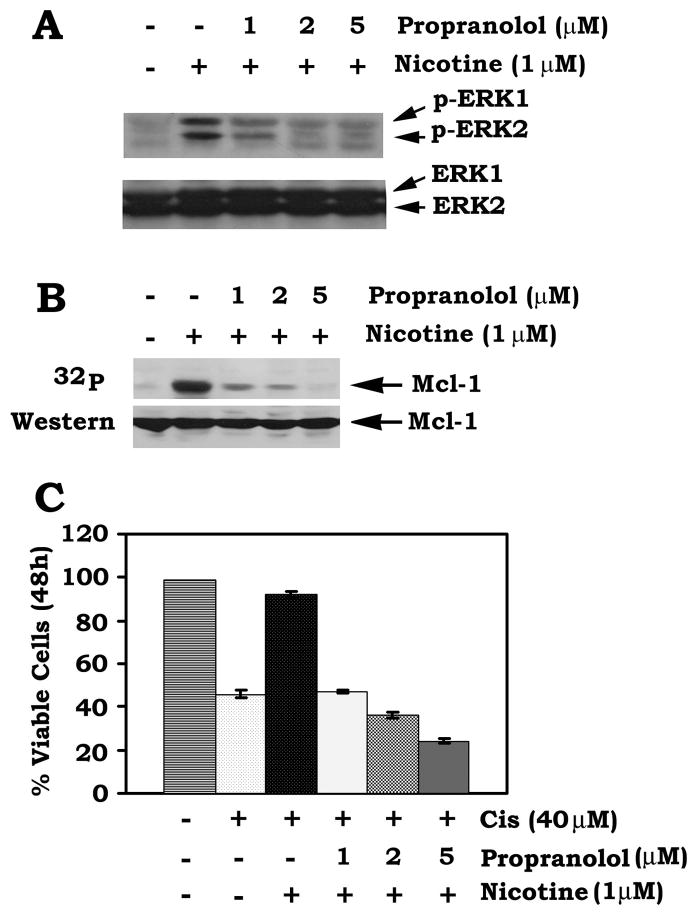
Nicotinic acetylcholine receptors (nAChRs), especially α7nAChR or β-adrenergic receptor, have been found to play an important role in nicotine signaling in human lung cancer cells (24). Thus, α-bungartoxin (α-BTX), a non-competent potent α7nAChR specific inhibitor (25) and propranolol, a β-adrenergic receptor inhibitor (26), were selected to test which type of receptor may be involved in nicotine/Mcl-1 signaling. H1299 cells were treated with nicotine in the presence or absence of increasing concentrations of propranolol or α-BTX. Interestingly, propranolol but not α-BTX can inhibit nicotine-induced activation of ERK1/2 as well as Mcl-1 phosphorylation in a dose dependent manner (Fig. 5AB and data not shown). Functionally, propranolol also blocks nicotine-induced cell survival and promotes apoptosis following treatment with cisplatin (Fig. 5C). These results reveal that nicotine-induced Mcl-1 phosphorylation may occur in a mechanism involving the upstream β-adrenergic receptor in human lung cancer cells.
FIGURE 5. The β-adrenergic receptor specific inhibitor propranolol suppressed nicotine-induced Mcl-1 phosphorylation and enhances chemosensitivity of human lung cancer cells.
A, H1299 cells were treated with nicotine in the absence or presence of increasing concentrations of propranolol for 30 min. Western blot analysis was performed to detect the phosphorylated ERK1/2 (p-ERK1/2) using a phospho-specific ERK antibody. B, H1299 cells were metabolically labeled with 32P-orthophosphoric acid for 90 min and treated with nicotine in the absence or presence of increasing concentrations of propranolol for 30 min. Phosphorylation of Mcl-1 was determined by autoradiography. C, H1299 cells were treated with cisplatin (40μM) and nicotine (1μM) in the presence or absence of increasing concentrations of propranolol for 48h. Cell viability was determined by analyzing annexin-V binding on FACS. Data represent the mean ± S.D. of three separate determinations.
Nicotine-Induced Mcl-1 Phosphorylation Enhances Mcl-1 Expression and Its Half-Life
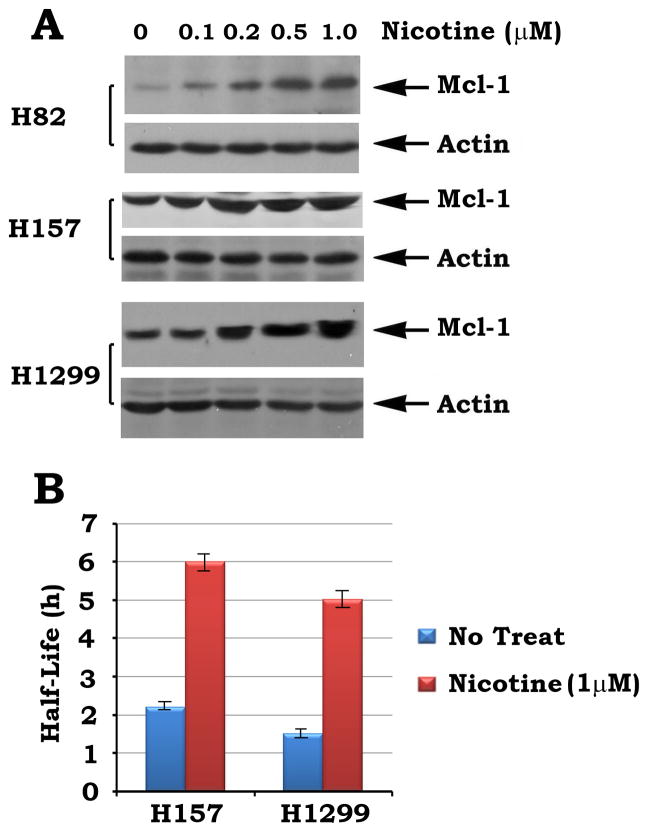
To test whether nicotine affects Mcl-1 expression, H82 cells expressing low levels of endogenous Mcl-1 or H157 and H1299 cells expressing high levels of endogenous Mcl-1 were treated with increasing concentrations of nicotine for 3h. Results indicate that nicotine induces a dose-dependent increase of Mcl-1 expression in various lung cancer cells (Fig. 6A). To further determine whether nicotine regulates the turnover rate of Mcl-1, the half-life of Mcl-1 was measured using the classical 35S-methionine pulse–chase method as described (27). H157 or H1299 cells were metabolically labeled with 35S-methionine for 60 min. The 35S-labeled cells were washed and incubated in fresh methionine-replete RPMI medium 1640 in the absence or presence of nicotine (1μM) for various time points up to 12 h. 35S-labeled Mcl-1 was immunoprecipitated using Mcl-1 antibody. The samples were subjected to SDS-PAGE. The Half-life of Mcl-1 was determined by electronic autoradiography. Intriguingly, exposure of cells to nicotine significantly prolong the half-life of Mcl-1 (i.e. from 2.2 h to 6 h in H157 cells or from 1.5h to 5h in H1299 cells, respectively; Fig. 6B). These data suggest that nicotine enhanced Mcl-1 expression may occur, at least in part, through prolonging the half-life of Mcl-1, which may contribute to its long-term survival activity. Mule has been identified as the E3 ubiquitin ligase for Mcl-1 (28). Nicotine-induced T163 phosphorylation of Mcl-1 may render Mcl-1 more resistant to Mule-induced polyubiquitination and degradation. This may be a potential mechanism by which nicotine-induced Mcl-1 phosphorylation increases Mcl-1’s stability. Further work is required to demonstrate this hypothesis.
FIGURE 6. Treatment of cells with nicotine prolongs the half-life of Mcl-1.
A, H82, H157 and H1299 cells expressing various levels of endogenous Mcl-1 were treated with increased concentrations of nicotine for 3h. Mcl-1 expression was analyzed by Western blot. B, H157 or H1299 cells expressing high levels of endogenous Mcl-1 were metabolically labeled with 35S-methionine. The half-life Mcl-1 in H157 and H1299 cells were determined by classic pulse-chase analysis.
Mcl-1 Is A Required Target in Nicotine-Induced Survival of Human Lung Cancer Cells
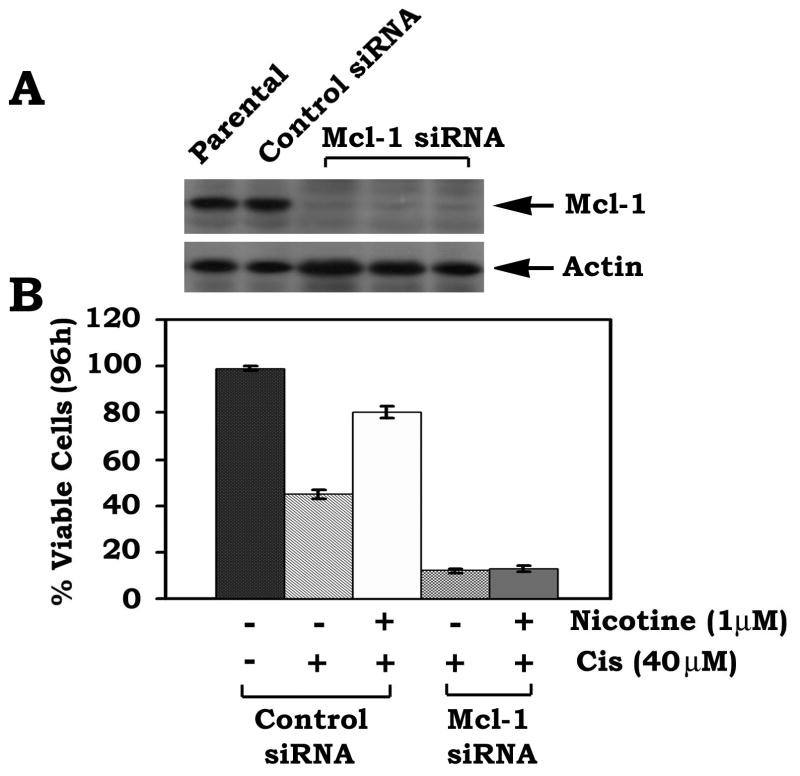
To test whether Mcl-1 is a required target for nicotine-induced survival of human lung cancer cells, an RNAi approach was employed. Recent studies have demonstrated that transfection of cells with siRNA concentrations greater than 100 nM frequently produces nonspecific off-target effects, and a concentration of 20–100 nM only occasionally produces effects (29). One group has reported that siRNA concentrations of 10–20 nM generally do not exert nonspecific effects (30). To minimize the nonspecific effect, a low concentration (i.e.15nM) of Mcl-1 siRNA was used in the experiment. H1299 cells were transfected with Mcl-1 siRNA or control siRNA. Cells expressingMcl-1 siRNA displayed a >95% reduction of Mcl-1 protein expression(Fig. 7A). Importantly, specific knockdown of Mcl-1 expression by RNAi significantly enhances apoptotic cell death following treatment withthe chemotherapeutic drug cisplatin in the absence or presenceof nicotine (Fig. 7B). Nicotine had no significant survival effect on cells expressing Mcl-1 siRNA, suggesting thatMcl-1 is a required target in nicotine-induced survival and/or chemoresistance of H1299 lung cancer cells.
FIGURE 7. Mcl-1 is essential for nicotine-induced chemoresistance in human lung cancer H1299 cells.
A, Mcl-1 siRNA (15nM) or control siRNA (15 nM) was transfected into H1299 cells using Lipofectamine 2000. After 24h, the levels of Mcl-1 expression were analyzed by Western blot. B, H1299 cells expressing Mcl-1 siRNA or control siRNA were treated with cisplatin (40 μM) in the absence or presence of nicotine (1 μM) for 96h. Cell viability was determined by analyzing annexin-V binding on FACS. Data represent the mean ± S.D. of three separate determinations.
Discussion
The antiapoptotic Bcl2 family member Mcl-1 has been originally identified in the ML-1 human myeloid leukemia cell line which undergoes phorbol ester-induced differentiation (30). Mcl-1 can be stimulated by multiple growth factors including interleukin-3 (IL-3), IL-5, IL-6, IL-7, granulocyte-macrophage colony-stimulating factor (GM-CSF), vascular endothelial growth factor, alpha interferon and epidermal growth factor (31). A recent report and our findings indicate that Mcl-1 is extensively expressed in both small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) cells (15; Fig. 1A). Importantly, nicotine, a major component of cigarette smoke, can mimic growth factor(s) to simulate Mcl-1 phosphorylation, which is associated with enhanced chemoresistance of human lung cancer cells (Fig. 1). ERK1 and ERK2 are physiological Mcl-1 kinases that can phosphorylate Mcl-1 at T163 site in the PEST region (aa 104-176) (20, 32). Intriguingly, nicotine not only stimulates phosphorylation and activation of ERK1/2 but also facilitates the phosphorylated, active ERK1/2 to co-localize with Mcl-1 in cytoplasm (Fig. 2AB). Since active ERK1 and ERK2 can directly phosphorylate the Mcl-1 protein isolated from human lung cancer H1299 cells (Fig. 2C) and the specific MEK/ERK inhibitor PD98059 blocks nicotine-stimulated Mcl-1 phosphorylation (Fig. 4), we propose that ERK1 and ERK2 function as nicotine-activated Mcl-1 kinases to phosphorylate Mcl-1 and regulate its activity in human lung cancer cells.
Genetic studies indicate that nicotine stimulates Mcl-1 phosphorylation exclusively at T163 site because only WT but not the T163A Mcl-1 mutant can be phosphorylated when cells were exposed to nicotine (Fig. 3A). The nonphosphorylatable T163A Mcl-1 mutant displays less antiapoptotic activity than WT Mcl-1. Since nicotine only prolongs survival of cells expressing WT but not the T163A Mcl-1 mutant, this suggests that phosphorylation of Mcl-1 at T163 is essential for nicotine-induced Mcl-1 phosphorylation and survival. Moreover, a substitution of T163 with glutamate (E), which mimics T163 site phosphorylation in the PEST region, also enhances Mcl-1’s antiapoptotic activity (Fig. 3). These findings indicate that either the phosphomimetic mutation at T163 or nicotine-induced T163 site phosphorylation can enhance Mcl-1’s survival activity. Thus, the T163 site should be a critical target for nicotine to positively regulate the antiapoptotic function of Mcl-1.
In addition to the nicotine acetylcholine receptors (nAChRs), high levels of β-adrengernic receptor have been found to be expressed in human lung cancer cells (33). Mounting evidence now indicates that nicotine can function as a β-adrengernic receptor agonist and its effect is abrogated by propranolol (a β-adrengernic receptor inhibitor; refs 26, 34). It has been reported that ERK1 and ERK2 are the signal downstream components in nicotine/β-adrenergic receptor signaling pathway (35–37). Importantly, ERK1 and ERK2 are physiological Mcl-1 kinases (20). Because treatment of cells with β-adrenergic receptor inhibitor (propranolol) not only suppresses nicotine-stimulated ERK1/2 activation (Fig. 5A) but also inhibits Mcl-1 phosphorylation (Fig. 5B), the β-adrenergic receptor should be linked to nicotine-stimulated Mcl-1 phosphorylation through ERK activation. Thus, the β-adrenergic receptor may function as the major upstream receptor in nicotine-stimulated ERK/Mcl-1 survival signal pathway. Importantly, inhibition of nicotine-induced ERK activation and Mcl-1 phosphorylation by propranolol suppresses nicotine-stimulated survival and enhances apoptotic cell death (Fig. 5). Thus, propranolol may have the potential to be developed as a clinically useful drug that specifically targets the β-adrenergic receptor to block nicotine-stimulated survival signal pathway in patients with lung cancer expressing high levels of β-adrenergic receptor and Mcl-1.
Mcl-1 protein is rapidly degraded in response to cell death signals and is immediately reinduced by survival stimuli (31). Mcl-1 has a very short half-life (i.e. 30 min~ 3h) in various cells (16–19). Because nicotine can induce Mcl-1 phosphorylation at T163 site through activation of ERK1/2 (Figs. 1–3), this may positively regulate Mcl-1 stability and expression level. As expected, nicotine not only markedly prolongs the half-life of Mcl-1 from 1.5–2.2h to 5–6h but also up-regulates its expression level in various human lung cancer cells (Fig. 6). This supports the notion that nicotine-stimulated Mcl-1 phosphorylation at T163 may prevent Mcl-1 from Mule-induced polyubiquitination and degradation, which could render Mcl-1 a longer half-life and long-term survival activity. H82 cells express very low levels of endogenous Mcl-1 (Fig. 1A) and exposure of H82 cells to nicotine upregulates Mcl-1 expression (Fig. 6A). However, nicotine failed to protect the vector-only H82 cells from cisplatin treatment (Fig. 3B). This consequence may result from the ability of cisplatin to block nicotine-enhanced Mcl-1 expression in H82 cells (data not shown). In support of this, cisplatin has also been reported to facilitate Mule-mediated Mcl-1 degradation (28). This may help explain why nicotine fails to protect H82 cells from cisplatin treatment.
Specific depletion of Mcl-1 from H1299 cells by RNAi blocks nicotine-stimulated survival and enhances sensitivity of human lung cancer cells to chemotherapeutic drug, cisplatin (Fig. 7). This indicates that Mcl-1 is an essential target for nicotine-induced survival and chemoresistance in human lung cancer cells. Thus, Mcl-1 should be a major target for development of new strategies for lung cancer treatment.
In summary, our studies demonstrate that nicotine promotes survival of human lung cancer cells in a novel mechanism by activating Mcl-1’s antiapoptotic function via its phosphorylation. Nicotine activates ERK1/2 through the upstream β-adrenergic receptor, which can induce Mcl-1 phosphorylation at T163 site in the PEST region. Nicotine-induced Mcl-1 phosphorylation at T163 enhances the half-life of Mcl-1, which leads to its long-term survival function and/or chemoresistance of human lung cancer cells. Thus, disruption of Mcl-1’s antiapoptotic function by blocking its T163 site phosphorylation may represent a new strategy for the treatment of tobacco-related cancer especially lung cancer and other malignancies that express Mcl-1.
Materials and methods
Materials
Nicotine, propranolol, cisplatin and VP-16 were purchased from Sigma (St. Louis, MO). Purified ERK1 and ERK2 were obtained from Calbiochem (San Diego, CA). ERK1, ERK2, phospho-specific ERK, Mcl-1 and actin antibodies as well as Mcl-1 siRNA were purchased from Santa Cruz Biotechnology(Santa Cruz, CA). Alexa Fluor 594-conjugated goat anti-mouse IgG antibody and Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody were obtained from Invitrogen (Carlsbad, CA). WT Mcl-1 cDNA in the pcDNA3.1/V5-His-TOPO was kindly provided by Dr. Ulrich Maurer (University of Ulm, Ulm, Germany). [32P] Orthophosphate and [32P] γ-ATP were purchased from MP Biomedicals (Irvine, CA). All reagents used were obtained from commercial sources unless otherwise stated.
Cell lines, Plasmids, cDNA, Mutagenesis and Transfections
H23, H69, H82, H157, H358, H460 and H1299 cells were maintained in RPMI 1640 with 10% fetal bovine serum (FBS). A549 cells weremaintained in F-12K medium with 10% fetal bovine serum. Wild type (WT) Mcl-1 cDNA was cut from Mcl-1 cDNA/pcDNA3.1/V5-His-TOPO construct using BamHI and XbaI restriction enzymes. The Mcl-1 cDNA insert was cloned in pUC19. Nucleotides corresponding to threonine (T) 163 residue was substituted to create a conservative alteration to alanine (A) or glutamic acid (E). The 5′ phosphorylated mutagenic primers for Mcl-1 mutants were synthesized as follow: T163A, 5′-GTC ACT ACC CTC GGC GCC GCC GCC AGC-3′; T163E, 5′-GTC ACT ACC CTC GGA GCC GCC GCC AGC AG-3′. The WT-Mcl-1/pUC19 construct was used as the target plasmid which contains a unique NdeI restriction site for selection against the unmutated plasmid. The NdeI selection primer is: 5′-GAG TGC ACC ATG GGC GGT GTG AAA-3′. The nonphosphorylatable (T163A) and the phosphomimetic (T163E) Mcl-1 mutants were created using a site directed mutagenesis kit (Clontech) according to the manufacturer’s instructions. Each single mutant was confirmed bysequencing of the cDNA and was then cloned into the pCIneo (Promega) mammalianexpression vector. The pCIneo plasmids containing WT, T163A or T163E were transfected into H82 cells using Lipofectamine ™ 2000 (Invitrogen).Clones stably expressing WT, T163A or T163E were selected in medium containing G418 (0.8 mg/ml). The expression levels of exogenousMcl-1 were compared by Western blot analysis using a Mcl-1 antibody. Three separate clonesfor each mutant expressing similar amounts of exogenous Mcl-1 were selected for analysis.
Metabolic Labeling, Immunoprecipitation, and Western Blot Analysis
Cells were washed with phosphate-free RPMI medium and metabolically labeled with 32P-orthophosphoric acid for 90 minutes (min). After agonist or inhibitor addition, cells were washed with ice-cold PBS and lysed in detergent buffer. Mcl-1 was immunoprecipitated and subjected to 10% SDS/PAGE, transferred to a nitrocellulose membrance and exposed to Kodak X-omat film at −80°C. Mcl-1 phosphorylation was determined by autoradiography. The same filter was then probed by Western blot analysis with a Mcl-1 antibody and developed by using an ECL Kit (Amersham) as described previously (12, 14).
Preparation of Cell Lysates
Cells were washed with 1× PBS and resuspended in ice-cold EBC buffer (0.5% NP-40, 50 mMTris, pH 7.6, 120 mM NaCl, 1 mM EDTA and 1mM β-mercaptoethanol)with a cocktail of protease inhibitors (EMD Biosciences). Cells were lysed by sonication and centrifuged at 14,000 × g for 10 min at 4°C. The resulting supernatant was collected as the total cell lysate.
Immunofluorescence
The cells were washed with 1× PBS, fixed with methanol and acetone (1:1) for 5 minutes and then blocked with 10% normal mouse or rabbit serum for 20 min at room temperature. Cells were incubatedwith a mouse or rabbit primaryantibody for 90 min. After washing, samples were incubatedwith Alexa 488 (green) conjugatedanti-mouse or Alexa 594 (red)-conjugated anti-rabbit secondary antibodies for 60 min. Cells were washedwith 1× PBS and observed under a fluorescent microscope (Zeiss). Pictures were taken and colored with the same exposure setting for each experiment. Individual green- and red-stained images derived from the samefield were merged using Openlab 3.1.5 software from Improvision, Inc. (Lexington, MA).
Mcl-1 Phosphorylation by ERK1 and ERK2 In Vitro
Mcl-1 was immunoprecipitated from lysates isolated from H1299 cells and incubated with purified, activated ERK1 or ERK2 enzyme (0.5ug) in the assay buffer (10mM Hepes, pH 8.0, 100uM ATP, 10mM MgCL2, 1mM DTT, 0.5mM benzamidine, 2uCi of [γ-32P] ATP) for 30 min at 30°C as described (38). The reaction was stopped by the addition of 2× SDS sample buffer and boiling the sample for 5 min. The samples were analyzed by SDS-PAGE. Mcl-1 phosphorylation was determined by autoradiography.
Metabolic Labeling and Pulse–Chase Analysis of Mcl-1 Half-Life
Cells were metabolically labeled with 35S-methionine for 60 min. The 35S-methionine-labeled cells were washed and incubated in fresh methionine-replete RPMI medium 1640 in the absence or presence of nicotine (1μM) for various time points up to 12 h. 35S-labeled Mcl-1 was immunoprecipitated by using Mcl-1 antibody. The samples were subjected to SDS/10–20% PAGE. The half-life ( t1/2) of Mcl-1 was determined by electronic autoradiography as described (27).
RNA Interference
Human Mcl-1 siRNA (GAAGACCAUAAACCAAGAAtt) was purchased from Santa Cruz Biotechnology(Santa Cruz, CA). H1299 cells were transfectedwith Mcl-1 siRNA using LipofectAMINE™ 2000 (Invitrogen). A control siRNA(nonhomologous to any known gene sequence) was used as a negativecontrol. The levels of Mcl-1 expression were analyzed by Westernblot. Specific silencing of the targeted Mcl-1gene was confirmed by at least three independent experiments.
Cell Viability Assay
Apoptotic and viable cells were detected using an ApoAlert Annexin-V kit (Clontech) according to the manufacturer’s instructions. The percentage of annexin-Vlow (i.e. viable) or annexin-Vhigh (i.e. apoptotic) cells was determined using thedata obtained by fluorescence-activated cell sorter analysis asdescribed (14). Cell viability was also confirmed using the trypan blue dye exclusion method.
Acknowledgments
We are grateful to Dr. Ulrich Maurer (University of Ulm, Ulm, Germany) for kindly providing Mcl-1 cDNA.
Abbreviations
- SCLC
small cell lung cancer
- NSCLC
non-small cell lung cancer
- Mcl-1
myeloid cell leukemia 1
- nAChR
nicotinic acetylcholine receptor
- α-BTX
α-bungartoxin
- MEK
mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- ERK
extracellular signal-regulated kinase
- BH
Bcl2 homology domain
- T
threonine
- S
serine
- A
alanine
- E
glutamate
- siRNA
small interfering RNA
- RNAi
RNA interference
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
This work was supported by a Lotus Scholars Program (200734), by a Flight Attendant Medical Research Institute Clinical Innovator Award, by an Award from Stop Children Cancer, Inc., by NCI, National Institutes of Health Grant R01 CA112183 and by the HHMI Science for Life Undergraduate Award (to TW).
References
- 1.Heusch WL, Maneckjee R. Signaling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis. 1998;19:551–556. doi: 10.1093/carcin/19.4.551. [DOI] [PubMed] [Google Scholar]
- 2.Dresler CM. Is it more important to quit smoking than which chemotherapy is used? Lung Cancer. 2003;39:119–124. doi: 10.1016/s0169-5002(02)00455-5. [DOI] [PubMed] [Google Scholar]
- 3.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1991;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz N. Drug therapy: pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med. 1988;319:1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- 5.Wright SC, Zhong J, Zheng H, Larrick JW. Nicotine inhibition of apoptosis suggests a role in tumor promotion. FASEB J. 1993;7:1045–1051. [PubMed] [Google Scholar]
- 6.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ. Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 9.Brambilla E, Negoescu A, Gazzeri S, Lantuejoul S, Moro D, Brambilla C, Coll JL. Apoptosis-related factors p53, Bcl2, and Bax in neuroendocrine lung tumors. Am J Pathol. 1996;149:1941–1952. [PMC free article] [PubMed] [Google Scholar]
- 10.Ferron PE, Bagni I, Guidoboni M, Beccati MD, Nenci I. Combined and sequential expression of p53, Rb, Ras and Bcl-2 in bronchial preneoplastic lesions. Tumori. 1997;83:587–593. doi: 10.1177/030089169708300222. [DOI] [PubMed] [Google Scholar]
- 11.Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97:584–592. doi: 10.1002/ijc.10096. [DOI] [PubMed] [Google Scholar]
- 12.Mai H, May WS, Gao F, Jin Z, Deng X. A functional role for nicotine in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem. 2003;278:1886–1891. doi: 10.1074/jbc.M209044200. [DOI] [PubMed] [Google Scholar]
- 13.Jin Z, Gao F, Flagg T, Deng X. Nicotine induces multisite phosphorylation of Bad in association with suppression of apoptosis. J Biol Chem. 2004;279:23837–23844. doi: 10.1074/jbc.M402566200. [DOI] [PubMed] [Google Scholar]
- 14.Xin M, Deng X. Nicotine inactivation of Bax’s proapoptotic function through phosphorylation. J Biol Chem. 2005;280:10781–10789. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- 15.Song L, Coppola D, Livingston S, Cress D, Haura E. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biology & Therapy. 2005;4:267–276. doi: 10.4161/cbt.4.3.1496. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Lee S, Meng X, Mott J, Bronk S, Werneburg N, Craig R, Kaufmann S, Gores G. Serine 64 phosphorylation enhances the antiapoptotic function of Mcl-1. J Biol Chem. 2007;282:18407– 18417. doi: 10.1074/jbc.M610010200. [DOI] [PubMed] [Google Scholar]
- 17.Chao J, Li J, Huang H, Chou C, Kuo M, Yen J, Yang Y. Mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol Cell Biol. 1998;18:4883–4898. doi: 10.1128/mcb.18.8.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Peng H, Cheng Y, Yuan H, Yang Y. Stabilization and Enhancement of the Antiapoptotic Activity of Mcl-1 by TCTP. Mol Cell Biol. 2005;25:3117–3126. doi: 10.1128/MCB.25.8.3117-3126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domina A, Vrana J, Gregory M, Hann S, Craig R. Mcl-1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–5315. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- 21.Domina A, Smith J, Craig R. Myeloid Cell Leukemia 1 Is Phosphorylated through Two Distinct Pathways, One Associated with Extracellular Signal-regulated Kinase Activation and the Other with G2/M Accumulation or Protein Phosphatase 1/2A Inhibition. J Biol Chem. 2000;275:21688–21694. doi: 10.1074/jbc.M000915200. [DOI] [PubMed] [Google Scholar]
- 22.Maurer U, Charvet C, Wagman A, Dejardin E, Green D. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of Mcl-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Niell HB. Extensive stage small cell lung cancer. Curr-Treat-Options-Oncol. 2001;2:71–76. doi: 10.1007/s11864-001-0018-4. [DOI] [PubMed] [Google Scholar]
- 24.Jin Z, Gao F, Flagg T, Deng X. Nicotine Induces Multi-site Phosphorylation of Bad in Association with Suppression of Apoptosis. J Biol Chem. 2004;279:23837–23844. doi: 10.1074/jbc.M402566200. [DOI] [PubMed] [Google Scholar]
- 25.Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- 26.Perissin L, Rapozzi V, Zorzet S, Giraldi T. Blockers of adrenergic neurons and receptors, tumor progression and effects of rotational stress in mice. Anticancer Res. 1996;16:3409–3413. [PubMed] [Google Scholar]
- 27.Deng X, Gao F, Flagg T, May WS. Mono- and multi-site phosphorylation enhances Bcl2’s anti-apoptotic function and inhibition of cell cycle entry functions. Proc Natl Acad Sci USA. 2004;101:153–158. doi: 10.1073/pnas.2533920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Semizarov D, Frost L, Sarthy A, Kroeger P, Halbert D, Fesik S. Specificity of short interfering RNA determined through gene expression signatures. Proc Natl Acad Sci USA. 2003;100:6347–6352. doi: 10.1073/pnas.1131959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozopas K, Yang T, Buchan H, Zhou P, Craig R. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci USA. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang H, Yen Y. Mcl-1, a highly regulated cell death and survival controller. Journal of Biomedical Science. 2006;13:201–204. doi: 10.1007/s11373-005-9064-4. [DOI] [PubMed] [Google Scholar]
- 32.Biasio AD, Vrana JA, Zhou P, Qian L, Bieszczad CK, Braley KE, Domina AM, Weintraub SJ, Neveu JM, Lane WS, Craig RW. N-terminal Truncation of Antiapoptotic MCL1, but Not G2/M-induced Phosphorylation, Is Associated with Stabilization and Abundant Expression in Tumor Cells. J Biol Chem. 2007;282:23919–23936. doi: 10.1074/jbc.M700938200. [DOI] [PubMed] [Google Scholar]
- 33.Arnold H, Sears R. Protein phosphatase 2A regulatory subunit B56α associates with c-Myc and negatively regulates c-Myc accumulation. Mol Cell Biol. 2008;26:2832–2844. doi: 10.1128/MCB.26.7.2832-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park PG, Merryman J, Orloff M, Schuller HM. s-Adrenergic Mitogenic Signal Transduction in Peripheral Lung Adenocarcinoma: Implications for Individuals with Preexisting Chronic Lung Disease. Cancer Res. 1995;55:3504–3508. [PubMed] [Google Scholar]
- 35.Shin V, Wu W, Chu K, Koo M, Wong H, Lam E, Tai E, Cho C. Functional role of β-adrenergic receptors in the mitogenic action of nicotine on gastric cancer cells. Toxicological Sciences. 2007;96:21–29. doi: 10.1093/toxsci/kfl118. [DOI] [PubMed] [Google Scholar]
- 36.Wong H, Yu L, Lam E, Tai E, Wu W, Cho C. Nicotine promotes colon tumor growth and angiohenesis through β-adrenergic activation. Toxicological Sciences. 2007;97:279–287. doi: 10.1093/toxsci/kfm060. [DOI] [PubMed] [Google Scholar]
- 37.Shin V, Jin H, Ng E, Yu J, Leung W, Cho C, Sung J. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induce cyclooxygenase-2 activity in human gastric cancer cells: Involvement of nicotinic acetylcholine receptor (nAChR) and beta-adrenergic receptor signaling pathways. Toxicology and Applied Pharmacology. 2008;233:254–261. doi: 10.1016/j.taap.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Deng X, Ruvolo P, Carr BK, May WS. Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci USA. 2000;97:1578–1583. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]