Abstract
Objective
The risk of atherosclerosis in the setting of chylomicronemia has been a topic of debate. In this study, we examined susceptibility to atherosclerosis in Gpihbp1-deficient mice (Gpihbp1−/−), which manifest severe chylomicronemia as a result of defective lipolysis.
Methods and Results
Gpihbp1−/− mice on a chow diet have plasma triglyceride and cholesterol levels of 2812 ± 209 and 319 ± 27 mg/dl, respectively. Even though nearly all of the lipids were contained in large lipoproteins (50–135 nm), the mice developed progressive aortic atherosclerosis. In other experiments, we found that both Gpihbp1-deficient “apo-B48–only” mice and Gpihbp1-deficient “apo-B100–only” mice manifest severe chylomicronemia. Thus, GPIHBP1 is required for the processing of both apo-B48– and apo-B100–containing lipoproteins.
Conclusions
Chylomicronemia causes atherosclerosis in mice. Also, we found that GPIHBP1 is required for the lipolytic processing of both apo-B48– and apo-B100–containing lipoproteins.
Keywords: lipoprotein lipase, chylomicronemia, lipolysis, GPIHBP1
Introduction
Human and mouse studies have proven that high levels of cholesterol-rich remnants cause atherosclerosis, but the relevance of triglyceride-rich lipoproteins (TRLs) to atherogenesis remains controversial.1 In part, this controversy stems from the fact that elevated levels of TRLs and remnants often coexist.
Humans with chylomicronemia have severe hypertriglyceridemia but low levels of cholesterol-rich remnants. Chylomicronemia patients are often assumed to be protected from atherosclerosis because experiments in rabbits had shown that very large lipoproteins cannot enter the arterial wall and were not particularly atherogenic.2 However, this assumption was recently challenged by the finding of atherosclerosis in four chylomicronemia patients with lipoprotein lipase (LPL) deficiency.3
Mice lacking GPIHBP1 (Gpihbp1−/−) have severe chylomicronemia, even on a low-fat chow diet, as a result of defective lipolysis.4 Very recently, chylomicronemia has been observed in a young man with a homozygous missense mutation in GPIHBP1.5 Thus far, no one has examined whether Gpihbp1−/− mice have an increased susceptibility to atherosclerotic lesions. In this study, we examined that issue.
We also tackled a second issue; we addressed whether GPIHBP1 is required for the lipolytic processing of both apo-B48–containing lipoproteins and apo-B100–containing lipoproteins.
Materials and Methods
Gpihbp1−/− mice (>90% C57BL/6)4 were housed in a barrier facility and fed a 4.5%-fat chow diet. We also bred “apo-B48–only” and “apo-B100–only” Gpihbp1−/− mice (Gpihbp1−/− Apob48/48, Gpihbp1−/− Apob100/100).6 All experiments were approved by the Animal Research Committee.
Plasma lipid levels were measured with enzymatic kits, and their distribution within lipoproteins was assessed by Fast Protein Liquid Chromatography (FPLC).6 Lipoprotein diameters were measured by laser light scattering.6 Western blots were performed as described.6
Sections of the aortic root were stained with Oil Red O and lesions were quantified as described.6 Macrophages were stained with a monoclonal antibody against CD68 (Serotec; 1:100).
Results
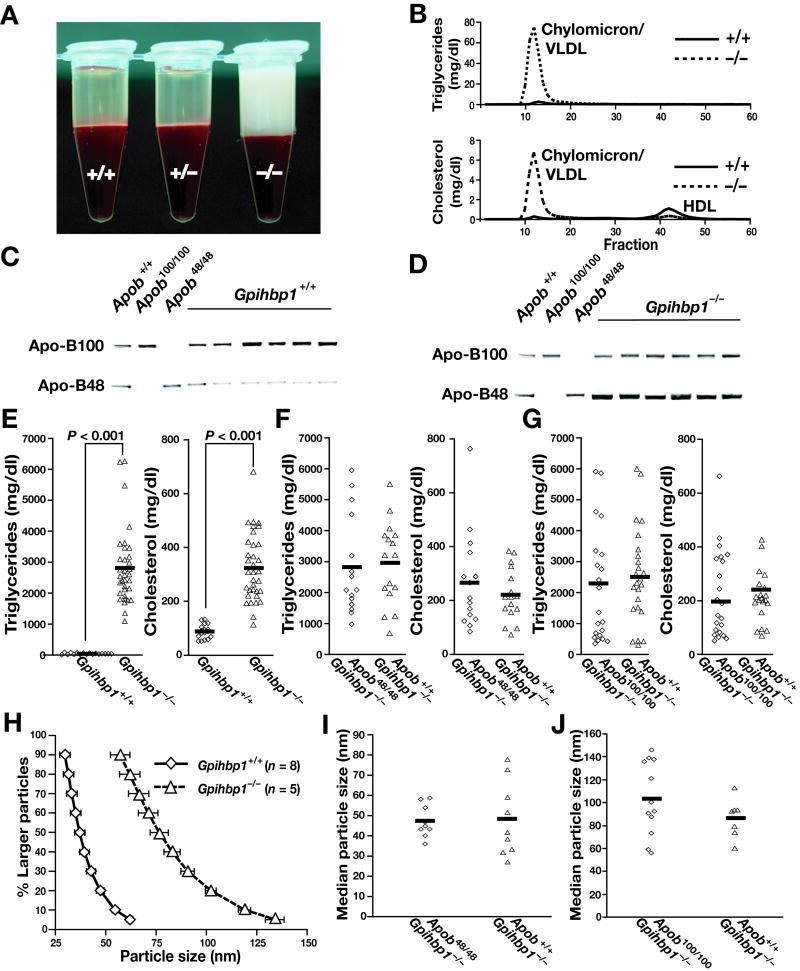
Gpihbp1−/− mice had lipemic plasma (Figure 1A) and nearly all of the plasma lipids were contained in large lipoproteins (as judged by FPLC) (Figure 1B). Also, plasma apo-B48 levels in Gpihbp1−/− mice were increased (Figures 1C–D). Gpihbp1−/− mice had triglyceride and cholesterol levels of 2812 ± 209 and 319 ± 27 mg/dl, respectively (n = 42) (vs. 35 ± 5 and 90 ± 6 mg/dl in littermate Gpihbp1+/+ mice, n = 24; p < 0.0001 for both) (Figure 1E).
Figure 1.
Lipids and lipoproteins in Gpihbp1−/− mice. A, Lipemic plasma in 15-month-old chow-fed Gpihbp1−/− mice. B, Distribution of lipids in FPLC-fractionated plasma. C–D, Western blots of Gpihbp1+/+ (C) and Gpihbp1−/− (D) plasma with an apo-B–specific antibody. Apob100/100 and Apob48/48 mice were used as controls. E–G, Plasma triglyceride and cholesterol levels in Gpihbp1−/−, Gpihbp1−/− Apob48/48, and Gpihbp1−/− Apob100/100 mice on a chow diet. H, Distribution of d < 1.006 g/ml lipoprotein diameters in Gpihbp1−/− mice on a chow diet (deciles plus the 95% point). The sizes of the d < 1.006 g/ml lipoproteins in Gpihbp1−/− mice were similar to those observed previously in mouse lymph chylomicrons during active lipid absorption.9 We did not compare the sizes of lipoproteins in chow-fed Gpihbp1−/− mice and diabetic rabbits (such as those studied by Nordestgaard et al.2). Because the diabetic rabbits examined by Nordestgaard et al.2 were on a high-fat diet, it is possible that their lipoproteins were larger than those in chow-fed Gpihbp1−/− mice. (I) Median diameters of d < 1.006 g/ml lipoproteins in Gpihbp1−/− Apob+/+ and littermate Gpihbp1−/− Apob48/48 mice. (J) Median diameters of d < 1.006 g/ml lipoproteins in Gpihbp1−/− Apob+/+ mice and littermate Gpihbp1−/− Apob100/100 mice. In the two different experiments shown in panels I and J, the median diameter of lipoproteins in Gpihbp1−/− Apob+/+ mice were different. We do not fully understand the lipoprotein size differences in the two different experiments. The experiments were performed independently, many months apart, on different sets of mice. Both experiments were performed on mice consuming ad libitum diets (not fasting mice), and the mice examined in panel I were three months younger than the mice examined in panel J. In any case, the important point (for the experiments in both panel I and panel J) is that lipoprotein sizes in littermates were not affected by Apob genotype.
The fact that amphibians and birds have neither apo-B48 nor GPIHBP1 led Beigneux et al.4 to speculate that GPIHBP1 might have arisen in mammals as a new protein specifically dedicated to the lipolytic processing of apo-B48–lipoproteins. They further hypothesized that GPIHBP1 might be unimportant for the processing of apo-B100–containing lipoproteins and that mice lacking both apo-B48 and GPIHBP1 might be normolipidemic. While this hypothesis initially seemed attractive, it was incorrect. Gpihbp1−/− Apob+/+ mice and littermate Gpihbp1−/− Apob48/48 mice had very similar plasma lipid levels (Figure 1F). Similarly, Gpihbp1−/− Apob+/+ mice and littermate Gpihbp1−/− Apob100/100 mice had very similar plasma lipid levels (Figure 1G).
Most of the d < 1.006 g/ml lipoproteins in Gpihbp1−/− mice were large (50–135 nm), overlapping little with the sizes of lipoproteins in Gpihbp1+/+ mice (Figure 1H). Lipoprotein sizes were similar in Gpihbp1−/− Apob+/+ mice and littermate Gpihbp1−/− Apob48/48 mice (Figure 1I). Also, lipoprotein sizes were similar in Gpihbp1−/− Apob+/+ mice and littermate Gpihbp1−/− Apob100/100 mice (Figure 1J).
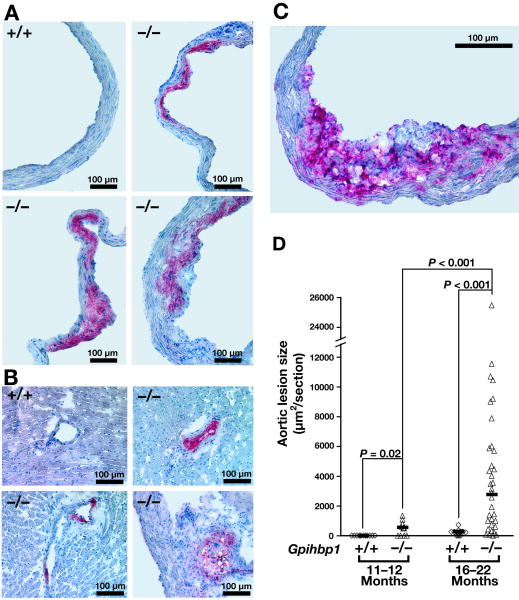
Chow-fed Gpihbp1−/− mice developed lipid-rich atherosclerotic lesions in the aortic root and coronary arteries (Figures 2A–B). The lesions also stained strongly for macrophages (Figure 2C). The lesions were small at 11–12 months (median 500 μm2/section) but were much larger at 16–22 months (median 3000 μm2/section) (Figure 2D). Lesions were evident in both males and females [3947; 3172–6071 (median; 33rd–66th percentile) and 1680; 1000–4688 μm2/section, respectively, p = 0.74].
Figure 2.
Atherosclerosis in chow-fed Gpihbp1−/− mice. A, Oil red O–stained atherosclerotic lesions in the aortic root of three 22-month-old Gpihbp1−/− mice. B, Oil red O–stained coronary atherosclerotic lesions in three 18-month-old Gpihbp1−/− mice. C, Staining of macrophages in an atherosclerotic lesion with an antibody against CD68. D, Atherosclerotic lesion sizes (mean μm2/section) at 11–12 months (Gpihbp1+/+, n = 12; Gpihbp1−/−, n = 12) and 16–22 months (Gpihbp1+/+, n = 20; Gpihbp1−/−, n = 18). Horizontal bars represent medians; differences tested with the Mann-Whitney test.
Discussion
GPIHBP1-deficient mice develop severe chylomicronemia as a result of defective lipolysis of triglyceride-rich lipoproteins.4 In this study, we address two timely and important issues. First, we show that mice with chylomicronemia develop spontaneous atherosclerosis, despite the fact that most of the lipids in these mice are found in large lipoproteins—lipoproteins that are often considered to be nonatherogenic.2 Thus, chylomicronemia leads to atherosclerotic lesions, even in mice fed a low-fat chow diet. These findings in mice add plausibility to the concept3 that chylomicronemia in humans could lead to increased susceptibility to atherosclerosis. Second, we show that the severe chylomicronemia in Gpihbp1−/− mice is not due to a selective defect in the processing of apo-B48–containing lipoproteins. Beigneux et al.4 had hypothesized that GPIHBP1 might be a mammalian protein specifically dedicated to the processing of apo-B48–containing lipoproteins and further speculated that the processing of apo-B100–containing lipoproteins might not depend on GPIHBP1. This speculation is incorrect. The plasma lipid levels and lipoprotein sizes in Gpihbp1−/− Apob+/+ mice are very similar to those in littermate Gpihbp1−/− Apob100/100 mice (or littermate Gpihbp1−/− Apob48/48 mice). Thus, GPIHBP1 is required for the processing of both apo-B48–containing lipoproteins and apo-B100–containing lipoproteins.
Finding atherosclerosis in Gpihbp1−/− mice is consistent with a recent report of aortic lesions in Lpl−/− mice that had been rescued with an injection of an LPL adenovirus.7, 8 The severity of the hypertriglyceridemia in Gpihbp1−/− mice and LPL adenovirus–rescued Lpl−/− mice is quite similar; however, Gpihbp1−/− mice appear to be the most reasonable choice for future research. Gpihbp1−/− mice can be bred in limitless numbers, while the production of adenovirus-rescued Lpl−/− mice is more challenging. Also, systemic infections with an adenovirus could cloud the interpretation of mouse atherosclerosis studies.
The lesions in Gpihbp1−/− mice are small and require longer to develop than those of Apoe−/− or Ldlr−/− mice.6 Nevertheless, these mice will be useful to the research community. With Gpihbp1−/− mice, it should be possible to determine if the atherogenicity of TRLs depends on their cholesterol content. Also, this model opens the door to defining the impact of different dietary fatty acids (including dietary oxidized fatty acids) on the atherogenicity of TRLs.
Acknowledgments
Sources of Funding HL094732-01 (APB); T32HL69766 (MMW); Texas AgriLife 8738 (RLW); HL090553 and HL087228 (SGY).
Footnotes
Disclosures None.
References
- 1.Criqui MH. Triglycerides and coronary heart disease revisited (again) Ann Intern Med. 2007;147:425–427. doi: 10.7326/0003-4819-147-6-200709180-00014. [DOI] [PubMed] [Google Scholar]
- 2.Nordestgaard BG, Zilversmit DB. Large lipoproteins are excluded from the arterial wall in diabetic cholesterol-fed rabbits. J Lipid Res. 1988;29:1491–1500. [PubMed] [Google Scholar]
- 3.Benlian P, De Gennes JL, Foubert L, Zhang H, Gagne SE, Hayden M. Premature atherosclerosis in patients with familial chylomicronemia caused by mutations in the lipoprotein lipase gene. N Engl J Med. 1996;335:848–854. doi: 10.1056/NEJM199609193351203. [DOI] [PubMed] [Google Scholar]
- 4.Beigneux AP, Davies B, Gin P, Weinstein MM, Farber E, Qiao X, Peale P, Bunting S, Walzem RL, Wong JS, Blaner WS, Ding ZM, Melford K, Wongsiriroj N, Shu X, de Sauvage F, Ryan RO, Fong LG, Bensadoun A, Young SG. Glycosylphosphatidylinositol-anchored high density lipoprotein–binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigneux AP, Franssen R, Bensadoun A, Gin P, Melford K, Peter J, Walzem RL, Weinstein MM, Davies BS, Kuivenhoven JA, Kastelein JJ, Fong LG, Dallinga-Thie GM, Young SG. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arterioscler Thromb Vasc Biol. 2009;29:956–962. doi: 10.1161/ATVBAHA.109.186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Véniant MM, Pierotti V, Newland D, Cham CM, Sanan DA, Walzem RL, Young SG. Susceptibility to atherosclerosis in mice expressing exclusively apolipoprotein B48 or apolipoprotein B100. J Clin Invest. 1997;100:180–188. doi: 10.1172/JCI119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Qi R, Xian X, Yang F, Blackstein M, Deng X, Fan J, Ross C, Karasinska J, Hayden MR, Liu G. Spontaneous Atherosclerosis in Aged Lipoprotein Lipase Deficient Mice With Severe Hypertriglyceridemia on a Normal Chow Diet. Circ Res. 2007 doi: 10.1161/CIRCRESAHA.107.156554. [DOI] [PubMed] [Google Scholar]
- 8.Strauss JG, Frank S, Kratky D, Hammerle G, Hrzenjak A, Knipping G, von Eckardstein A, Kostner GM, Zechner R. Adenovirus-mediated rescue of lipoprotein lipase-deficient mice. Lipolysis of triglyceride-rich lipoproteins is essential for high density lipoprotein maturation in mice. J Biol Chem. 2001;276:36083–36090. doi: 10.1074/jbc.M104430200. [DOI] [PubMed] [Google Scholar]
- 9.Lo CM, Nordskog BK, Nauli AM, Zheng S, Vonlehmden SB, Yang Q, Lee D, Swift LL, Davidson NO, Tso P. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol. 2008;294:G344–352. doi: 10.1152/ajpgi.00123.2007. [DOI] [PubMed] [Google Scholar]