Abstract
Objective
The present study analyzed the risk factors, prevalence and clinical results following revision surgery for adjacent segment degeneration (ASD) in patients who had undergone lumbar fusion.
Methods
Over an 8-year period, we performed posterior lumbar fusion in 81 patients. Patients were followed a minimum of 2 years (mean 5.5 years). During that time, 9 patients required revision surgery due to ASD development. Four patients underwent autogenous posterolateral arthrodesis and extended transpedicle screw fixation, 4 patients underwent decompressive laminectomy and interspinous device implantation, and 1 patient underwent simple decompression.
Results
Of the 9 of patients with clinical ASD, 33.3% (3 of 9) of patients did not have radiographic ASD on plain radiographs. Following revision surgery, the clinical results were excellent or good in 8 patients (88.9%). Age > 50 years at primary surgery was a significant risk factor for ASD development, while number of fusion levels, initial diagnosis and type of fusion were not.
Conclusion
The incidence of ASD development after lumbar surgery was 11.1% (9 of 81) in this study. Age greater than 50 was the statistically significant risk factor for ASD development. Similar successful clinical outcomes were observed after extended fusion with wide decompression or after interspinous device implantation. Given the latter procedure is less invasive, the findings suggest it may be considered a treatment alternative in selected cases but it needs further study.
Keywords: Adjacent segment degeneration, Risk factor, Fusion, Interspinous implant
INTRODUCTION
Lumbar fusion is a common procedure for managing low back instability such as degenerative disc disease or high-grade spondylolisthesis2,18,20). However, spinal arthrodesis alters the normal spine biomechanics of the spine and a significant amount of additional force is placed on the facet joints of the adjacent segments, which can accelerate degenerative change at the non-fused adjacent segments above or below the fusion site5,13). This adjacent segment degeneration (ASD) has been a concern for spine surgeons for many years12).
An increasing number of patients who have undergone lumbar fusion show symptoms of adjacent instability and stenosis1,14). Radiographic evidence of ASD reported to occur in 30% of patients followed for 5 years or more after lumbar fusion surgery8,16,17). The revision surgery rate due to ASD is reported in the literature to be 0-18%, while the success rate for such surgery is reported to be 60-80%, lower than that for the primary surgery6,8,9,11,14,16,19). Patients with ASD usually suffer from recurrent back pain and sciatica. Complications of revision surgery include dural tears, root injury and incomplete neural decompression are common in revision spine surgery.
In this study, the authors analyzed the clinical, radiological features and risk factors of patients who underwent revision surgery for ASD and evaluate the results of the surgical methods of ASD.
MATERIALS AND METHODS
A total of 81 adult patients with degenerative lumbar pathologies underwent posterior fusion with pedicle screw fixation between January 2000 and December 2007 in a single institution. The minimum follow-up was 2 years (mean 5.5 years). Patients undergoing single, 2- and multilevel fusion using pedicle screws were included in the study. The pathologies were degenerative spondylolisthesis in 33 patients, spinal stenosis in 44 patients and spondylolysis in 4 patients. All patients reported no complications during the first year after surgery. Patients were analyzed in terms of radiographic ASD and clinical ASD. Radiographic ASD was defined as collapse of the disc space, dynamic instability with slippage more than 4 mm and/or angle change more than 10° on flexion and extension on plain radiographs. Clinical ASD was defined as symptomatic spinal stenosis and mechanical back pain with/without radiographic ASD. Preoperative evaluation included plain radiographs and MRI. Patients underwent postoperative radiograph examination before discharge, and were then followed up 1, 3 and 6 months postoperatively and then annually. In addition to radiographic analysis, postoperative follow-up evaluation included a review of medical records and a postoperative visit to the out patient department. Radiographs included standing anteroposterior, lateral lumbosacral and dynamic flexion/extension views, and were analyzed with particular attention to degeneration at adjacent levels.
Revision surgery for ASD was undertaken in 9 patients. All patients had symptoms of radicular pain, neuralgic claudication, or both but not low back pain alone Revision surgery involved, extended fusion in 4 patients, interspinous device implantation in 4 patients [DIAM implants (Medtronic, Memphis, TN, USA) in 2 patients, and Corflex implants (Spine motion, Germany) in 2 patients] and simple decompression in 1 patient due to old age.
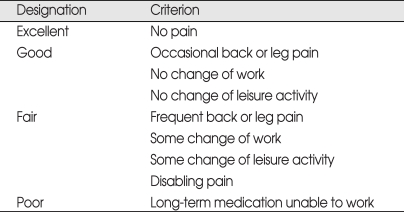
The choice of extended fusion or interspinous device implantation was made on the basis of radiographic findings and patients' condition. The surgical indications for extended fusion were collapse of the disc space, dynamic instability on plain radiographs and severe spinal stenosis on MRI scan. The indications for device implantation were no radiographic ASD and no dynamic instability on plain radiographs. Patients' condition was evaluated based on age, osteoporosis, pain, activity, analgesic use and overall satisfaction. Clinical outcomes were measured before surgery and at the final follow-up in the out-patient department using modified Brodsky's criteria (Table 1)3). Factors associated with revision surgery were investigated, including sex, age, diagnosis, initial spinal fusion and decompression methods. Data were analyzed in a 2 × 2 cross contingency table using Fisher's exact probability test. A probability of less than 0.05 was defined as statistically significant.
Table 1.
Modified Brodsky's criteria for clinical results
RESULTS
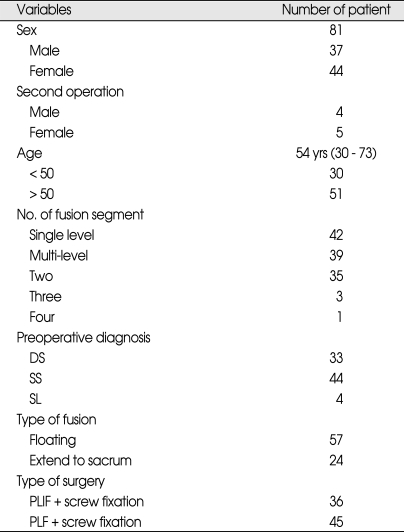
Of 81 patients who had undergone lumbar fusion surgery, 37 were male and 44 were female.
The mean patient age at the time of surgery was 54 years (range, 30-73 years). The mean follow-up period was 5.5 years. Forty-two patients underwent a single-level fusion, 35 patients underwent 2-level fusion, and 4 patients underwent three-or four-level fusion. Of the 81 lumbar fusion patients, 9 (11.1%) required a second operation due to ASD symptoms (Table 2). The mean period between the first and second operations was 4 years 6 months (range, 2 years-7 years 2 months). Degeneration occurred in the upper adjacent segments in 8 patients (88.9%) and in the lower adjacent segments in one patient (11.1%).
Table 2.
Patient population who treated with posterior fusion and pedicle screw fixation
DS : degenerative spondylolisthesis, PLF : posterolateral fusion, PLIF : posterior lumbar interbody fusion, SL : spondylolysis, SS : spinal stenosis
Factors associated with adjacent segment disease
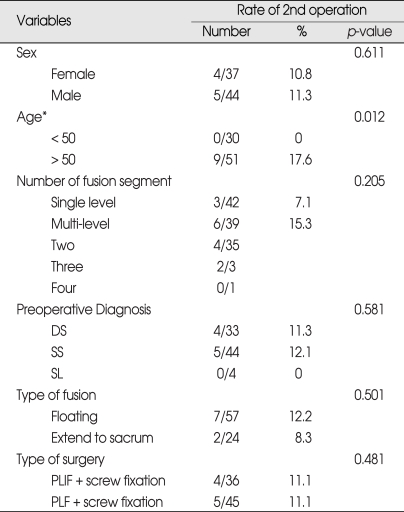
ASD development was assessed in relation to sex, age, initial diagnosis, type of fusion and decompression method (Table 3). Patients > 50 years old were at a higher risk of developing clinical ASD than those < 50 years (p = 0.012). Specifically, 17.6% (9 of 51) of patients over the age 50 developed clinical ASD compared with 0% (0 of 30) of patients 50 years old or younger. Clinical ASD developed in 7.1% (3 of 42) of single-level fusion, 11.4% (4 of 35) of 2-level fusion patients and 66.6% (2 of 3) of 3 level fusion patients. Incidence of the ASD had no correlation between single-level and multi-level fusion on analysis (p = 0.205).
Table 3.
Rate of reoperation related to variables
DS : degenerative spondylolisthesis, PLF : posterolateral fusion, PLIF : posterior lumbar interbody fusion, SL : spondylolysis, SS : spinal stenosis
ASD developed in 11.3% (5 of 44) of spinal stenosis patients and 12.1% (4 of 33) of with degenerative spondylolisthesis patients. It has no relation to the initial diagnosis (p = 0.581). In the type of fusion, 12.2% (7 of 57) of patients with floating fusion developed ASD compared with 8.3% (2 of 24) of patients who underwent the extended fusion to the sacrum (p = 0.501). There was no significant risk factor for the development ASD between floating fusion and extended fusion to the sacrum. The type of primary surgery (i.e., circumferential fusion vs. posterior only fusion) did not contribute to the occurrence of clinical ASD : 11.1% (4 of 36) of circumferential fusion patients and 11.1% (5 of 45) of posterior only fusion patients (p = 0.481).
Treatment and clinical outcomes
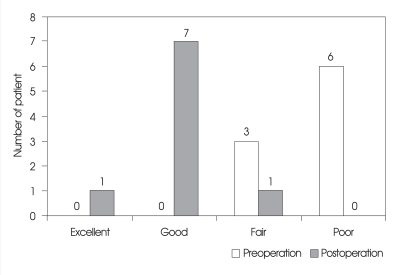
Of the 9 ASD patients who underwent revision surgery, 4 patients with dynamic instability on plain radiographs underwent extended fusion, while 4 patients had interspinous devices implanted (3 patients had no radiographic ASD and 1 patient had a collapse of the disc but no dynamic instability on plain radiographs). A simple laminectomy was performed in 1 patient due to old age. The type of instrumentation used depended on imaging finding and availability. Bone grafting was routinely performed in extended fusion cases. Assessment at the final follow-up, 1 patient had excellent result, 7 patients had good results and 1 patient had a fair result (Fig. 1). Thus, clinical outcomes were good or better in 88.9% (8 of 9) of patients. Residual low back pain was more common than sciatica or neurologic claudication. There were no compli-cations related to the revision surgery, such as dural tear, root injury or wound complication.
Fig. 1.
Results of revision surgery based on modified Brodsky's criteria.
Radiological assessment
Nine of 81 (11.1%) initial lumbar fu-sion patients showed clinical evidence of ASD. Of those, 33.3% (3 of 9) showed no radiographic evidence of ASD in plain radiographs (Fig. 2). Three patients had normal intervertebral discs appearance on plain radiographs, underlying stenosis at the adjacent segments can lead to clinically significant symptoms.
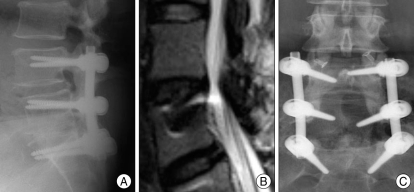
Fig. 2.
Adjacent segment degeneration (ASD) occurred at 5 years after primary surgery. On plain radiographs, there are no evidences of ASD such as disc space narrow and segmental instability at L3-L4 (A) but clinically significant spinal stenosis confirmed by magnetic resonance image (B). The patient implanted interspinous device on L3-4 (C).
DISCUSSION
Decompression and instrumented spinal fusion to treat degenerative lumbar disease has become a standardized operation in recent years, with predictable results and outcomes. Despite the high fusion rate and satisfactory clinical outcome, fusion of a motion segment increases stress in neighboring segments that can result in pathologic changes such as instability of the motion segment, disc space narrowing, and stenosis caused by facet degeneration and ligament flavum hypertrophy. In the present study, 9 of 81 patients (11.1%) who underwent lumbar fusion surgery required revision surgery for ASD. All of the 9 patients showed neurological symptoms and spinal stenosis. Compare to patients with no second operation, it revealed that patients who were older than fifty showed a high incidence of ASD. Other factors, sex, initial diagnosis, multisegment fusion, floating fusion and type of surgery were not risk factors in our study.
Factors that contributting to adjacent segment disease
Controversy exists regarding whether adjacent segment disease increases as more levels are included in lumbar fusion. Wiltse et al.19), reported an increased incidence of ASD with the use of a pedicle screw, but no relationship between fusion length and ASD development. In another study, Ghiselli et al.7) reported that patients who had a single-level fusion were more likely to have clinical ASD than those who had a multilevel fusion.
In our study, we found that there was no difference in ASD development between single-level and multilevel fusion patients (p = 0.205). Gillet8) reported that, when 5 or more levels were included, there was no increased risk of ASD. He believed this was due to the bracing effect of the rib cage in the thoracic spine, and hence he recommended fusion extension up to the thoracic area when treating ASD proximal to a prior fusion.
We also found that there was no difference in ASD incidence in patients undergoing the circumferential fusion (i.e., posterior fusion with a lumbar interbody fusion) compared to those undergoing a posterior only fusion. These findings are similar to those of Penta et al.15) who reported that circumferential fusion did not increase the incidence of ASD in a study of 26 patients followed for a minimum of 5 years following surgery.
Radiological assessment
Many reports show that the rate of adjacent segment disease increases over time following fusion surgery, but that only a small number of patients require revision surgery for ASD (0-18%)4,8,14,17).
In contrast, not all patients with symptoms of spinal stenosis had evidence of radiographic ASD.
In our study, 3 of 9 ASD patients (33.3%) had no radiographic evidence such as end plate erosion, disc space narrowing or instability on plain radiographs. These patients had only clinical symptoms such as back pain and radiating pain (leg pain) with stenosis at the adjacent levels on MRI scans. Lee12) explained that ASD causes premature degeneration of the facet joints. Facets degeneration can induce facet hypertrophy and thickening of the ligamentum flavum. Degeneration and damage of the posterior elements (e.g., ligament, facet) contribute to disc collapse and may be the main cause of compression of the thecal sac.
These factors may induce clinical symptoms without disc degeneration. Based on these observations, we recommend that any patient who presents with recurrent leg pain several years after fusion be evaluated for the presence of spinal stenosis at the adjacent segments regardless of the appearance of the intervertebral disc on plain radiographs.
Surgical treatment of adjacent segment disease
Revision surgery is always stressful for patients and challenging for surgeons. Dural tear and root injury are potential complications. We follow certain practices when performing revision surgery. First, when we approach the lesion, we always approach the lesion from the lateral side to the medial side. The major hindrance to revision surgery is scar tissue and adhesion, and we have observed that scar tissue is less adherent in the lateral area than the midline area. We always commence the laminectomy from the lateral side (the non stenotic area, where the scar is less developed) to the medial side.
Second is the wide decompression by laminectomy and facetectomy. Adjacent instability has been recognized as an important type of failed back surgery syndrome. We had thought that the main cause of the failed back surgery syndrome is inappropriate decompression. Decompression was carried through the bilateral lateral recess by an extensive medial facetectomy. Medial facetectomy can reduce the risk of root injury and manipulation of the nerve root.
Implantation of an interspinous device for adjacent segment disease
Of the 9 patients requiring revision surgery due to ASD, 4 patients were implanted with interspinous devices and all 4 patients showed good results after revision surgerys at the final follow-up. Interspinous device implantation is less invasive and clinical results appeared very satisfactory in patients whose symptoms deteriorated by spinal stenosis21). Kim et al.10) reported that range of motion (ROM) at the instrumented level was significantly decreased in both posterolateral fusion group and interspinous device group. This means that the interspinous device has some restabilizing effect on an unstable segment. Although the interspinous device group may improve less the instability at the instrumented level compared to the extended fusion group, patients showed a similar clinical result. If longer-term studies show comparable outcomes, interspinous implantation may be indicated in the surgical treatment of mild ASD. This minimally invasive surgery would be particularly beneficial for elderly patients or those with osteoporosis or in otherwise poor general condition.
CONCLUSION
We found that 11.1% of lumbar spinal fusion patients required the revision surgery due to ASD, consistent with incidence of the ASD reported in other literature. The average period between the first and second operation was 4 years 6 months. Patients over the age of 50 have a higher risk of developing clinical ASD than those who were 50 years old or younger. Circumferential fusion and fusion length were not a risk factors for ASD. Patients implanted with interspinous device remained in a satisfactory condition for at least 2 years postoperatively. It appears that interspinous device implantation and simple laminectomy can preserve the posterior complex integrity, making it an alternative treatment for ASD in selected cases. However, further studies and longer follow-up are required to better determine whether, and under what conditions, extended fusion or interspinous device implantation is the best option.
References
- 1.Aota Y, Kumano K, Hirabayashi S. Postfusion instability at the adjacent segments after rigid pedicle screw fixation for degenerative lumbar spinal disorders. J Spinal Disord. 1995;8:464–473. [PubMed] [Google Scholar]
- 2.Bridwell KH, Sedgewick TA, O'Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993;6:461–472. doi: 10.1097/00002517-199306060-00001. [DOI] [PubMed] [Google Scholar]
- 3.Brodsky AE, Hendricks RL, Khalil MA, Darden BV, Brotzman TT. Segmental ("floating") lumbar spine fusions. Spine (Phila Pa 1976) 1989;14:447–450. doi: 10.1097/00007632-198904000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Buttermann GR, Garvey TA, Hunt AF, Transfeldt EE, Bradford DS, Boachie-Adjei O, et al. Lumbar fusion results related to diagnosis. Spine (Phila Pa 1976) 1998;23:116–127. doi: 10.1097/00007632-199801010-00024. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham BW, Kotani Y, McNulty PS, Cappuccino A, McAFee PC. The effect of spinal destabilization and instrumentation on lumbar intradiscal pressure : an in vitro biomechanical analysis. Spine (Phila Pa 1976) 1997;22:2655–2663. doi: 10.1097/00007632-199711150-00014. [DOI] [PubMed] [Google Scholar]
- 6.Fritsch EW, Heisel J, Rupp S. The failed back surgery syndrome : reason, intraoperative findings and long-term results : a report of 182 operative treatments. Spine (Phila Pa 1976) 1996;21:626–633. doi: 10.1097/00007632-199603010-00017. [DOI] [PubMed] [Google Scholar]
- 7.Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86:1497–1503. doi: 10.2106/00004623-200407000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Gillet P. The fate of the adjacent motion segments after lumbar fusion. J Spinal Disord Tech. 2003;16:338–345. doi: 10.1097/00024720-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kim SS, Michelsen CB. Revision surgery for failed back surgery syndrome. Spine (Phila Pa 1976) 1992;17:957–960. doi: 10.1097/00007632-199208000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Kong DS, Kim ES, Eoh W. One-year outcome evaluation after interspinous implantation for degenerative spinal stenosis with segmental instability. J Korean Med Sci. 2007;22:330–335. doi: 10.3346/jkms.2007.22.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeheration following lumbar spine fusion. Eur Spine J. 2001;10:314–319. doi: 10.1007/s005860000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976) 1988;13:375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 13.Lee CK, Langrana NA. Lumbosacral spinal fusion : a biomechanical study. Spine (Phila Pa 1976) 1984;9:574–581. doi: 10.1097/00007632-198409000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann TR, Spratt KF, Tozzi JE, Weinstein JN, Reinarz SJ, el-Khoury GY, et al. Long-term follow-up of lower lumbar fusion patients. Spine (Phila Pa 1976) 1987;12:97–104. doi: 10.1097/00007632-198703000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Penta M, Sandhu A, Fraser RD. Magnetic resonance imaging assessment of disc degeneration 10 years after anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1995;20:743–747. doi: 10.1097/00007632-199503150-00018. [DOI] [PubMed] [Google Scholar]
- 16.Rahm MD, Hall BB. Adjacent-segment degeneration after lumbar fusion with instrumentation : a retrospective study. J Spinal Disord. 1996;9:392–400. [PubMed] [Google Scholar]
- 17.Roy-Camille R, Benazet JP, Desauge JP, Kuntz F. Lumbosacral fusion with pedicular screw plating instrumentation. A 10-year follow-up. Acta Orthop Scand Suppl. 1993;251:100–104. doi: 10.3109/17453679309160135. [DOI] [PubMed] [Google Scholar]
- 18.West JL, 3rd, Bradford DS, Ogilvie JW. Results of spinal arthrodesis with pedicle screw-plate fixation. J Bone Joint Surg Am. 1991;73:1179–1184. [PubMed] [Google Scholar]
- 19.Wiltse LL, Radecki SE, Biel HM, DiMartino PP, Oas RA, Farjalla G, et al. Comparative study of the incidence and severity of degenerative change in the transition zones after instrumented versus noninstrumented fusions of the lumbar spine. J Spinal Disord. 1999;12:27–33. [PubMed] [Google Scholar]
- 20.Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results. Spine (Phila Pa 1976) 1993;18:983–991. doi: 10.1097/00007632-199306150-00006. [DOI] [PubMed] [Google Scholar]
- 21.Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, et al. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant : 1-year results. Eur Spine J. 2004;13:22–31. doi: 10.1007/s00586-003-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]