Abstract
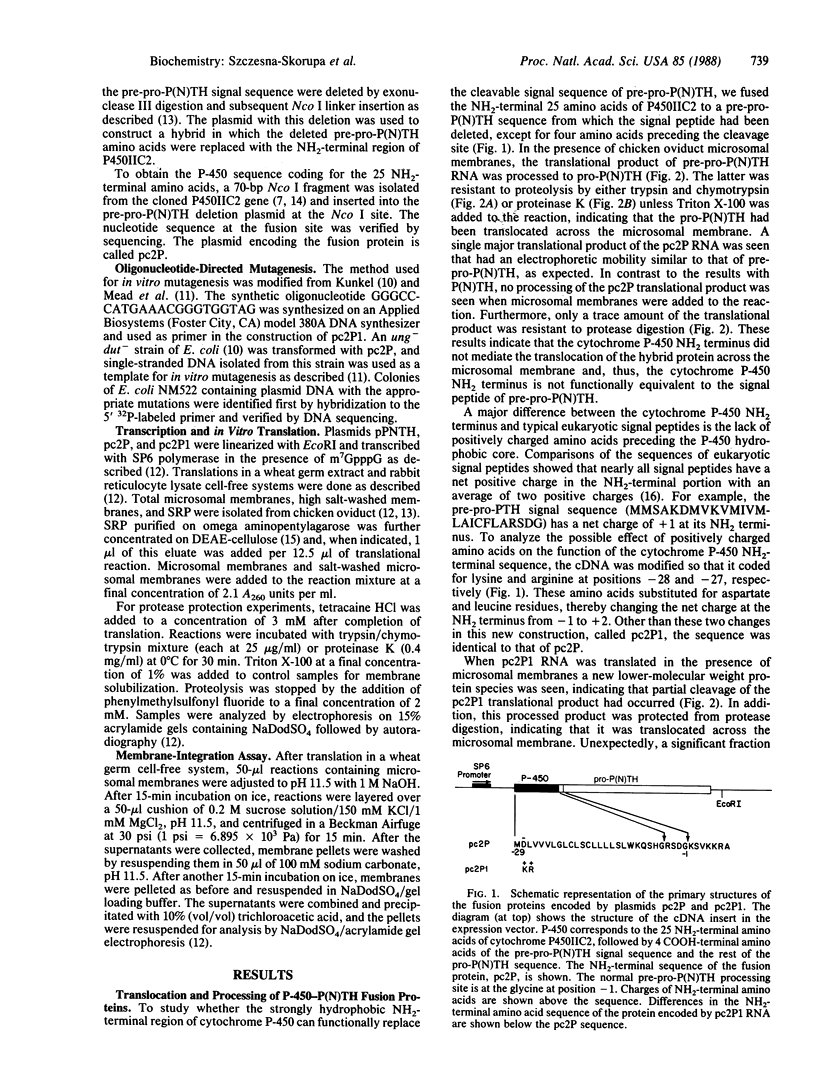
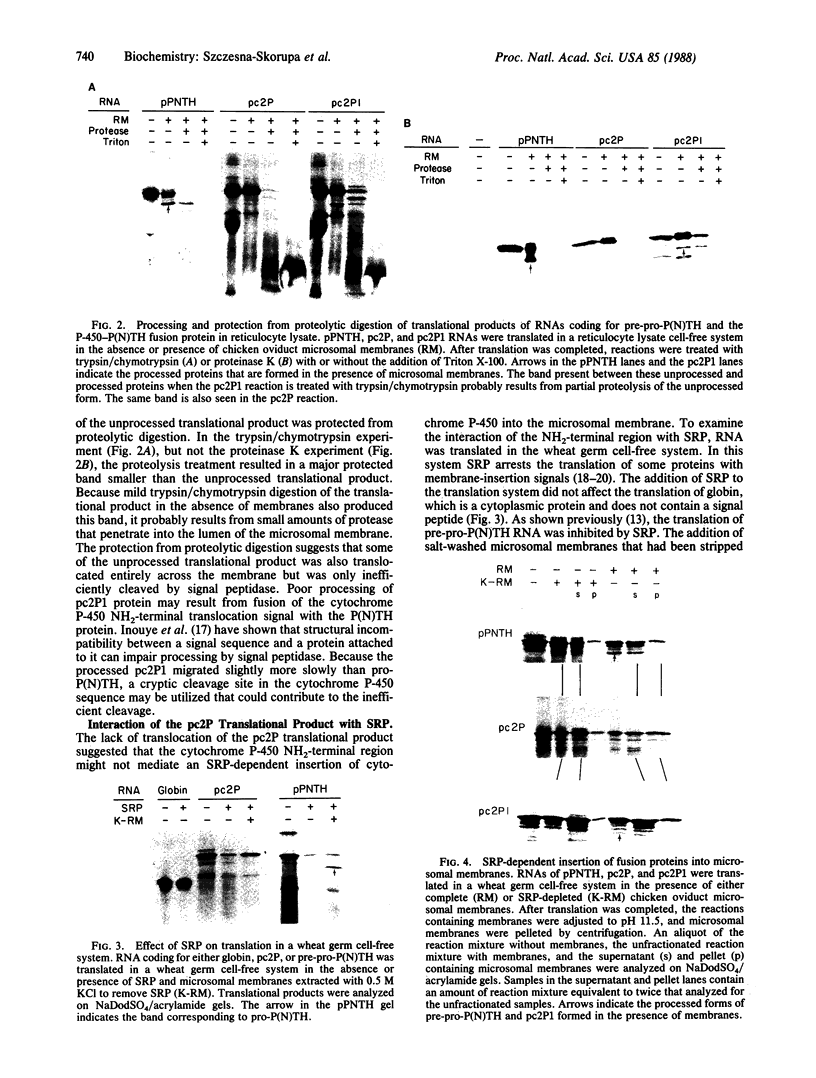
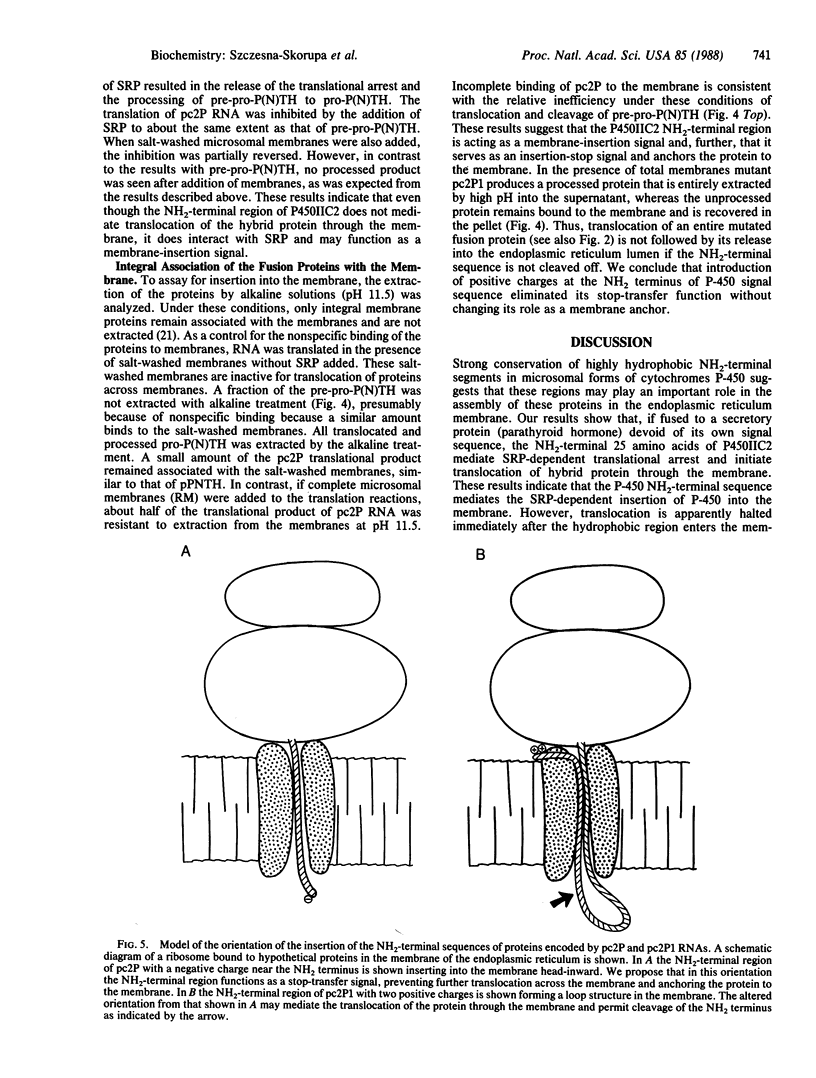
The NH2-terminal sequences of cytochromes P-450 resemble signal peptides, but these sequences are not cleaved during the insertion of these integral membrane proteins into the microsomes. To examine whether these putative signal peptides are functionally equivalent to signal peptides of secretory proteins, cDNA coding for a fusion protein was produced, in which the signal peptide for preproparathyroid hormone was replaced with the putative signal peptide of cytochrome P450IIC2. The translational product of RNA synthesized in vitro from the cDNA was neither processed nor translocated by chicken oviduct microsomal membranes in a reticulocyte cell-free system but was resistant to extraction from the membranes by alkaline solutions. In addition, the translation of the hybrid RNA was arrested by signal recognition particle. Unlike most signal peptides, the cytochrome P450IIC2 NH2-terminal sequence does not contain basic amino acids preceding the hydrophobic core. Introduction by oligonucleotide-directed mutagenesis of lysine and arginine at the NH2 terminus resulted in a fusion protein that was partially processed by the microsomal membranes, with translocation across the membrane of both the processed and unprocessed proteins. The positive charges convert the cytochrome P450IIC2 NH2 terminus from a combination membrane insertion-halt transfer signal to a more classical secretory membrane-insertion signal, possibly by altering the orientation of the signal peptide in the membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Mostov K. E., Blobel G. Mechanisms of integration of de novo-synthesized polypeptides into membranes: signal-recognition particle is required for integration into microsomal membranes of calcium ATPase and of lens MP26 but not of cytochrome b5. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7249–7253. doi: 10.1073/pnas.80.23.7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nun S., Kreibich G., Adesnik M., Alterman L., Negishi M., Sabatini D. D. Synthesis and insertion of cytochrome P-450 into endoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 1980 Feb;77(2):965–969. doi: 10.1073/pnas.77.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos T. J., Davis A. R., Nayak D. P. NH2-terminal hydrophobic region of influenza virus neuraminidase provides the signal function in translocation. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2327–2331. doi: 10.1073/pnas.81.8.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J., Inukai M., Inouye M. Dual functions of the signal peptide in protein transfer across the membrane. Cell. 1985 Nov;43(1):351–360. doi: 10.1016/0092-8674(85)90040-6. [DOI] [PubMed] [Google Scholar]
- Davis N. G., Model P. An artificial anchor domain: hydrophobicity suffices to stop transfer. Cell. 1985 Jun;41(2):607–614. doi: 10.1016/s0092-8674(85)80033-7. [DOI] [PubMed] [Google Scholar]
- De Lemos-Chiarandini C., Frey A. B., Sabatini D. D., Kreibich G. Determination of the membrane topology of the phenobarbital-inducible rat liver cytochrome P-450 isoenzyme PB-4 using site-specific antibodies. J Cell Biol. 1987 Feb;104(2):209–219. doi: 10.1083/jcb.104.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia P. D., Ghrayeb J., Inouye M., Walter P. Wild type and mutant signal peptides of Escherichia coli outer membrane lipoprotein interact with equal efficiency with mammalian signal recognition particle. J Biol Chem. 1987 Jul 15;262(20):9463–9468. [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Govind S., Bell P. A., Kemper B. Structure of genes in the cytochrome P-450PBc subfamily: conservation of intron locations in the phenobarbital-inducible family. DNA. 1986 Oct;5(5):371–382. doi: 10.1089/dna.1986.5.371. [DOI] [PubMed] [Google Scholar]
- Haugen D. A., Armes L. G., Yasunobu K. T., Coon M. J. Amino-terminal sequence of phenobarbital-inducible cytochrome P-450 from rabbit liver microsomes: similarity to hydrophobic amino-terminal segments of preproteins. Biochem Biophys Res Commun. 1977 Aug 8;77(3):967–973. doi: 10.1016/s0006-291x(77)80072-7. [DOI] [PubMed] [Google Scholar]
- Holland E. C., Drickamer K. Signal recognition particle mediates the insertion of a transmembrane protein which has a cytoplasmic NH2 terminus. J Biol Chem. 1986 Jan 25;261(3):1286–1292. [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Inouye S., Duffaud G., Inouye M. Structural requirement at the cleavage site for efficient processing of the lipoprotein secretory precursor of Escherichia coli. J Biol Chem. 1986 Aug 25;261(24):10970–10975. [PubMed] [Google Scholar]
- Kaiser C. A., Preuss D., Grisafi P., Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987 Jan 16;235(4786):312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton J. K., DeBrunner-Vossbrinck B. A., Kemper B. Isolation and sequence analysis of three cloned cDNAs for rabbit liver proteins that are related to rabbit cytochrome P-450 (form 2), the major phenobarbital-inducible form. Biochemistry. 1984 Jan 17;23(2):204–210. doi: 10.1021/bi00297a005. [DOI] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. Signal recognition particle-dependent membrane insertion of mouse invariant chain: a membrane-spanning protein with a cytoplasmically exposed amino terminus. J Cell Biol. 1986 Jun;102(6):2169–2175. doi: 10.1083/jcb.102.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. The membrane-spanning segment of invariant chain (I gamma) contains a potentially cleavable signal sequence. Cell. 1986 Sep 26;46(7):1103–1112. doi: 10.1016/0092-8674(86)90710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Skorupa E. S., Kemper B. Single stranded DNA SP6 promoter plasmids for engineering mutant RNAs and proteins: synthesis of a 'stretched' preproparathyroid hormone. Nucleic Acids Res. 1985 Feb 25;13(4):1103–1118. doi: 10.1093/nar/13.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Mize N. K., Andrews D. W., Lingappa V. R. A stop transfer sequence recognizes receptors for nascent chain translocation across the endoplasmic reticulum membrane. Cell. 1986 Dec 5;47(5):711–719. doi: 10.1016/0092-8674(86)90514-3. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., DeFoor P., Fleischer S., Blobel G. Co-translational membrane integration of calcium pump protein without signal sequence cleavage. Nature. 1981 Jul 2;292(5818):87–88. doi: 10.1038/292087a0. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B., Levin W. The P450 gene superfamily: recommended nomenclature. DNA. 1987 Feb;6(1):1–11. doi: 10.1089/dna.1987.6.1. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Mihara K., Sato R. A short amino-terminal segment of microsomal cytochrome P-450 functions both as an insertion signal and as a stop-transfer sequence. EMBO J. 1987 Aug;6(8):2425–2431. doi: 10.1002/j.1460-2075.1987.tb02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M., Mihara K., Sato R. Signal recognition particle is required for co-translational insertion of cytochrome P-450 into microsomal membranes. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3361–3364. doi: 10.1073/pnas.81.11.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. An internal signal sequence: the asialoglycoprotein receptor membrane anchor. Cell. 1986 Jan 17;44(1):177–185. doi: 10.1016/0092-8674(86)90496-4. [DOI] [PubMed] [Google Scholar]
- Szczesna-Skorupa E., Mead D. A., Kemper B. Mutations in the NH2-terminal domain of the signal peptide of preproparathyroid hormone inhibit translocation without affecting interaction with signal recognition particle. J Biol Chem. 1987 Jun 25;262(18):8896–8900. [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle: a ribonucleoprotein required for cotranslational translocation of proteins, isolation and properties. Methods Enzymol. 1983;96:682–691. doi: 10.1016/s0076-6879(83)96057-3. [DOI] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Zerial M., Melancon P., Schneider C., Garoff H. The transmembrane segment of the human transferrin receptor functions as a signal peptide. EMBO J. 1986 Jul;5(7):1543–1550. doi: 10.1002/j.1460-2075.1986.tb04395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Analysis of the distribution of charged residues in the N-terminal region of signal sequences: implications for protein export in prokaryotic and eukaryotic cells. EMBO J. 1984 Oct;3(10):2315–2318. doi: 10.1002/j.1460-2075.1984.tb02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]