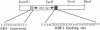Abstract
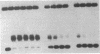
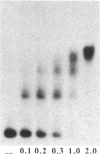
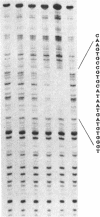
We have isolated a protein from Saccharomyces cerevisiae that binds specifically to a nucleotide sequence associated with the autonomously replicating sequence (ARS) ARS120, located in the telomeric region of a yeast chromosome. "Footprinting" analysis revealed that a 26-base-pair DNA sequence, 5'-CAAGTGCCGTGCATAATGATGTGGGT-3', was protected by this protein from DNase I digestion. A plasmid containing 48 direct tandem repeats of this oligonucleotide was constructed and used to affinity-purify the binding activity. The purified protein, OBF1 (origin binding factor), showed specific binding to ARS120. The 26-base-pair OBF1-protected sequence was sufficient for the recognition and binding of the protein, since the mobility of a DNA fragment containing the synthetic binding site was retarded in agarose gels when incubated with OBF1. By performing competition experiments with a number of different ARSs, we showed that OBF1 binds tightly to some but not all ARSs. Interestingly, OBF1 does not appear to have a discernible affinity for ARS1 or the ARSs associated with mating type loci, HML alpha and HMRa, which are substrates for a DNA-binding activity reported by others. Since OBF1 appears to bind to DNA associated with a number of ARSs, we suggest that this protein may have a function related to ARS activity, perhaps in the initiation of DNA replication at selected ARSs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broach J. R., Li Y. Y., Feldman J., Jayaram M., Abraham J., Nasmyth K. A., Hicks J. B. Localization and sequence analysis of yeast origins of DNA replication. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1165–1173. doi: 10.1101/sqb.1983.047.01.132. [DOI] [PubMed] [Google Scholar]
- Button L. L., Astell C. R. The Saccharomyces cerevisiae chromosome III left telomere has a type X, but not a type Y', ARS region. Mol Cell Biol. 1986 Apr;6(4):1352–1356. doi: 10.1128/mcb.6.4.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S. E., Campbell J. L. Yeast DNA replication in vitro: initiation and elongation events mimic in vivo processes. Cell. 1982 Nov;31(1):201–213. doi: 10.1016/0092-8674(82)90420-2. [DOI] [PubMed] [Google Scholar]
- Celniker S. E., Sweder K., Srienc F., Bailey J. E., Campbell J. L. Deletion mutations affecting autonomously replicating sequence ARS1 of Saccharomyces cerevisiae. Mol Cell Biol. 1984 Nov;4(11):2455–2466. doi: 10.1128/mcb.4.11.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Organization of DNA sequences and replication origins at yeast telomeres. Cell. 1983 Jun;33(2):563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Hice R. H., Chlebowicz-Sledziewska E. ARS replication during the yeast S phase. Cell. 1983 Mar;32(3):831–838. doi: 10.1016/0092-8674(83)90069-7. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Niedzwiecka A., Edelman G. M. In vitro association of a replication complex with a yeast chromosomal replicator. J Biol Chem. 1983 Mar 10;258(5):2754–2757. [PubMed] [Google Scholar]
- Johnson A. D., Meyer B. J., Ptashne M. Interactions between DNA-bound repressors govern regulation by the lambda phage repressor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5061–5065. doi: 10.1073/pnas.76.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearsey S. Structural requirements for the function of a yeast chromosomal replicator. Cell. 1984 May;37(1):299–307. doi: 10.1016/0092-8674(84)90326-x. [DOI] [PubMed] [Google Scholar]
- Kojo H., Greenberg B. D., Sugino A. Yeast 2-micrometer plasmid DNA replication in vitro: origin and direction. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7261–7265. doi: 10.1073/pnas.78.12.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine G. T., Sinha P., Tye B. K. Mutants of S. cerevisiae defective in the maintenance of minichromosomes. Genetics. 1984 Mar;106(3):365–385. doi: 10.1093/genetics/106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., Kelly T. J. Purification of nuclear factor I by DNA recognition site affinity chromatography. J Biol Chem. 1986 Jan 25;261(3):1398–1408. [PubMed] [Google Scholar]
- Saffer L. D., Miller O. L., Jr Electron microscopic study of Saccharomyces cerevisiae rDNA chromatin replication. Mol Cell Biol. 1986 Apr;6(4):1148–1157. doi: 10.1128/mcb.6.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D., Stillman D. J., Brand A. H., Nasmyth K. A. Identification of silencer binding proteins from yeast: possible roles in SIR control and DNA replication. EMBO J. 1987 Feb;6(2):461–467. doi: 10.1002/j.1460-2075.1987.tb04776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Ramaswamy R. On the dynamics of controlled metabolic network and cellular behaviour. Biosystems. 1987;20(4):341–354. doi: 10.1016/0303-2647(87)90052-9. [DOI] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Sequence of a yeast DNA fragment containing a chromosomal replicator and the TRP1 gene. Gene. 1980 Jul;10(2):157–166. doi: 10.1016/0378-1119(80)90133-x. [DOI] [PubMed] [Google Scholar]
- Xiao H., Lis J. T. A consensus sequence polymer inhibits in vivo expression of heat shock genes. Mol Cell Biol. 1986 Sep;6(9):3200–3206. doi: 10.1128/mcb.6.9.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]