Abstract
Background
Stearoyl-CoA desaturase 1 (SCD1) is a critical regulator of energy metabolism and inflammation. We have previously reported that inhibition of SCD1 in hyperlipidemic mice fed a saturated fatty acid (SFA)-enriched diet prevented development of the metabolic syndrome, yet surprisingly promoted severe atherosclerosis. In this study we tested whether dietary fish oil supplementation could prevent the accelerated atherosclerosis caused by SCD1 inhibition.
Methods and Results
LDLr−/−, Apob100/100 mice were fed diets enriched in saturated fat or fish oil in conjunction with antisense oligonucleotide (ASO) treatment to inhibit SCD1. As previously reported, in SFA-fed mice, SCD1 inhibition dramatically protected against development of the metabolic syndrome, yet promoted atherosclerosis. In contrast, in mice fed fish oil, SCD1 inhibition did not result in augmented macrophage inflammatory response or severe atherosclerosis. In fact, the combined therapy of dietary fish oil and SCD1 ASO treatment effectively prevented both the metabolic syndrome and atherosclerosis.
Conclusions
SCD1 ASO treatment in conjunction with dietary fish oil supplementation is an effective combination therapy to comprehensively combat the metabolic syndrome and atherosclerosis in mice.
Keywords: saturated fat, fish oil, atherosclerosis, inflammation, metabolic syndrome
Introduction
It has been estimated that nearly a quarter of American adults have the metabolic syndrome.1–3 Inhibition of stearoyl-CoA desaturase 1 (SCD1) has been proposed as an attractive strategy for preventing most aspects of the metabolic syndrome including obesity4–9, insulin resistance4,6,10,11, hypertriglyceridemia6,12–14, and hepatic steatosis.8,9,12,15,16 However, several unwanted side effects are associated with SCD1 inhibition or deletion, including alopecia17–20 and accelerated atherosclerosis.21,22 We have previously shown that the accelerated atherosclerosis seen with SCD1 ASO-mediated inhibition is associated with saturated fatty acid (SFA) enrichment of macrophage membranes, and enhanced proinflammatory signaling through toll-like receptor 4 (TLR4).21 Likely through a similar mechanism, mice lacking SCD1 have enhanced dextran sulfate sodium (DSS)- and bacterial-induced inflammatory gene expression and exaggerated colitis.23 These recent studies21–23 suggest that SCD1 may serve a protective function against proinflammatory signaling.
It is reasonable to assume that many of the inflammation-linked side effects seen with SCD1 inhibition stems from the abnormal accumulation of SCD1 substrates, saturated fatty acids, in multiple tissues. Indeed, there is a large body of evidence that SFAs are potent proinflammatory molecules, linking these SCD1 substrates to a number of inflammatory diseases.24–34 In fact, recent evidence suggests that SFAs can activate multiple toll-like receptors (TLRs), which play a key role in innate immunity.24,28,29,31–34 Furthermore, TLR4 is necessary for SFAs to induce obesity, insulin resistance, and vascular inflammation in rodents.24–27 Therefore, one of the key roles of SCD1 may be to suppress inflammation by preventing excessive accumulation of SFA-derived TLR4 ligands. Interestingly, long chain ω-3 polyunsaturated fatty acids (ω-3 PUFA) have been shown to counteract SFA-induced TLR4 activation in cultured macrophage and dendritic cell systems.28,30–33 In parallel, there is strong evidence that dietary ω-3 PUFA supplementation can effectively blunt inflammation and related diseases in vivo.34–36 Therefore, we reasoned that dietary supplementation with fish oil derived ω-3 PUFAs may prevent the SFA-driven TLR4 hypersensitivity and accelerated atherosclerosis seen with SCD1 inhibition.21
Methods
Male low density lipoprotein receptor deficient (LDLr−/−), apolipoprotein B 100 only (ApoB100/100) mice were treated with antisense oligonucleotides (ASOs) to inhibit SCD1 while consuming diets containing 0.1% (w/w) cholesterol and 12% of energy as primarily either SFA-enriched fat (palm oil) or long chain ω-3 PUFA-enriched fat (fish oil) for 20 weeks as previously described.21 Detailed descriptions of diets, materials, and experimental methods are availablein the online supplement.
Results
SCD1 ASO treatment and dietary fish oil reduce SCD1 expression in a tissue-specific manner, resulting in protection against diet-induced obesity and insulin resistance
There is a large body of evidence that either SCD1 inhibition or dietary fish oil alone can protect against diet induced obesity and insulin resistance in rodents.4–11,30–36 However, the possibility that the combination of these two treatments can act synergistically to improve the metabolic syndrome has never been addressed. Interestingly, both SCD1 ASO treatment and dietary fish oil can diminish SCD1 expression in a tissue-specific manner (Supplemental Figure 1), resulting in dramatic protection against diet-induced obesity and insulin resistance (Supplemental Figure 2). A detailed description of these anticipated metabolic improvements is provided in the online supplement.
Dietary fish oil supplementation prevents SCD1 ASO-driven atherosclerosis
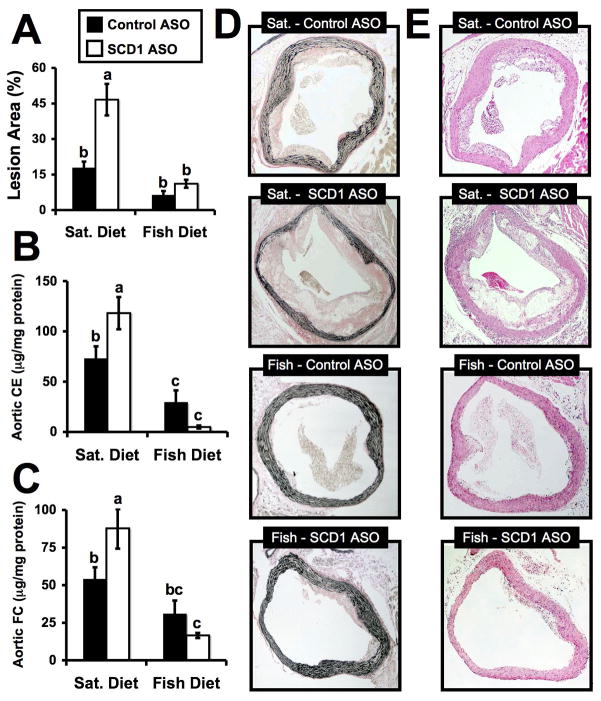
We have previously shown that the SCD1 inhibition in hyperlipidemic mice fed either SFA-rich or monounsaturated fatty acid (MUFA)-rich diets results in more extensive atherosclerosis.21 This unforeseen side effect of SCD1 inhibition was associated with SFA enrichment of plasma lipids and macrophage membranes, together with enhanced TLR4-driven proinflammatory signaling. Importantly, long chain ω-3 PUFAs have been shown to prevent SFA-induced TLR4 activation in cultured cells.28,31–34 Therefore, we set out to test whether this relationship held up in vivo by examining atherosclerosis, a complex disease with an inflammatory component that is promoted by both SFA37,38 and TLR4 activation.39,40 As previously demonstrated, en face morphometric analysis showed that SCD1 ASO treatment in mice fed a SFA-rich diet resulted in a 2.7-fold increase in total aortic lesion area, compared to mice treated with a control ASO (Figure 1A). In parallel, SCD1 inhibition in mice fed a SFA-rich diet also resulted in increased concentrations of aortic cholesteryl ester (Figure 1B), aortic free cholesterol (Figure 1C), and abundant areas of necrosis with visible cholesterol crystals (Figure 1D), all indicating accelerated atherosclerosis. In contrast, in mice fed a fish oil diet, SCD1 ASO treatment had no significant effects on lesion area (Figure 1A), aortic cholesteryl ester (Figure 1B), and aortic free cholesterol (Figure 1C), or lesion complication (Figure 1D and 1E), compared to control ASO treated mice. The chemical measurement may have been the most precise quantification of atherosclerosis, and mice fed a fish oil diet had significantly less aortic cholesteryl ester, compared to SFA-fed mice regardless of ASO treatment (Figure 1B). After 20 weeks of induction, the rank order of aortic cholesteryl ester for the groups was: SFA-fed/SCD1 ASO (118 μg/mg protein) > SFA-fed/Control ASO (73 μg/mg protein) > Fish-oil fed/Control ASO (29 μg/mg protein) > Fish oil-fed/SCD1 ASO (5 μg/mg protein).
Figure 1.
Dietary fish oil supplementation prevents SCD1 ASO-driven atherosclerosis in LDLr−/−, ApoB100/100 mice. Starting at six weeks of age, male mice were fed diets containing 0.1% (w/w) cholesterol enriched in either saturated (Sat.) or long chain ω-3 fatty acids (Fish) for 20 weeks in conjunction with biweekly injections (25 mg/kg) of a non-targeting control ASO ■ or SCD1 ASO □. A. En face morphometric analysis of total aortic lesion area. Data shown in panel A represent the mean ± SEM from 6 mice per group. GLC analysis of aortic cholesteryl ester (B) and free cholesterol (C) was determined. Data in panels B and C represents the mean ± SEM from 8–15 mice per group. Values not sharing a common superscript differ significantly (p<0.05). D. Representative Verhoeff-van Giesen stained sections of proximal aortae from mice treated with diet and ASO for 20 weeks. E. Representative hematoxylin and eosin stained sections of proximal aortae from mice treated with diet and ASO for 20 weeks.
Dietary fish oil supplementation and SCD1 ASO treatment improve atherogenic hyperlipidemia in a complimentary fashion
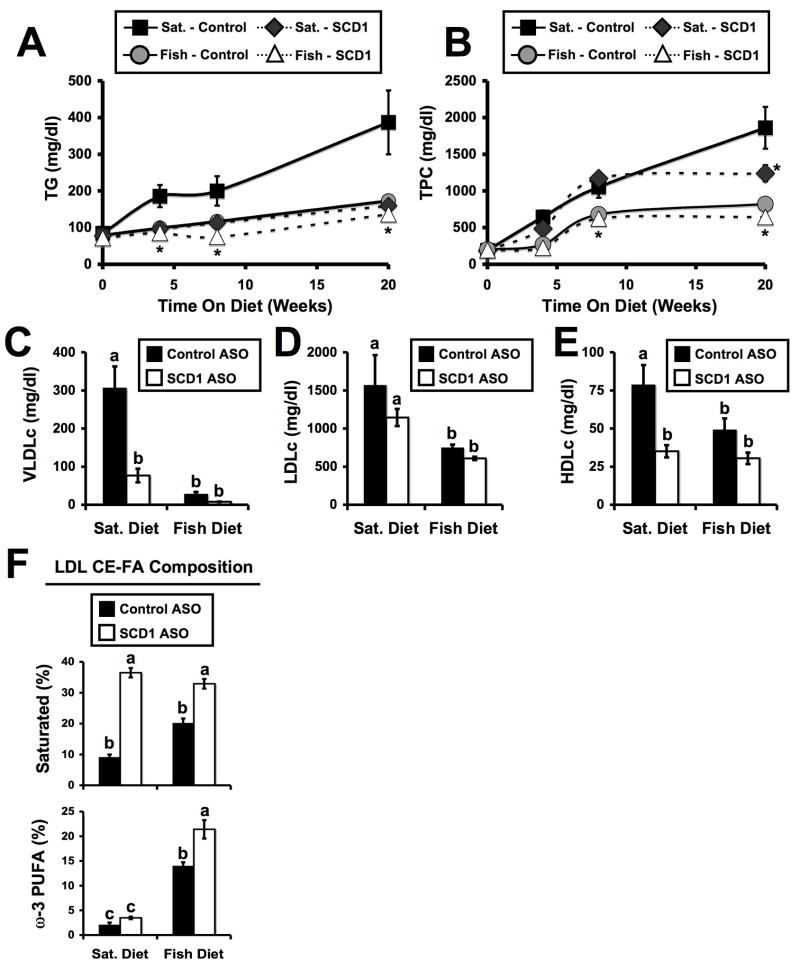
In agreement with previous reports,6,12–14 SCD1 inhibition alone and dietary fish oil alone prevented diet-induced hypertriglyceridemia, which was apparent after only four weeks of either treatment (Figure 2A). After eight weeks of treatment, the rank order of the groups for plasma TG was: SFA-fed/Control ASO (201 mg/dl) > SFA-fed/SCD1 ASO (118 mg/dl) > Fish oil-fed/Control ASO (113 mg/dl) > Fish oil-fed/SCD1 ASO (76 mg/dl). In contrast to plasma TG, SCD1 ASO treatment only modestly reduced total plasma cholesterol (TPC) after 20 week in mice fed the SFA diet. TPC was not significantly altered after 4 or 8 weeks of this treatment (Figure 2B). Furthermore, in the fish oil fed group, SCD1 ASO treatment did not produce a TPC lowering effect (Figure 2B). When lipoprotein cholesterol distribution was analyzed, we discovered that SCD1 inhibition alone and fish oil feeding alone decreased VLDL cholesterol compared to their respective controls (Figure 2C), but the two treatments together were not synergistic in lowering VLDLc. Interestingly, SCD1 ASO treatment had no effect on LDLc regardless of diet (Figure 2D). In contrast, dietary fish oil significantly reduced LDLc, compared to SFA-fed groups, regardless of ASO treatment (Figure 2D). Furthermore, both SCD1 ASO treatment and dietary fish oil caused significant reductions in plasma HDLc (Figure 2E). As previously described,21 SCD1 ASO treatment caused marked enrichment of LDL cholesteryl esters (LDL-CE) with saturated FA in both diet groups (Figure 2F). Also, dietary fish oil supplementation resulted in the expected ω-3 PUFA-enrichment in LDL-CE (Figure 2F). Interestingly, ASO-mediated inhibition of SCD1 in fish oil fed mice caused significantly more ω-3 PUFAs to be incorporated in LDL-CE, compared to control ASO treated mice. It seems the primary effects of SCD1 inhibition alone are: diminished plasma triglyceride, decreased VLDLc, and enrichment of plasma lipoproteins with SFA. In contrast, dietary fish oil effectively reduces both plasma TG and LDLc levels. Collectively, the effects of SCD1 ASO and dietary fish oil synergistically improve atherogenic hyperlipidemia, likely through independent mechanisms.
Figure 2.
Combined therapy of dietary fish oil and SCD1 ASO synergistically improves hyperlipidemia in LDLr−/−, ApoB100/100 mice. Starting at six weeks of age, male mice were fed diets containing 0.1% (w/w) cholesterol enriched in either saturated (Sat.) or long chain ω-3 fatty acids (Fish) for 20 weeks in conjunction with biweekly injections (25 mg/kg) of a non-targeting control ASO (Control) or SCD1 ASO (SCD1). Plasma samples were collected at baseline (6 weeks of age), and after 4, 8, or 20 weeks of diet and ASO treatment. Plasma triglycerides (A) and total plasma cholesterol (TPC) (B) were measured enzymatically. Data shown in panels (A) and (B) represents the mean ± SEM from 5–8 mice per group, * = significantly different than the saturated diet fed control ASO treated group, within each time point (p<0.05). Panels C–E represents cholesterol levels in very-low-density lipoproteins (VLDLc), low-density lipoproteins (LDLc), and high-density lipoproteins (HDLc) in mice receiving dietary and ASO treatment for 20 weeks. Data shown in panel C–E represent the mean ± SEM from 6 mice per group, and values not sharing a common superscript differ significantly (p<0.05). F. Fatty acid (FA) composition [% of total FA as saturated or long chain ω-3 (eicosapentaenoic and docosahexaenoic) fatty acids] of LDL cholesteryl esters (LDL CE-FA). Data shown in panel (F) represents the mean ± SEM (n=5 per group), and values not sharing a common superscript differ significantly (p<0.05).
Dietary fish oil supplementation prevents SCD1 ASO-driven TLR4 hypersensitivity in macrophages
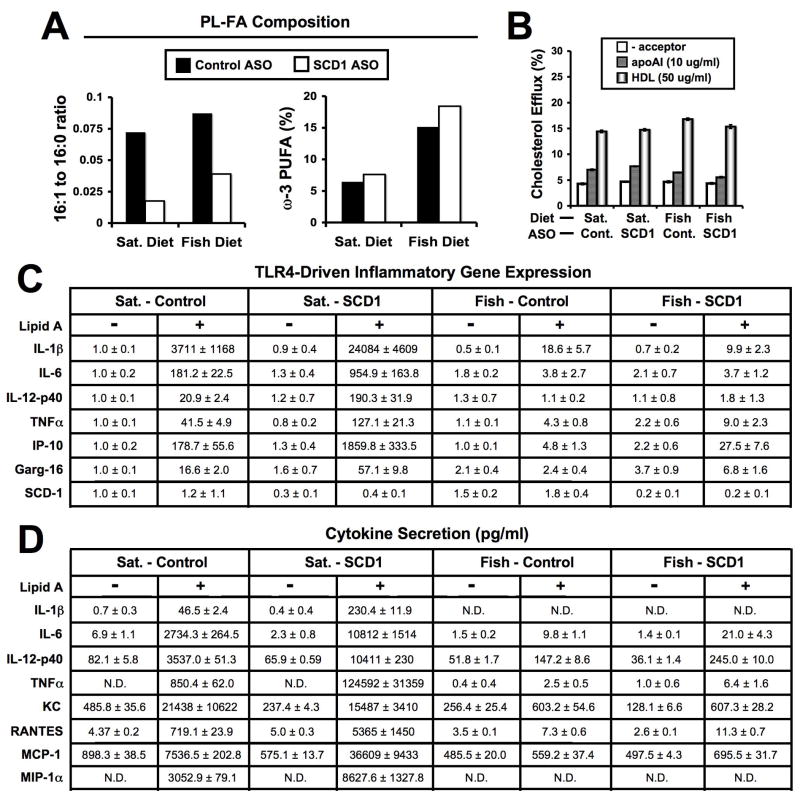
SCD1 ASO treatment for six weeks reduced the palmitoleate (16:1) to palmitate (16:0) ratio in macrophage PL in both diet groups (Figure 3A). This was anticipated since we have previously demonstrated that SCD1 ASO treatment reduces macrophage SCD1 expression.21 Dietary fish oil alone did not alter the 16:1 to 16:0 ratio in macrophage PL, compared to PL isolated from SFA-fed mice (Figure 3A), and did not alter macrophage SCD1 mRNA levels (Figure 3C). However, dietary fish oil supplementation resulted in expected ω-3 PUFA-enrichment in macrophage PL (Figure 3A). As seen in plasma (Figure 2F) and the liver (Supplemental Figure 3D), ASO-mediated inhibition of SCD1 in mice fed a fish oil diet caused a trend towards increased ω-3 PUFA incorporation into macrophage PL, compared to control ASO treated mice. It has previously been demonstrated that macrophage SCD1 plays a role in cellular cholesterol efflux41, which could subsequently impact atherogenesis. However, we did not see any appreciable effects of diet or SCD1 ASO treatment on cellular cholesterol efflux to either lipid-free apoAI or HDL (Figure 3B). More importantly, when macrophages isolated from SCD1 ASO-treated mice fed a SFA-rich diet were challenged with a specific TLR4 agonist (10 ng/ml Kdo2-Lipid A), marked hypersensitivity was apparent, both at the mRNA level (Figure 3C) and the level of protein secretion (Figure 3D) as previously described.21 In support of this, TLR4-dependent upregulation of inflammatory gene expression was much more robust in macrophages isolated from SFA-rich diet fed mice treated with SCD1 ASO, compared to macrophages isolated from their counterparts treated with control ASO (Figure 3C). Likewise, TLR4-dependent promotion of inflammatory cytokine secretion was much more robust in macrophages isolated from SFA-rich diet fed SCD1 ASO treated mice, compared to macrophages isolated from their counterparts treated with control ASO (Figure 3D). In contrast, in mice fed a ω-3 PUFA-rich diet, SCD1 ASO treatment did not result in TLR4 hypersensitivity (Figure 3C and 3D). Interestingly, plasma levels of inflammatory cytokines were relative low in all the experimental groups (Supplemental Table II), and were not significantly different with SCD1 ASO treatment. Collectively, these data suggest that SCD1 ASO-driven TLR4 hypersensitivity in macrophages can be prevented by dietary fish oil supplementation.
Figure 3.
Dietary fish oil supplementation prevents SCD1 ASO-driven TLR4 hypersensitivity in macrophages. Starting at six weeks of age, male mice were fed diets containing 0.1% (w/w) cholesterol enriched in either saturated (Sat.) or long chain ω-3 fatty acids (Fish) for 6 weeks in conjunction with biweekly injections (25 mg/kg) of a non-targeting control ASO (Cont.) or SCD1 ASO (SCD1). Following six weeks of diet and ASO treatment, freshly isolated thioglycollate-elicited macrophages were pooled (n=5–7 mice per pool) and cultured as described in materials and methods. A. Phospholipid fatty acid (PL-FA) composition [16:1 to 16:0 ratio and % of total FA as long chain ω-3 (eicosapentaenoic and docosahexaenoic) fatty acids] of freshly isolated (2h culture) macrophages. B. Macrophage cholesterol efflux to apoAI (10 μg/ml) or high density lipoprotein (HDL, 50 μg/ml). C. TLR4-driven gene expression: Freshly isolated macrophages were treated with vehicle or 10ng/ml Kdo2-Lipid A (TLR4 agonist) for 6 hours, and mRNA levels were measured for interleukins 1 beta (IL-1β), 6 (IL-6), and 12p40 (IL-12p40), tumor necrosis factor alpha (TNFα), C-X-C motif ligand 10 (IP-10), and gluocorticoid attenuated response gene 16 (Garg-16), and normalized to GAPDH. Data shown in panel C are expressed as the mean relative mRNA expression, where all values were normalized to the levels in the Sat. – Control vehicle treated group, and the standard error of the mean (SEM) was calculated from triplicate plates for each pool. D. TLR4-driven cytokine secretion: Freshly isolated macrophages were treated with vehicle or 10ng/ml Kdo2-Lipid A (TLR4 agonist) for 6 hours, and cytokine levels (pg/ml) were measured for IL-1β, IL-6, IL-12p40, TNFα, Chemokine C-X-C motif ligand 1 (KC), Regulated upon Activation Normal T-cell Expressed and Secreted (RANTES,) monocyte chemotactic protein 1 (MCP-1), and macrophage inflammatory protein 1 alpha (MIP-1α) using conditioned media. Data shown in panel D the mean ± SEM from triplicate plates for each pool. N.D. = values not detectable
Combined therapy of dietary fish oil and SCD1 ASO prevents diet-induced hepatic steatosis
Mice lacking SCD1 are protected against diet- and genetically-induced hepatic steatosis.13,14,17,20,21 Likewise, dietary ω-3 PUFA supplementation protects against hepatic steatosis in a number of experimental models.6,22 Interestingly, the dual therapy of dietary fish oil and SCD1 ASO treatment resulted in near complete prevention of diet-induced hepatic steatosis (Supplemental Figure 3). A detailed description of these data is provided in the online supplement.
Discussion
The search for metabolic syndrome targets has strongly supported SCD1 inhibitors as an attractive option for preventing obesity, insulin resistance, hypertriglyceridemia, and hepatic steatosis.4–16 Unfortunately, there are severe side effects associated with diminished SCD1 activity in mice, including skin pathology17–20 and accelerated atherosclerosis.21,22 These warning signs have unfortunately impeded many SCD1 inhibitor programs,42 without complete understanding of the etiology of these complex side effects. It is logical to assume many of the side effects seen with SCD1 inhibition stem from the abnormal accumulation of SFAs in multiple tissues. Indeed, SFAs are potent proinflammatory molecules24–34, and have been linked to innate immunity. 24,28,29,31–34 Therefore, SCD1 may indirectly suppress inflammation by preventing SFA-induced activation of TLR4. The concept of fatty acids regulating inflammation is not unique to SFAs. In fact, long chain ω-3 PUFA from fish oil have been shown to inhibit inflammation, and more importantly, counteract SFA-induced TLR4 activation in cultured cells. 28,30–33 This study provides new evidence that this reciprocal relationship between SFA and ω-3 PUFA in modulating inflammation also holds true in vivo, and can be exploited to protect against multiple metabolic diseases.
The question now becomes: how does the combination of SCD1 ASO treatment and dietary fish oil synergize to comprehensively prevent the development of obesity, insulin resistance, hyperlipidemia, hepatic steatosis, and atherosclerosis? There are likely both shared and independent mechanisms by which these treatments mediate their effects. When given as a monotherapy, SCD1 ASO treatment results in striking protection against diet-induced obesity7,21, insulin resistance7,10,21, hypertriglyceridemia7,21, and hepatic steatosis.7,21 Unfortunately, SCD1 ASO treatment promotes severe atherosclerosis in hyperlipidemic mice fed either a SFA- or MUFA-rich diet.21 We believe that dietary fish oil supplementation is able to prevent SCD1 ASO-driven atherosclerosis through at least three independent mechanisms: 1) lowering LDLc, 2) enriching the remaining LDL-CE in atheroprotective ω-3 PUFAs, and 3) counteracting SFA-driven inflammation. In support of this, dietary fish oil lowered LDLc by 47–53%, compared to SFA-fed mice regardless of ASO treatment (Figure 2D). However, the LDL-CE remaining in fish oil fed mice treated with SCD1 ASO was highly enriched in ω-3 PUFAs, yet enriched in SFA to the same extent as SFA-fed SCD1 ASO-treated mice (Figure 2F). These results indicate that dietary fish oil does not diminish SCD1 ASO-mediated SFA enrichment of plasma lipids, but rather shifts the fatty acid composition to be more polyunsaturated, which has the potential to diminish the production of VLDLc (Figure 2C,45). In this regard, it has been previously demonstrated that long chain fatty acids such as docosahexaenoic acid (DHA) can inhibit VLDL secretion by promoting post-ER presecretory proteolysis (PERPP)-mediated degradation of apolipoprotein B46 or endoplasmic reticulum (ER) stress related-degradation of apoB47. Although we did not directly measure oxidant stress or PERPP, we saw no indication that either fish oil or SCD1 ASO promoted hepatic ER stress (Supplemental Figure 4).
It is important to note that SCD1 ASO treatment unexpectedly results in a dramatic HDLc reduction in hyperlipidemic mice fed a SFA-rich diet.21 Based on this, it has recently been speculated that SCD1 ASO-driven HDLc lowering may be the cause of accelerated atherosclerosis seen under these conditions.22 However, our data suggests that HDLc lowering plays little, if any, role in SCD1 ASO-driven accelerated atherosclerosis. In support of this, dietary fish oil alone actually decreased HDLc, compared to SFA fed mice (Figure 2E), yet atherosclerosis was also decreased in fish oil fed mice. Most importantly, fish oil supplementation did not prevent SCD1 ASO-mediated reductions in HDLc seen in SFA-fed mice (Figure 2E). In fact, the mice treated with SCD1 ASO and fed dietary fish oil had the lowest HDLc of any group with the rank order of the four groups being: SFA-fed/Control ASO (79 mg/dl) > Fish oil-fed/Control ASO (49 mg/dl) > SFA-fed/SCD1 ASO (35 mg/dl) > Fish oil-fed/SCD1 ASO (30 mg/dl). Collectively, these data suggest that HDLc modulation is not the primary mechanism by which dietary fish oil protects against SCD1 ASO-driven atherosclerosis.
In addition to reducing plasma lipoprotein levels, dietary fish oil prevents SCD1 ASO-driven TLR4 hypersensitivity (Figure 3C). This may be due, in part, to the enrichment of macrophage membranes with long chain ω-3 PUFAs (Figure 3A), which are known to prevent SFA-driven TLR4 activation.28,30–33 Importantly, SCD1 ASO treatment results in marked accumulation of SFA in plasma, multiple tissues, and isolated macrophages (Figure 2F, Supplemental Figure 3D, and Figure 3A,21). However, this SFA enrichment is not prevented by dietary fish oil supplementation (Figure 2F, Supplemental Figure 3D, and Figure 3A). Hence, even in the face of massive SCD1 ASO-driven SFA accumulation, moderate dietary ω-3 PUFA supplementation can prevent SFA-driven inflammation (Figure 3C) and atherosclerosis (Figure 1). This makes it tempting to speculate that the other diverse pathologies associated with genetic deletion of SCD1,11,17–20,23 including alopecia, might likewise be ameliorated by the anti-inflammatory effects of dietary fish oil. Interestingly, during the preparation of this manuscript, a recent study warned that previous work29–33 describing SFA-mediated activation of TLR4 or TLR2 may have been confounded by contamination of the fatty acid vehicle (BSA) with LPS and/or bacterial lipoproteins.48 Importantly, since in vivo dietary feeding of long-chain fatty acids does not require a BSA vehicle, this is likely not the only explanation for SFA-induced TLR4 activation. Rather, our results provide in vivo evidence that saturated fatty acids do indeed promote TLR4-dependent signaling and that n-3 PUFAs can antagonize SFA-driven TLR4 hypersensitivity (Figure 3). However, whether fatty acids exert their effects through direct TLR4 agonism or by modulating membrane organization is still a matter of debate, and requires further work.
It is important to point out that ASO-mediated inhibition of SCD1 does not alter SCD1 protein expression in the skin or result in alopecia.21 This tissue specific pattern of inhibition seen with in vivo ASO administration has been documented previously,43,44 and is ideal for SCD1 inhibition where tissue specificity is required to avoid unwanted side effects. In a recent study by MacDonald and colleagues,22 it was speculated that the accelerated atherosclerosis seen with genetic SCD1 deficiency was in part due to dermal inflammation.22 This is unlikely to be the primary mechanism for the accelerated atherosclerosis, since SCD1 ASO treatment also results in severe atherosclerosis, without affecting skin SCD1 expression or alopecia.21 Therefore, ASO-mediated inhibition may provide a unique tissue-specific therapeutic strategy to avoid the skin pathology17–20 that would likely accompany small molecule SCD1 inhibitors without tissue specificity.
In summary, we have demonstrated that SCD1 ASO treatment protects against development of the metabolic syndrome, but unfortunately promotes atherosclerosis in mice fed diets enriched in either SFA or MUFA.6 However, SCD1 ASO-driven atherosclerosis can be completely prevented by dietary fish oil (Figure 1). Importantly, the pro-inflammatory effects of SCD1 ASO treatment can be overcome by dietary ω-3 PUFA supplementation, and the dual therapy provides dramatic protection against atherogenic hyperlipidemia. Therefore, this synergistic dual therapy may provide a novel therapeutic approach for the metabolic syndrome and atherosclerosis.
Supplementary Material
Acknowledgments
Sources of funding: This work was supported by grants from the National Center for Complimentary and Alternative Medicine (NCCAM-P50AT002782 to L.L.R. and J.S.P.), the National Institutes of Health (NIH-P01-HL49373 to L.L.R. and J.S.P. R01-HL094525 to J.S.P., and 1K99HL096166-01 to J.M.B.), the American Heart Association (AHA postdoctoral fellowships # 0625400U to J.M.B, # 0825445E to S.C., and #09POST2250225 to X.Z.), and Howard Hughes Medical Institute (Gilliam Fellowship to T.M.N.). We thank Rosanne Crooke and Mark Graham (ISIS Pharmaceuticals, Inc. Carlsbad, CA USA) for providing ASOs used in this study.
References
- 1.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus SC, Jr, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 4.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song B, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald ML, Singaraja RR, Bissada N, Ruddle P, Watts R, Karasinska JM, Gibson WT, Fievet C, Vance JE, Staels B, Hayden MR. Absence of stearoyl-CoA desaturase-1 ameloriates features of the metabolic syndrome in LDLR-deficient mice. J Lipid Res. 2008;49:217–229. doi: 10.1194/jlr.M700478-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, Bergeron R, Doebber T, Zhang BB. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115:1030–1038. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki M, Dobrzyn A, Sampath H, Lee SH, Man WC, Chu K, Peters JM, Gonzalez FJ, Ntambi JM. Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferators-activated receptor-alpha. J Biol Chem. 2004;279:35017–35024. doi: 10.1074/jbc.M405327200. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rosetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116:1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, Keller MP, Ntambi JM, Attie AD. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56:1228–1239. doi: 10.2337/db06-1142. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–30128. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 13.Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyeridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- 14.Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol. 2006;26:6786–6798. doi: 10.1128/MCB.00077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, Friedman JM. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113:414–424. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and –independent mechanisms. J Biol Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, Stenn KS, Parimoo S. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- 18.Sundberg JP, Boggess D, Sundberg BA, Eilertsen K, Parimoo S, Filippi M, Stenn K. Asebia-2J (Scd1(ab2j): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067–2075. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase 1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 20.Binczek E, Jenke B, Holz B, Gunter RH, Thevis M, Stoffel W. Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1−/−) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol Chem. 2007;388:405–418. doi: 10.1515/BC.2007.046. [DOI] [PubMed] [Google Scholar]
- 21.Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen T, Zhu X, Duong M, Wibley AL, Shah R, Davis MA, Kelly K, Wilson MD, Kent C, Parks JS, Rudel LL. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008;118:1467–1475. doi: 10.1161/CIRCULATIONAHA.108.793182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macdonald ML, van Eck M, Hildebrand RB, Wong BW, Bissada N, Ruddle P, Kontush A, Hussein H, Pouladi MA, Chapman MJ, Fievet C, van Berkel TJ, Staels B, McManus BM, Hayden MR. Arterioscler Thromb Vasc Biol. 2008;29:341–347. doi: 10.1161/ATVBAHA.108.181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Shah YM, Morimura K, Krausz KW, Miyazaki M, Richardson TA, Morgan ET, Ntambi JM, Idel JR, Gonzalez FJ. Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab. 2008;7:135–147. doi: 10.1016/j.cmet.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 26.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediated vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 27.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fats. Obesity. 2008;16:1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 28.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 29.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Role of the Toll-like receptor4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 30.Weatherhill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Zhao L, Youn HS, Weatherhill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 32.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Renier G, Skamene E, DeSanctis J, Radzioch D. Dietary n-3 polyunsaturated fatty acids prevent the development of atherosclerotic lesions in mice. Modulation of macrophage secretory activities. Arterioscler Thromb. 1993;13:1515–1524. doi: 10.1161/01.atv.13.10.1515. [DOI] [PubMed] [Google Scholar]
- 35.Blok WL, Katan MB, van der Meer JW. Modulation of inflammation and cytokine production by dietary (n-3) fatty acids. J Nutr. 1996;126:1515–1533. doi: 10.1093/jn/126.6.1515. [DOI] [PubMed] [Google Scholar]
- 36.Hansen SN, Harris WS. New evidence for the cardiovascular benefits of long chain omega-3 fatty acids. Curr Atheroscler Rep. 2007;9:434–440. doi: 10.1007/s11883-007-0058-8. [DOI] [PubMed] [Google Scholar]
- 37.Rudel LL, Parks JS, Sawyer JK. Compared with dietary monounsaturated and saturated fat, polyunsaturated fat protects African green monkeys from coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:2101–2110. doi: 10.1161/01.atv.15.12.2101. [DOI] [PubMed] [Google Scholar]
- 38.Rudel LL, Kelley K, Sawyer JK, Shah R, Wilson MD. Dietary monounsaturated fatty acids promote aortic atherosclerosis in LDL receptor-null, human ApoB100-overexpressing transgenic mice. Arterioscler Thromb Vasc Biol. 1998;18:1818–1827. doi: 10.1161/01.atv.18.11.1818. [DOI] [PubMed] [Google Scholar]
- 39.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-lik receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 40.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y, Hao M, Luo Y, Liang CP, Silver DL, Cheng C, Maxfield FR, Tall AR. Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J Biol Chem. 2003;278:5813–5820. doi: 10.1074/jbc.M208687200. [DOI] [PubMed] [Google Scholar]
- 42.Wertheimer SJ, Bolin D, Erickson S, Conde-Knape K, Belunis C, Konkar A, Taub R, Rondinone CM, et al. Drug Discovery Today: Therapeutic Strategies. 2007;4:129–135. [Google Scholar]
- 43.Levin AA, Yu RZ, Geary RS. Antisense Drug Technology. 2. Boca Raton, Fl: CRC Press; 2008. Basic principles of the pharmacokinetics of antisense oligonucleotide drug. [Google Scholar]
- 44.Yu XX, Murray SF, Pandey SK, Booten SL, Bao D, Song XZ, Kelly S, Chen S, McKay R, Monia BP, Bhanot S. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 2005;42:362–371. doi: 10.1002/hep.20783. [DOI] [PubMed] [Google Scholar]
- 45.Van Vlijmen BJ, Mensink RP, van’t Hof HB, Offermans RF, Hofker MH, Havekes LM. Effects of dietary fish oil on serum lipids and VLDL kinetics in hyperlipidemic apolipoprotein E*3-Leiden transgenic mice. J Lipid Res. 1998;39:1181–1188. [PubMed] [Google Scholar]
- 46.Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J Clin Invest. 2004;113:1277–1287. doi: 10.1172/JCI19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate toll-like receptor signaling. Arterioscler Thromb Vasc Biol. doi: 10.1161/ATVBAHA.109.194050. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.