Abstract
Testicular cancer is the most common solid tumor among males in the 20–39 year-old age range. Moreover, testicular cancer has unique biological associations, clinical features, and psychosocial impacts that establish this tumor as a prototypic malignancy of young adults. The biology of testicular germ cell tumors after puberty is distinctive. Epidemiologic patterns of testicular cancer suggest etiologic factors that may be congenital, racial, and geographic. The clinical management of a cancer common among young adults, but rare among adults in general, requires expertise so as not to jeopardize the high rates of survivorship associated with modern therapy. The concurrent but separate development of staging, prognostic systems and treatment recommendations, between the fields of pediatric and adult oncology, highlight the need for increased integration and cooperation across these subspecialties. And the high rate of survival, combined with the need for long term monitoring for relapse or late effects, demonstrates the challenge of delivering longitudinal care in this mobile and active young adult population.
Introduction
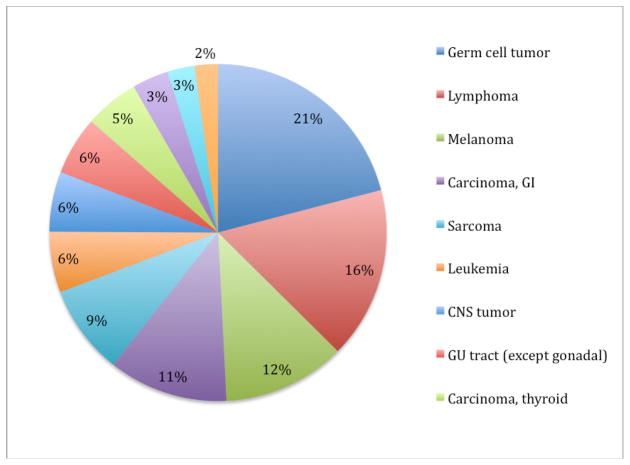
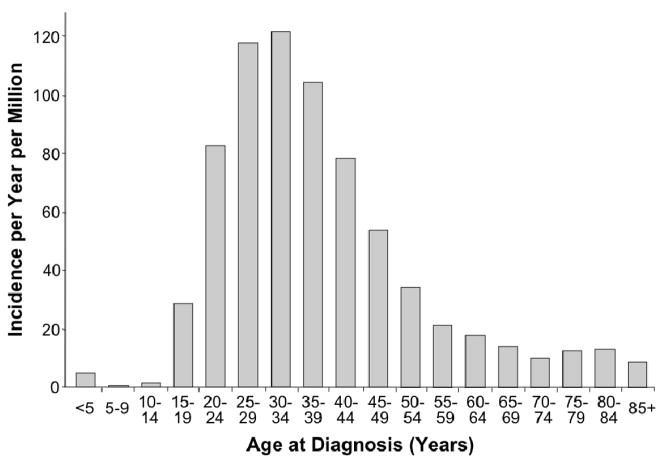
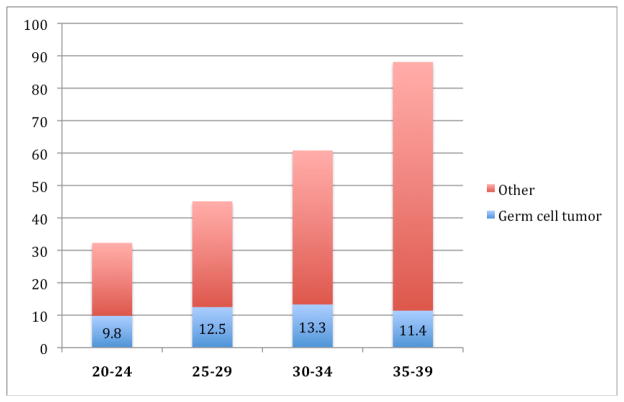
Germ cell tumor is the most common cancer among males in the 20–39 year-old age range, representing 21% of invasive cancer diagnoses (Figure 1).1 The vast majority of testicular tumors in this age range are germ cell tumors. In the period 2001–2005, the incidence of germ cell tumor in the US was estimated to be 11.8 per 100,000 males aged 20–39.1 Overall, there are about 8200 new cases of testicular germ cell tumor in the United States annually. The incidence of germ cell tumors varies significantly with age [Figure 2).2 Cases are rare before age 15, peak in the age range 20–39, then decline, leading many to consider testicular germ cell tumor a prototypic malignancy of young adults. The incidence of germ cell tumors in males is relatively steady through the age range 20–39, but the proportion of germ cell tumor relative to other cancer diagnoses decreases as other cancer types emerge (Figure 3).1
Figure 1.
SEER incidence of cancer in males, age 20 – 39, 2001–2005.1
Figure 2.
Incidence of testicular cancer, SEER 1975–2000. (from Fallon, et al)2
Figure 3.
Age-adjusted SEER incidence of cancer, including germ cell tumor, among males, by age, 2001 – 2005. Rates are per 100,000 and are age-adjusted to the 2000 US Population.1
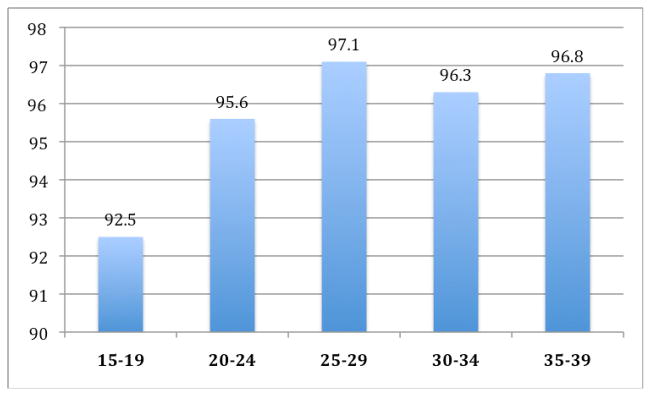
With modern therapy, testicular cancer represents a highly curable malignancy. The 5-year relative survival rate is over 96% (Figure 4) and very significant improvements in mortality rates were observed from 1976–2005 (Figure 5).1 However there remain a number of unique challenges to delivering optimal treatment and care to young adults with testicular cancer. It is important to recognize that, while testicular germ cell tumor is common among young males who are diagnosed with cancer, it remains rare among malignancies in adults, accounting for only 1% of all cancer in males.2 Moreover, testicular cancer has unique biological associations, clinical features, and psychosocial impacts that separate its care from that of the general oncologic population.
Figure 4.
5 year relative survival rate for testicular cancer, by age, 1996 – 2004.1
Figure 5.
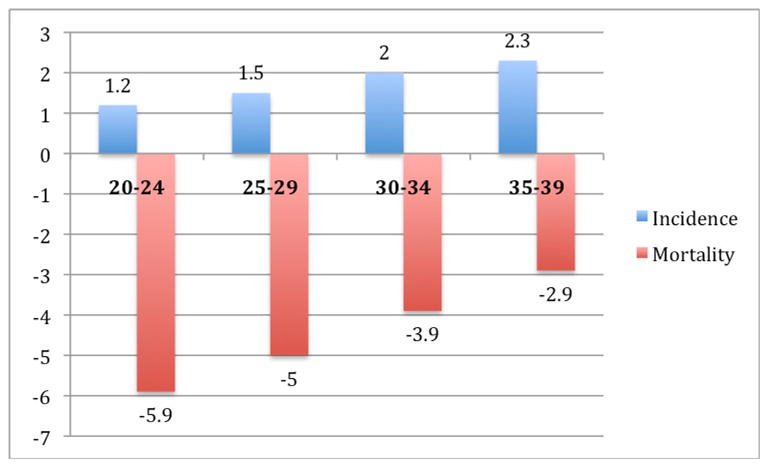
Average annual percentage change in SEER incidence and US mortality rates for testicular cancer, by age, 1976 – 2005.1
Biology
The biology of testicular germ cell tumors after puberty is distinctive. Germ cell tumors are felt to arise from a precursor lesion called intratubular germ cell neoplasia that can be found growing in situ within seminiferous tubules and which express transcription factors common to embryonic stem cells, suggesting the cell of origin is a pluripotent gonocyte. The primordial germ cells arise in the yolk sac during the fourth week of gestation and migrate to the gonadal ridge, differentiate, and further migrate to the iliac fossa and finally to the scrotum. Abnormalities in the differentiation and migration of these cells lead to the variety of presentations of germ cell tumors. Despite a common cell of origin, testicular cancers are histologically and clinically separated into seminoma and non-seminoma.
Seminoma is the most common histology after the age of 35. Among testicular cancer patients with a history of cryptorchidism, seminoma accounts for 60% of tumors. Based on clinical outcome differences, only tumors with pure seminomatous features are categorized as seminoma, and tumors with mixed features (including those associated with elevations in serum AFP) are treated as non-seminomas. The only histologically and clinically distinct subtype of seminoma is spermatocytic seminoma, which rarely metastasizes and is almost always cured with orchiectomy alone.
Non-seminoma includes any or all of the histologic subtypes, yolk sac tumor, choriocarcinoma, embryonal cell carcinoma, and teratoma. While yolk sac elements are present in approximately half of non-seminomatous germ cell tumors, the pure form of yolk sac tumor is rarely seen in post-pubertal males. Progression from precursor lesions to invasive germ cell tumor among pubertal and post-pubertal males is often associated with acquisition of excess genetic material from the short arm of chromosome 12, including isochromosome 12p.3 This is in contrast to prepubertal males who develop pure yolk sac tumors that are commonly diploid or tetraploid.4 Yolk sac elements are responsible for tumor production of alpha-fetoprotein (AFP). Choriocarinoma is a less common element of germ cell tumors and rarely seen in its pure form, but is associated with an aggressive course of visceral and brain metastases. The diagnosis requires a combination of cytotrophoblasts and syncytiotrophoblasts, the latter associated with production of human chorionic gonadotropin (HCG). Embryonal cell carcinoma elements are present in up to 90% of germ cell tumors and are associated with high-level elevations in HCG. Finally, teratoma is a tumor that contains elements of endoderm, mesoderm and ectoderm layers. The pure form of teratoma is also rare, most often seen in children under the age of 4 years.
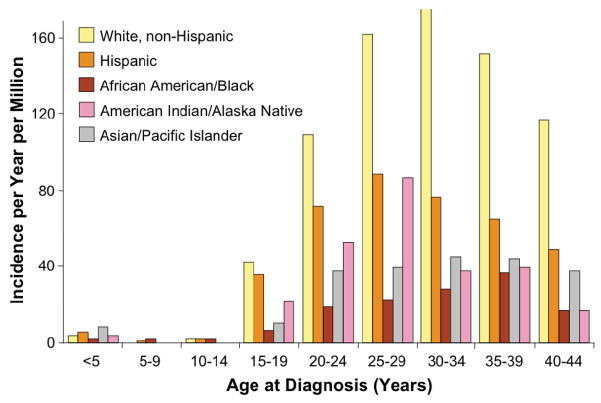
Epidemiologic patterns of testicular cancer suggest unique etiologic factors in its development. The preponderance of this tumor in the young adult age group, along with its association with a history of cryptorchidism (including the contralateral testis), testicular atrophy, hypogonadism, infertility, testicular dysgenesis syndromes, inguinal hernias, and first degree relatives with testicular cancer, point to some genetic predisposition and/or in utero environmental event.5,6 Additional associations have been made with the dysplastic nevus syndrome and, among those with mediastinal primary germ cell tumors, with Klinefelter’s syndrome.7,8 The incidence of testicular cancer varies markedly by race/ethnicity, with rates that are significantly highest among non-Hispanic whites across the age span (Figure 6).2 This unusual racial distribution is associated with geographic variations across the world. The incidence of germ cell tumors has been rising significantly from 1976–2005, with an average annual percentage change of 1.5–2.3% [Figure 5).2 The reasons for this increased incidence are not known.
Figure 6.
Incidence of testicular cancer by race/ethnicity, SEER 1975–2000. (from Fallon, et al)2
Clinical management
The clinical management of a cancer common among young adults but rare among adults in general requires particular expertise, so as not to jeopardize the high rates of survivorship associated with modern therapy. The most common presentation of testicular cancer is a painless mass within the testis, a situation that requires clinical suspicion among young male patients and their primary health care providers to consider cancer. A solid mass within the testis in a young male should prompt urgent evaluation with ultrasound. Baseline serum tumor markers may assist in making a diagnosis and include AFP, HCG, and lactate dehydrogenase (LDH). When a testicular mass is confirmed, the diagnostic procedure is a radical inguinal orchiectomy. Trans-scrotal biopsy should not be performed as seeding the scrotum with malignant cells may lead to a disruption in the pattern of regional lymphatic drainage or spread, and compromise surveillance and surgical therapy. Unfortunately, approximately 25–35% of young men with testicular cancer present with abdominal or back pain, hemoptysis, headache, or other signs of metastatic disease. There is, however, evidence that in more recent years, more are presenting with less advanced metastatic disease.9 Due to predictable patterns of lymphatic or hematogenous spread, staging is completed with abdominal CT, chest xray or CT scan, and in symptomatic patients a brain MRI.
The concurrent but separate development of staging and prognostic systems, as well as treatment recommendations, between the fields of pediatric and adult oncology highlight the need for increased integration and cooperation across these subspecialties in caring for young adults with cancer. The pediatric staging system, outlined in the protocols of the Children’s Oncology group (COG), is listed in Table 1. In contrast, the adult staging system, outlined by the American Joint Committee on Cancer Clinical Staging, is listed in Table 2.10 The AJCC clinical staging utilizes the TNM system, and limits the stages of testicular cancer to I–III, as opposed to stages I–IV in the pediatric system.
Table 1.
Children’s Oncology Group Guidelines for Staging of Testicular Germ Cell Tumors
| Stage | Extent of Disease |
|---|---|
| I | Limited to testis (testes), completely resected by high inguinal orchiectomy; no clinical, radiographic, or histologic evidence of disease beyond the testes. Patients with normal or unknown tumor markers at diagnosis must have a negative ipsilateral retroperitoneal node sampling to confirm Stage I disease if radiographic studies demonstrate lymph nodes > 2 cm |
| II | Trans-scrotal biopsy; microscopic disease in scrotum or high in spermatic cord (< 5 cm from proximal end). Failure of tumor markers to normalize or decrease with an appropriate half-life. |
| III | Retroperitoneal lymph node involvement, but no visceral or extra-abdominal involvement. Lymph nodes > 4 cm by CT or > 2 cm and < 4 cm with biopsy proof. |
| IV | Distant metastases, including liver. |
Table 2.
American Joint Committee on Cancer Clinical Staging for Testicular Germ Cell Tumors9
| Tumor (pathologic) | T1: Limited to testis/epididymis without lymphovascular invasion. May invade tunica albuginea but not tunica vaginalis |
| T2: Limited to testis/epididymis with lymphovascular invasion or extending to involve tunica vaginalis | |
| T3: Invades spermatic cord with or without vascular/lymphatic invasion | |
| T4: Invades scrotum with or without vascular/lymphatic invasion | |
| Node | N1: Lymph node mass ≤ 2 cm in greatest dimension or multiple lymph nodes, not > 2 cm in greatest dimension |
| N2: Lymph node mass > 2 cm but not > 5 cm in greatest dimension, or multiple lymph nodes, any one mass > 2 cm but not > 5 cm in greatest dimension | |
| N3: Lymph node mass > 5 cm in greatest dimension | |
| Metastasis | M1a: Non-regional lymph node or pulmonary metastasis |
| M1b: Distant metastasis other than to non-regional lymph node and lungs | |
| Stage | Extent of Disease |
| I | Confined to the testis (any T, N0, M0) |
| II | Metastatic disease to the nodes of the periaortic or vena caval zone without pulmonary or visceral involvement (any T, any N, M0) |
| III | Metastasis above the diaphragm or involving other viscera (any T, any N, M1) |
Moreover, patients with newly diagnosed testicular cancer are given prognostic information and steered to treatment recommendations using different risk models across pediatric versus adult practice (Tables 3 and 4). The pediatric system is defined among those with non-seminoma, as seminoma is rare in children. The adult system was derived from the combined analysis of over 5000 patients from numerous studies who were treated for disseminated (AJCC stage III) germ cell tumor, including patients with seminoma and non-seminoma.11
Table 3.
Children’s Oncology Group Risk Stratification for Newly Diagnosed Malignant Germ Cell Tumors
| Prognosis | Characteristics |
|---|---|
| Low/Intermediate Risk-100%/97% 6-year survival | Testicular stage I/II |
| High Risk-90% 6-year survival | Testicular stage III/IV Extragonadal stage III/IV |
Table 4.
International Germ Cell Consensus Classification for Metastatic Germ Cell Testicular Cancer10
| Prognosis | Characteristics | |
|---|---|---|
| Good |
Non-Seminoma Testis or retroperitoneal primary, and no non-pulmonary visceral metastases, and α-fetoprotein < 1,000 ng/mL, βHCG < 5,000 IU/L and LDH < 1.5 upper limit of normal −92% 5-year survival |
Seminoma Any primary site, and no non-pulmonary visceral metastases, and normal α-fetoprotein, any βHCG, any LDH −86% 5 year survival |
| Intermediate |
Non-Seminoma Testis or retroperitoneal primary, and no non-pulmonary visceral metastases, and α-fetoprotein > 1,000 ng/mL and < 10,000 ng/mL or βHCG > 5,000 IU/L and < 50,000 IU/L or LDH > 1.5 normal and< 10 normal −80% 5-year survival |
Seminoma Any primary site, and non-pulmonary visceral metastases, and normal α-fetoprotein, any βHCG, any LDH −72% 5-year survival |
| Poor |
Non-Seminoma Mediastinal primary, or non-pulmonary visceral metastases, or α-fetoprotein > 10,000 ng/mL or βHCG > 50,000 IU/L or LDH > 10 upper limit of normal −48% 5-year survival |
Seminoma None |
Patients without clinical evidence of involvement beyond the testis are treated routinely with an orchiectomy followed by a choice of active surveillance, retroperitoneal lymph node dissection (RPLND), adjuvant radiation (for seminoma), or chemotherapy.12,13 Given the high rate of success in salvage therapy for relapsed disease on surveillance, that choice is becoming more popular.
In the current COG trial AGCT0132, patients with low risk non-seminomatous disease are treated with surgery followed by observation. Patients with progression are treated as those with intermediate risk and given 3 cycles of compressed BEP.14 This regimen differs from the adult BEP regimen by reducing the dose of bleomycin from 15 U/m2 weekly to 15 U/m2 every 3 weeks, and by compressing the doses of cisplatin (total dose 100 mg/m2 per cycle) and etoposide (total dose 500 mg/m2 per cycle) over 3 days instead of 5 days.15 However, in a study of 3-day versus 5-day BEP in adults, increased nausea, ototoxicity, and neurotoxcity were observed without any improvement in outcomes.16 Good risk adult patients with stage II or III non-seminoma should receive 3 cycles of standard BEP, and only those who can not receive bleomycin due to allergic reaction or concerns for underlying pulmonary function should be offered 4 cycles of EP.17 Adult patients with intermediate and poor prognosis non-seminoma should receive 4 cycles of BEP. For all patients, carboplatin should not be substituted for cisplatin. In patients with non-seminoma and significant residual radiographic abnormalities after primary chemotherapy, RPLND is considered as both a diagnostic and therapeutic procedure.
The treatment of seminoma differs from non-seminoma, due to the less aggressive biological behavior as well as the exquisite sensitivity of seminoma to radiation therapy and chemotherapy. Approximately 75% of patients with seminoma present with clinical stage I disease. Surveillance studies have demonstrated that 80–85% of patients with clinical stage I seminoma are cured with orchiectomy alone, although late relapses do occur.18 The addition of low-dose [20 Gy in 2 Gy fractions) adjuvant para-aortic radiation reduces relapse rates by two thirds to only approximately 4%, with most patients responsive to additional radiation or chemotherapy after a relapse. Similar reductions in relapse rates have been shown with one cycle of adjuvant carboplatin.19 For patients presenting with bulky stage II or stage III disease, modern cisplatin-based chemotherapy is associated with cure rates of more than 90%.20 RPLND is rarely used for removal of residual masses after chemotherapy; and PET scanning is of value in this setting.
Treatment of recurrent or refractory germ cell tumor is reviewed elsewhere21, but several important principles deserve mention. Secondary chemotherapeutic strategies are remarkably effective, with the majority of patients being cured by second line treatment that oftens includes high dose chemotherapy and adjunctive surgery. Caution should be used when establishing a diagnosis of relapsed or refractory disease based on elevations in serum tumor markers. False positive elevation of AFP is quite rare, with differential considerations including laboratory error, other tumor types such as hepatoma, or liver inflammation. False elevations of HCG may occur in patients who use marijuana, and there is some cross-reactivity in the radioimmunoassay with luteinizing hormone. In cases of persistently elevated HCG, patients should be asked about marijuana use and testosterone should be administered to ensure that a hypogonadal state with resultant high levels of luteinizing hormone is not interfering with the HCG measurement. If the serum tumor markers remain elevated, restaging procedures and investigation of sanctuary sites, including the brain and contralateral testis, are in order.
Psychosocial impact and late effects
The very high rate of survival, combined with the need for long term monitoring for relapse or late effects, demonstrates the challenge of delivering longitudinal care in this mobile and active young adult population. Late psychosocial effects among testicular cancer survivors have been reported to include fear of recurrence, survivor guilt, sleep disturbance, cognitive dysfunction, anxiety or depression, difficulty with formation of relationships and sexual dysfunction.22,23
Medical late effects among testicular cancer survivors can be significant and are related both to the underlying disease and associated treatments. Chemotherapy carries specific risks. Cisplatin causes both renal glomerular and tubular dysfunction with decreases in glomerular filtration rate and wasting of magnesium, which fortunately is often subclinical.24 Cisplatin is also associated with a dose-related high-frequency hearing loss among 20–40% of patients, which is permanent, and peripheral neuropathy, which often improves over time.25,26 Bleomycin may be responsible for lung disease in a dose-related fashion, with approximately 5% developing pulmonary fibrosis.27 Risk factors for bleomycin lung toxicity also include increased age, concomitant chest radiation, decreased renal function, and high concentrations of inspired oxygen. It is important to recognize that one radiographic presentation of bleomycin-induced lung disease may be subpleural-based nodules that may be mistaken on imaging studies for relapsed or refractory disease.
Vascular toxicity may take several forms in survivors of testicular cancer. Raynaud’s phenomenon is the most common vascular toxicity, most often associated with bleomycin exposure.28 However, large-vessel ischemia, including myocardial infarctions and cerebrovascular events, have been associated with testicular cancer chemotherapy.29 This risk may be complicated by the findings from long-term follow-up studies suggesting an association between testicular cancer and a metabolic syndrome of hypertension, glucose intolerance, unfavorable lipid profiles, and vascular events.30
Fertility after testicular cancer may be complicated not only by therapy but by other biologic factors. Beyond the physical effects of orchiectomy and the now rare complication of retrograde ejaculation after RPLND, it is apparent that some men have testicular atrophy as well as baseline abnormalities in sperm counts and/or testosterone levels prior to the diagnosis and treatment of testicular cancer.31 Gonadal dysfunction is common in patients with a history of testicular cancer even when treated with orchiectomy only. However, most patients return to their baseline fertility with one report of the probability of spermatogenesis after orchiectomy and cisplatin-based chemotherapy increasing from 48% at 2 years to 80% by 5 years.32
The risk of second malignancy after testicular cancer is highest for a second primary testicular cancer in the contralateral testis. Therapy-associated risks include carcinomas in prior radiation fields or myelodysplasia and leukemia, often associated with etoposide dose and characterized by abnormalities in the MLL gene.33
Conclusions
The epidemiology and basic biology of testicular cancer place it as a prototypic young adult malignancy. Survival rates with modern therapy are high, but delivery of quality care for testicular cancer, a rare tumor among all adults, requires specialized expertise. The challenges of developing standardized approaches to prognostication and therapy across the worlds of pediatric and adult oncology are apparent in testicular cancer. Finally, with the high rate of survival in this disease, quality care must include attention to the long-term outcomes of these young adult patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brandon Hayes-Lattin, Knight Cancer Institute, Oregon Health and Science University, Portland, Oregon.
Craig R. Nichols, Earle A. Chiles Research Institute, Providence Cancer Center, Portland, Oregon.
References
- 1.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2005. http://seer.cancer.gov/csr/1975_2005/, posted to the SEER web site, 2008. [Google Scholar]
- 2.Fallon R, Olson A, Saxman S. Male genital tract cancer. In: Bleyer A, O’Leary M, Barr R, et al., editors. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. Bethesda, MD: National Cancer Institute; 2006. NIH Pub. No. 06-5767. [Google Scholar]
- 3.Chaganti RS, Rodriguez E, Bosl GJ. Cytogenetics of male germ cell tumors. Urol Clin North Am. 1993;20:55–66. [PubMed] [Google Scholar]
- 4.Houldsworth J, Korkola JE, Bosl GJ, et al. Biology and genetics of adult male germ cell tumors. J Clin Oncol. 2006;24:5512–5518. doi: 10.1200/JCO.2006.08.4285. [DOI] [PubMed] [Google Scholar]
- 5.Dow JA, Mostofi FK. Testicular tumors following orchioplexy. South Med J. 1967;60:193–195. doi: 10.1097/00007611-196702000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Tollerud DJ, Blattner WA, Fraser MC, et al. Familial testicular cancer and urogenital developmental anomalies. Cancer. 1985;55:1849–1854. doi: 10.1002/1097-0142(19850415)55:8<1849::aid-cncr2820550834>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Raghavan D, Zalcberg JR, Grygiel JJ, et al. Multiple atypical nevi: a cutaneous marker of germ cell tumors. J Clin Oncol. 1994;12:2284–2287. doi: 10.1200/JCO.1994.12.11.2284. [DOI] [PubMed] [Google Scholar]
- 8.Nichols CR, Heerema MA, Palmer C, et al. Klinefelter’s syndrome associated with mediastinal germ cell neoplasms. J Clin Oncol. 1987;5:1290–1294. doi: 10.1200/JCO.1987.5.8.1290. [DOI] [PubMed] [Google Scholar]
- 9.Carver BS, Serio AM, Bajorin D, et al. Improved clinical outcome in recent years for men with metastatic nonseminomatous germ cell tumors. J Clin Oncol. 2007;25:5603–5608. doi: 10.1200/JCO.2007.13.6283. [DOI] [PubMed] [Google Scholar]
- 10.American Joint Committee on Cancer. Testis. In: Fleming ID, Cooper JS, Henson DE, et al., editors. AJCC cancer staging manual. 5. Philadelphia, PA: Lippincott-Raven; 1997. pp. 225–230. [Google Scholar]
- 11.International Germ Cell Cancer Collaborative Group. International germ cell consensus classification: A prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 12.Moore CJ, Daneshmand S, Hayes-Lattin B, et al. Outcomes of surveillance in unselected patients with clinical stage I testicular germ cell tumors: Results of a modern single institution series. J Clin Oncol. 2007;25(18S) abstract 5085. [Google Scholar]
- 13.Groll RJ, Warde P, Jewett MA. A comprehensive systemic review of testicular germ cell tumor surveillance. Crit Rev Oncol Hematol. 2007;64:182–197. doi: 10.1016/j.critrevonc.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Cullen JW, Olson TA, Ciller R, et al. Low dose bleomycin every three weeks with cisplatin and etoposide results in excellent EFS and survival rates for children with gonadal germ cell tumors (MGCT): A POG/CCG intergroup report. In: Harden P, Joffe JK, Jones WG, editors. Germ Cell Tumors V. London: Springer-Verlag; 2002. [Google Scholar]
- 15.Williams SD, Birch R, Einhorn LH, et al. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Eng J Med. 1987;316:1435–1440. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]
- 16.de Wit R, Roberts JT, Wilkinson PM, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol. 2001;19:1629–1640. doi: 10.1200/JCO.2001.19.6.1629. [DOI] [PubMed] [Google Scholar]
- 17.Bajorin DF, Geller NL, Weisen SF, et al. Two-drug therapy in patients with metastatic germ cell tumors. Cancer. 1991;67:28–32. doi: 10.1002/1097-0142(19910101)67:1<28::aid-cncr2820670106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Warde PR, Gospodarowicz MK, Goodman PJ, et al. The National Cancer Data Base report on patterns of care for testicular carcinoma. Cancer. 1996;86:2171–2183. [PubMed] [Google Scholar]
- 19.Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomized trial. Lancet. 2005;366:293–300. doi: 10.1016/S0140-6736(05)66984-X. [DOI] [PubMed] [Google Scholar]
- 20.Horwich A, Oliver RT, Wilkinson PM, et al. a Medical Research Council randomized trial of single agent carboplatin versus etoposide and cisplatin for advanced metastatic seminoma. Br J Cancer. 2000;83:1623–1629. doi: 10.1054/bjoc.2000.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes-Lattin B, Nichols CR. Hematopoietic cell transplantation in germ cell tumors. In: Blume KG, editor. Thomas’ Hematopoietic Cell Transplantation. 3. Boston, MA: Blackwell Publishing; 2004. pp. 1308–1319. [Google Scholar]
- 22.Gritz ER, Wellisch DK, Landsverk JA. Psychosocial sequelae in long-term survivors of testicular cancer. J Psychosoc Oncol. 1988;6:41–63. [Google Scholar]
- 23.Gritz ER, Wellisch DK, Wang HJ, et al. Long-term effects of testicular cancer on sexual functioning in married couples. Cancer. 1989;64:1560–1567. doi: 10.1002/1097-0142(19891001)64:7<1560::aid-cncr2820640735>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Fossa SD, Aass N, Winderen M, et al. Long-term renal function after treatment for malignant germ-cell tumours. Ann Oncol. 2002;13:222–228. doi: 10.1093/annonc/mdf048. [DOI] [PubMed] [Google Scholar]
- 25.Reddel RR, Kefford RF, Grant JM, et al. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treat Rep. 1982;66:19–23. [PubMed] [Google Scholar]
- 26.Thompson SW, Davis LE, Kornfeld M, et al. Cisplatin neuropathy. Clinical, electrophysiologic, morphologic, and toxicologic studies. Cancer. 1984;54:1269–1275. doi: 10.1002/1097-0142(19841001)54:7<1269::aid-cncr2820540707>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Haugnes HS, Aass N, Fossa SD, et al. Pulmonary function in long-term survivors of testicular cancer. J Clin Oncol. 2009;27:2779–2786. doi: 10.1200/JCO.2008.18.5181. [DOI] [PubMed] [Google Scholar]
- 28.Vogelsang NJ, Bosl GJ, Johnson K, et al. Raynaud’s phenomenon: a common toxicity after combination chemotherapy for testicular cancer. Lancet. 1981;2:970–973. doi: 10.7326/0003-4819-95-3-288. [DOI] [PubMed] [Google Scholar]
- 29.Doll DC, List AF, Greco FA, et al. Acute vascular ischemic events after cisplatin-based combination chemotherapy for germ-cell tumors of the testis. Ann Intern Med. 1986;105:48–51. doi: 10.7326/0003-4819-105-1-48. [DOI] [PubMed] [Google Scholar]
- 30.Haugnes HS, Aass N, Fossa SD, et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann Oncol. 2007;18:241–248. doi: 10.1093/annonc/mdl372. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen-Bjergaard J, Philip P, Larsen SO, et al. Therapy-related myelodysplasia and acute myeloid leukemia. Cytogenetic characteristics of 115 consecutive cases and risk in seven cohorts of patients treated intensively for malignant diseases in the Copenhagen series. Leukemia. 1993;7:1975–1986. [PubMed] [Google Scholar]
- 32.Hendry WF, Stedronska J, Jones CR, et al. Semen analysis in testicular cancer and Hodgkin’s disease: pre- and posttreatment findings and implications for cryopreservation. Br J Urol. 1983;55:769–773. doi: 10.1111/j.1464-410x.1983.tb03423.x. [DOI] [PubMed] [Google Scholar]
- 33.Lampe H, Horwich A, Norman A, et al. Fertility after chemotherapy for testicular germ cell cancers. J Clin Oncol. 1997;15:239–245. doi: 10.1200/JCO.1997.15.1.239. [DOI] [PubMed] [Google Scholar]