Abstract
Regulatory T cells (Tregs) are CD4+Foxp3+ cells that modulate autoimmune responses. Tregs have been shown to be also involved during the immune response against infectious agents. The aim of this work is to study the role of Tregs during the infection with the intracellular protozoan Toxoplasma gondii. Resistant BALB/c mice were injected with 200 μg of anti-CD25 mAb (clone PC61) and 2 days later they were infected with 20 cysts of the ME49 strain of T. gondii. We observed that depleted mice showed 50–60% mortality during the acute infection. When FACS analysis was carried out, we observed that although injection of PC61 mAb eliminated 50% of Tregs, infected-depleted mice showed a similar percentage of CD25+Foxp3− (activated T cells, Tact) to those observed in infected nondepleted animals, demonstrating that in our depletion/infection system, injection of PC61 mAb did not hamper T cell activation while percentage of Tregs was reduced by 75% 10 days post infection. We concluded that Tregs are essential during protection in the acute phase of T. gondii infection.
1. Introduction
Toxoplasma gondii (T. gondii) is an intracellular parasite and the etiological agent of toxoplasmosis. Although the infection is asymptomatic in most immunocompetent individuals, toxoplasmosis may cause severe complications in immunocompromised individuals [1, 2]. If T. gondii infection occurs during pregnancy, transplacental transmission can occur, leading to abortion or congenital malformations [2–5].
The immune response against T. gondii has been largely characterized and it has been demonstrated that cell mediated immunity is essential to control infection [2, 6, 7], involving synergy between CD4+ and CD8+ T cells [8, 9]. T. gondii triggers the production of IL-12, mainly by dendritic cells [10–12], which stimulate NK cells and T lymphocytes to secrete large amounts of IFN-γ, a key cytokine for protection against this parasite [10, 13, 14]. Thus, protection against T. gondii is dependent on a TH1 response [15]. However IL-10 is also required for prevention of development of IFN-γ mediated pathology [16].
Regulatory T cells (Tregs) are a subset of CD4+ T cells that control the immune response by suppressing many lymphocyte effector functions [17–19]. Natural Tregs constitutively express CD25 (the α chain of the IL-2 receptor) [20], CTLA-4 [21], and the forkhead family transcription factor Foxp3 [22, 23], which is required for their development and function [22]. Although initially described for preventing autoimmune responses [20, 24, 25], it has also been demonstrated that they can regulate the immune response against infectious agents [26–29]. For example, in vivo depletion of CD25+ cells leads to an increase in the production of IFN-γ in animals infected with Plasmodium chabaudi adami [30] and Trypanosoma congolense [31], or in animals infected with Schistosoma mansoni to an increased production of IFN-γ, IL-4, IL-5, and IL-13 [32], indicating that Tregs can control both TH1 and TH2 responses. During infection with Plasmodium berghei [33] or Litomosoides sigmodontis [34], depletion of Tregs leads to control or elimination of the parasites, respectively.
The aim of this paper is to study the role of Tregs during the acute infection of T. gondii in the resistant BALB/c strain of mice. We carried out depletion experiments by injection of the PC61 mAb in mice followed by infection with the type II strain ME49, and analyzed mortality. Since PC61 mAb injection could also eliminate other cell subtypes expressing CD25, mainly activated T cells (Tact), we also studied the CD4+ T cell subsets affected by injection of the PC61 mAb.
2. Materials and Methods
2.1. Mice
Six—eight-week-old female BALB/cAnN mice, weighing 18–20 g, and Swiss mice, were bred in our animal house and maintained in pathogen-free conditions. All protocols depicted in this paper were approved by the local Bioethics Committee for Animal Research.
2.2. Parasites
The ME49 strain of T. gondii was maintained in Swiss mice as previously described [35]. Briefly, brains from infected mice were removed and homogenized in Dulbecco's Phosphate Buffered Saline (DPBS); the number of cysts was enumerated and mice were infected intraperitoneally with 10 cysts; this procedure was carried out every 2–4 months. For peroral infection, mice anesthetized with Sevorane (ABBOTT, Mexico City, Mexico) were infected with 20 cysts by gavage in 0.1 mL DPBS.
2.3. Hybridomas and mAbs
The PC61 hybridoma secreting rat IgG1 against murine CD25 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). The F41D1 hybridoma, secreting an unrelated rat IgG1 mAb (isotype control), was a kind gift of Dr. Olivier Denis (Institut Scientifique de Santé Publique, Brussels, Belgium). Hybridomas were grown on CD Hybridoma Medium (GIBCO, Grand Island, NY, USA) and mAbs were obtained after ammonium sulfate (45% w/v) precipitation. After extensive dialysis against PBS, antibody concentration was determined by spectrophotometry at 280 nm. Antibodies were resuspended in PBS at 1-2 mg/mL and stored at −20°C until used.
2.4. CD25+ T Cells Depletion In Vivo and Infection
Unless otherwise stated, mice were injected intraperitoneally (ip) with 200 μg of purified PC61 mAb or control isotype at day −2. Two days later (day 0), mice were infected perorally as described above. Depletion was confirmed by analyzing CD4+CD25+ or CD4+CD25+ Foxp3+ cells, in peripheral blood samples when mice were infected. Weight and cumulative mortality were recorded daily; survival curves were compared by the logrank test using the Prism software (GraphPad, San Diego, CA).
2.5. Immunofluorescence
Spleen cells (1 × 106) were incubated (30 minutes, 4°C) with anti-CD4-FITC or -TC (clone RM4-5, Caltag, Burlingame, CA), anti-CD4-PerCP (Biolegend, San Diego CA), anti-CD25-PE or –APC (clone PC61 5.3, Caltag), or anti-CD25-PE (Clone 7D4, Miltenyi Biotec, Auburn, CA) in 100 μL of washing buffer (DPBS + 1% FCS + 0.1% NaN3). After washing 3 times, cells were suspended in DPBS and analyzed immediately. For Foxp3 detection, we used the Foxp3 Staining Buffer Set (eBioscience, San Diego, CA) with anti-Foxp3-Alexa Fluor 488 (clone FJK-16s, eBioscience) following the indications provided by the manufacturer.
2.6. Flow Cytometry
Flow cytometry analysis was performed on a FACScalibur or a FACScan cytometer (Becton Dickinson, San Jose, CA), running the Cell Quest program (Becton Dickinson). Lymphocytes were identified by forward scatter (FSC) and side scatter (SSC) characteristics, gated and further analyzed. Detailed analysis of each experiment is indicated in each figure legend. Samples were analyzed using the FlowJo software V. 5.7.2 (Tree Star, Ashland OR).
3. Results and Discussion
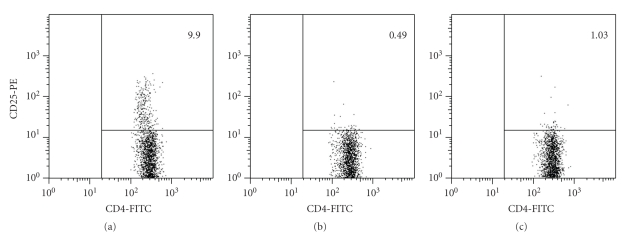
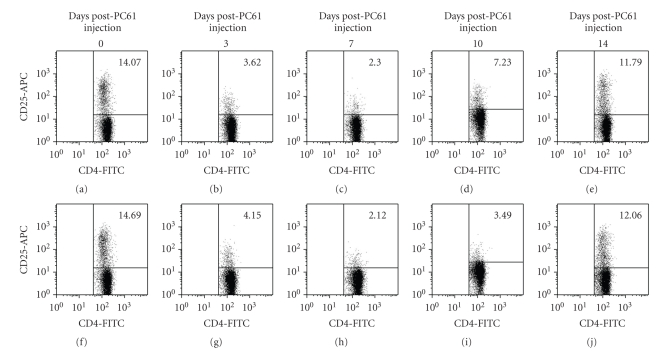
We carried out depletion studies by injection of the PC61 mAb to analyze the role of Tregs in the resistant BALB/c strain of mice during the infection with the type II strain ME49 of T. gondii. In the literature we found many protocols for Tregs depletion using the PC61 mAb in different models, using a wide range of mAb concentrations, from 100 μg to 1 mg [32, 33, 36–41], or even several injections of the PC61 mAb before and during infection [30, 42]. To reduce the possibility of Tact elimination due to high antibody concentrations, we tested the lowest doses reported of PC61 mAb (100 μg and 200 μg) by ip injection. Two days later we analyzed CD4+CD25+ cells to determine if depletion was achieved, since mice would be infected at this time point. We used the PC61 mAb in the FACS analysis because McNeill et al. [37], using Foxp3-GFP transgenic mice, demonstrated that detection of CD25+ cells in PC61-depleted mice using either the 7D4 or the PC61 mAbs showed similar results [37]. As can be seen in Figure 1, injection of either 100 (Figure 1(b)) or 200 μg (Figure 1(c)) of the PC61 mAb leads to similar levels of depletion of CD4+CD25+ cells in the spleen. Even though depletion efficiency was the same by the day we intended to infect the animals, we were concerned about how long would the depletion lasted. During the first week after injection of the PC61 mAb (Figure 2), depletion efficiency was very similar using both doses, but at 10 days post-depletion, a higher number of CD4+CD25+ cells were observed in animals treated with 100 μg when compared to cells from animals treated with 200 μg. At 14 days post depletion, about 83% of cells were already detected in both groups of animals. These results show that injection with 200 μg leads to a depletion of CD25+ cells for at least 10 days. Recovery of CD4+CD25+ is slightly faster in animals treated with 100 μg mAb; at 14 days, CD4+CD25+ cells from both groups reached almost normal levels. We thus chose 200 μg of PC61 mAb for the subsequent experiments.
Figure 1.
Injection of PC61 mAb depletes CD4+CD25+ cells. BALB/c mice (2 animals per group) were injected ip with DPBS (a), 100 μg (b), or 200 μg (c) of purified anti-CD25 mAb (PC61). Two days later, spleen cells were obtained; immunofluorescence was carried out using anti-CD4-FITC and anti-CD25-PE mAbs, and cells were analyzed by FACS. The lymphocyte region was first identified by FSC and SSC characteristics, gated, and 10,000 gated events were captured. Numbers indicate % of CD4+CD25+ cells within the CD4+ population.
Figure 2.
Kinetics analysis of the depletion of CD4+CD25+ cells. BALB/c mice were injected ip with 100 μg ((a)–(e)) or 200 μg ((f)–(j)) of PC61 mAb. Spleen cells were obtained at different time points and immunofluorescence was carried out as described in Figure 1 using anti-CD4-FITC and anti-CD25-APC mAbs. The lymphocyte region was first identified and gated by FSC and SSC characteristics, the CD4+ region was subgated and 10,000 CD4+ events were captured. Numbers indicate % of CD4+CD25+ cells within the CD4+ population.
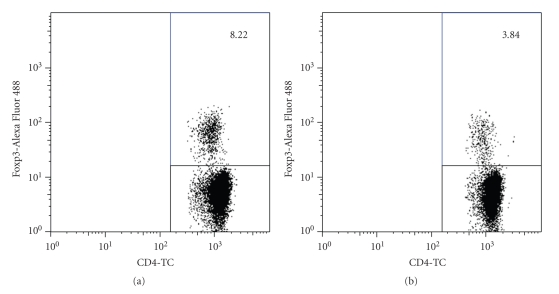
In order to study the role of Tregs during infection with T. gondii, resistant BALB/c mice were depleted of CD25+ cells, and 2 days later they were perorally infected with 20 cysts of the ME49 strain. Since CD25 is a molecule expressed by other cell types, including activated CD4+ T cells, and Foxp3 is exclusively expressed by Tregs and is required for their development and function [22], we thus analyzed the depletion of Foxp3+ cells (Tregs) the day of infection (2 days after depletion). We found that only 53% of Foxp3+ cells were eliminated by injection of the PC61 mAb (Figure 3), confirming results previously reported [37].
Figure 3.
Depletion with PC61 mAb partially eliminates Foxp3+ cells. BALB/c mice were injected with 200 μg of isotype (a) or PC61 (b) mAb ip. Two days later, before infection, animals were bled and immunofluorescence for detection of Foxp3 was carried out. The lymphocyte region was first identified and gated by FSC and SSC characteristics, the CD4+ region was subgated, and 10,000 CD4+ events were captured. Numbers indicate % of Foxp3+ cells within the CD4+ population. A representative analysis from one mouse per group is shown.
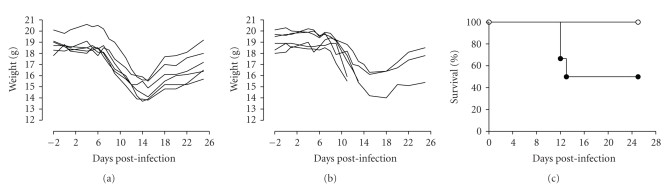
Weight changes and mortality were analyzed for 25 days after depletion and infection (Figure 4). All mice lost up to 25% weight due to the parasite; it has to be noted that depleted mice lost 5% less weight compared to isotype treated mice. After 15 days the latter started to gain weight (Figures 4(a) and 4(b)), but did not reach their initial weight.
Figure 4.
Depletion of CD25+ cells induces mortality in BALB/c mice infected with T. gondii. Animals (n = 6) were injected ip with 200 μg of either isotype mAb (a) (∘ in (c)) or PC61 mAb (b) (• in (c)) and 2 days later they were infected perorally with 20 cysts of the ME49 strain of T. gondii. Weight ((a),(b)) and cumulative mortality (c) were recorded daily for 25 days. In (a) and (b), each line corresponds to one mouse.
When mortality was analyzed, isotype-treated mice survived during the 25 day experiment, but 50% of PC61-treated mice died <13 days post infection (dpi) (Figure 4(c)), although this result was not statistically significant (P = .0549). A similar result was observed when mice were infected with 50 cysts, although we observed a higher mortality rate (data not shown).
Analysis of blood samples of the same animals from this experiment showed that although CD25+ cells were eliminated, including Foxp3+ and Foxp3− cells, at later time points, a marked increase in CD25+Foxp3− cells (Tact) was observed in depleted animals, while the percentage of Foxp3+ cells (Tregs) was still decreased (data not shown). Therefore, we carried out an experiment to confirm these observations. We depleted and infected mice as described above, they were killed 10 days pi, when animals showed symptoms of toxoplasmosis (2–4 days before death) and an exhaustive analysis of spleen cells was performed.
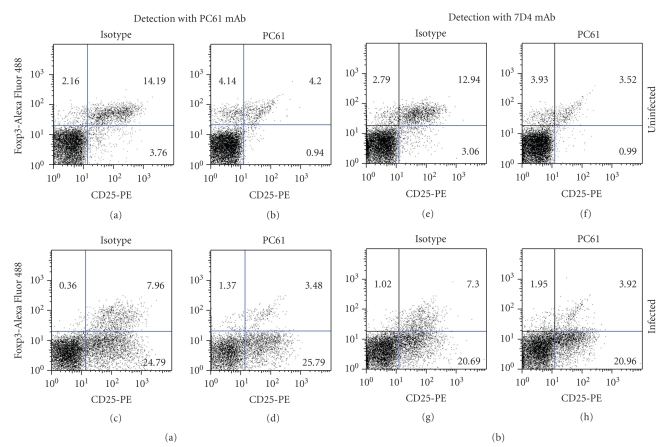
Analysis of Tregs and Tact cells from infected animals at this time point (Figure 5) showed that infection induced an expansion of Tact cells (3.76 versus 24.79). Depleted infected animals, however, showed a dramatic expansion of Tact when compared to depleted/noninfected animals (0.94 versus 25.79), but no difference was detected between Tact from infected nondepleted or depleted mice (24.79 versus 25.79), demonstrating that depletion did not prevent activation of T cells. On the other hand, a nearly 50% reduction in percentage of Tregs was observed in infected-nondepleted animals, when compared to control mice (8.32 versus 16.35); depleted noninfected animals still showed a 50% decrease in Tregs at this time point (8.34 versus 16.35), while depleted infected animals had 4.85% of Tregs, which represents a 50% reduction when compared to noninfected-depleted mice (4.85 versus 8.34). All these results were similar when mAb 7D4 was used to detect CD25 cells (Figure 5), demonstrating no interference in the detection of CD25 with PC61 mAb.
Figure 5.
Analysis of Tregs and Tact cells after PC61 injection and infection with T. gondii. Animals were depleted and infected as described in Figure 4. At 10 dpi spleens were obtained and immunofluorescence was carried out using anti-CD4-PerCP, anti-Foxp3-Alexa Fluor 488 and anti-CD25-PE mAb, either PC61 (a)–(d) or 7D4 clone (e)–(h). The lymphocyte region was first identified and gated by FSC and SSC characteristics, the CD4+ region was subgated, and 10,000 CD4+ events were captured. Numbers indicate % of cells from the CD4+ gating. A representative analysis from one mouse per group is shown.
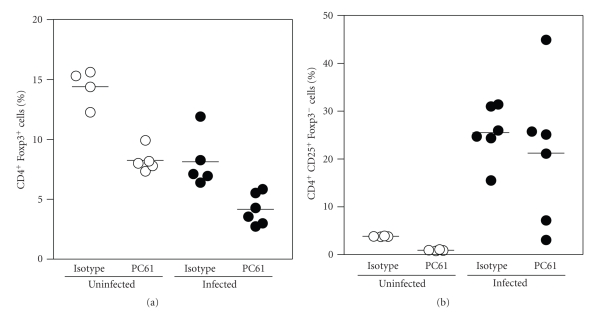
A summary of the results obtained from all mice studied is depicted in Figure 6. Analysis shows that at 10 dpi (12 days post depletion) 50% of Tregs are still lacking in depleted noninfected animals. Interestingly, infection induces a 50% reduction of Tregs, but depleted infected animals have 50% less Tregs than nondepleted infected mice, and only 25% of Tregs when compared to control noninfected mice. Analysis of Tact cells confirmed that infection leads to a dramatic expansion of this subset, which is not altered by depletion, since mean percentage of Tact cells was unaffected, although a high dispersion in data was observed (Figure 6).
Figure 6.
Summary of the Treg and Tact cell detection in mice after PC61 injection and infection with T. gondii. Percentage of Tregs (CD4+ Foxp3+) and Tact cells (CD4+CD25+Foxp3−) within the CD4+ gate obtained from all the animals described in Figure 5 is shown.
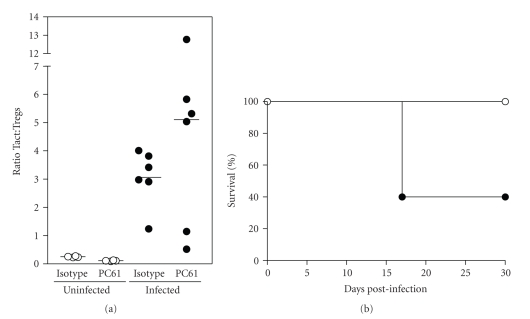
When the ratio of Tact/Tregs cells was calculated (Figure 7), we found that in noninfected animals, whether depleted or nondepleted, ratios remained nearly unchanged (≤0.28). In infected mice, a higher ratio was observed (1.2–4.0), indicating a expansion of Tact cells due to the parasite. In depleted/infected mice, however, the ratio was 0.5–12.7 (Figure 7(a)): a group of 4 mice showed a very high ratio (>5.0), while another group presented a low ratio (0.5–1.15). The day we carried out this experiment, one animal was moribund, and its spleen cells showed the highest proportion of Tact/Tregs (ratio = 12.7, Figure 7(a)). It has to be noted that in this experiment, an additional group of mice was used to evaluate mortality (Figure 7(b)), and we found that 60% of animals died 15 days pi, which correlates to the percentage of mice with the higher proportion of Tact/Tregs (Figure 7(a)). All these experiments show that although the generation of Tact cells due to infection is not altered by depletion, the percentage of Tregs remained very low, turning susceptible a resistant strain of mice.
Figure 7.
Infection with T. gondii induces an imbalance in Tact: Tregs cell ratio and is exacerbated by depletion. (a) Ratio of Tact: Tregs (CD4+CD25+Foxp3− cells : CD4+Foxp3+ cells) was calculated from data obtained from Figure 6. (b) An additional group of mice (n = 5) treated with isotype ( ) or PC61 (
) or PC61 ( ) was used to evaluate cumulative mortality for 30 days.
) was used to evaluate cumulative mortality for 30 days.
In the present work we analyzed the role of Tregs during infection with T. gondii in the resistant BALB/c strain of mice. We observed that infected animals showed a decreased number of Treg cells, a result which agrees with that reported by Ge et al. [43], who showed that in pregnant and nonpregnant mice, infection with T. gondii induced a reduction in Foxp3 mRNA expression levels in spleen and a reduction in both percentage and absolute number of Tregs. Thus, this parasite induces a decline in Tregs percentage [43]. These results differ from those observed during the acute phase of other infections (P. berghei, S. mansoni, Heligmosomoides polygyrus, Mycobacterium tuberculosis, Plasmodium yoelii, Brugia malayi, and L. sigmodontis), in which an increase in Foxp3+ cell number is observed [32, 39, 44–48]. In T. gondii infection, it is possible that Tregs die or migrate to other sites in order to control the immune response locally, as it has been shown during P. berghei infection [39]. It has to be noted that T. gondii can infect all cell types and disseminates to most organs [49, 50]. The fate of Tregs during T. gondii infection, however, remains currently unknown.
Injection of PC61 to deplete CD25+ cells has been widely used to study the role of Tregs in different models [32, 36, 37, 39, 42, 51, 52]. In our depletion experiments, we observed that 50–60% of BALB/c mice died during the acute phase of infection, an observation that suggests that Tregs play an important role in toxoplasmosis. However, since CD25 is also expressed by other cell types, injection of the mAb leads also to the depletion of Tact cells and interpretation of results should be taken carefully.
We used a low single dose (200 μg) of PC61 mAb, which was enough to eliminate 50% of Tregs. These observations agree with those reported previously [37], in which a partial depletion of Tregs cells is observed after injection of PC61 mAb.
Injection of PC61 mAb initially eliminates 50% of Tregs, but in infected mice this reduction is exacerbated due to the infection, reaching 75% Tregs reduction in these animals at 10 days pi, when compared to nondepleted-noninfected mice. However, depletion did not hinder the activation of T cells after T. gondii infection. In fact, levels of CD69 expression and percentage of CD69+ cells were similar in Foxp3− cells from nondepleted infected and depleted infected mice (data not shown), demonstrating that both groups generate the same proportion of Tact cells.
While this work was carried out, Couper et al. [41] reported that depletion using 1 mg of the PC61 mAb leads to an increased susceptibility of male C57BL/6J mice to the ME49 strain of T. gondii. Although the results in mortality obtained in our work are similar, we used the highly resistant BALB/c strain of mice, in contrast to the highly susceptible C57BL/6J strain. Moreover, we depleted animals with a lower concentration of PC61 mAb to lessen undesirable effects in other cell populations; a single dose of mAb (200 μg), 2 days before infection was enough to achieve a 50% elimination of Tregs. We avoided the use of higher concentrations of PC61 mAb because we think that this would induce a broad and long lasting depletion of CD25+ cells (both Tact and Tregs) making more difficult the interpretation of the results.
The use of a suitable concentration of the PC61 mAb allowed us to detect a difference in the proportions of Tact and Treg cells 10 days pi. Thus, the amount of PC61 antibody did not prevent the generation of Tact cells due to the infection. The levels of Treg, however, remained low during infection, leading to the loss of resistance of the BALB/c strain. These results show that the absence of Tregs alters the protective immune response against T. gondii and thus mice become susceptible.
T. gondii induces the activation of CD4+ and CD8+ T cells that are crucial to control infection [53]. These cells could in turn be controlled by Tregs, as it has been demonstrated in the T. congolense infection [31]. Our results show that Tregs play an important role in the modulation of the protective immune response against T. gondii. Since infection with this parasite drives a powerful TH1 immune response [53], it is tempting to speculate that the absence of Tregs induces an uncontrolled inflammatory response by Tact cells. The specific molecules and cells directly responsible for the death of animals, however, remain to be established.
Acknowledgments
This work was supported by Grant IN-200608 from PAPIIT (DGAPA, UNAM, Mexico), and by Grants 79775 and 102399 from CONACYT (Mexico). The authors are grateful to MVZ Georgina Díaz, MVZ Alfonso Malagón, and MVZ Jorge Omar García for their expert advice and help in the care of the animals. J. E. Olguín is a recipient of a scholarship from DGAPA. E. P. Tenorio is recipient of a PhD scholarship from CONACYT (Registro 199991) and part of the Biomedical Sciences PhD Program of the Universidad Nacional Autónoma de México.
References
- 1.Hughes HP. Toxoplasmosis: the need for improved diagnostic techniques and accurate risk assessment. Current Topics in Microbiology and Immunology. 1985;120:105–139. doi: 10.1007/978-3-662-09197-5_6. [DOI] [PubMed] [Google Scholar]
- 2.Montoya JG, Liesenfeld O. Toxoplasmosis. The Lancet. 2004;363(9425):1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 3.McCabe R, Remington JS. Toxoplasmosis: the time has come. The New England Journal of Medicine. 1988;318(5):313–315. doi: 10.1056/NEJM198802043180509. [DOI] [PubMed] [Google Scholar]
- 4.Remington JS, Krahenbuhl JL. Immunology of Human Infection, Part II. New York, NY, USA: Plenum; 1982. Immunology of Toxoplasma gondii; p. 327. [Google Scholar]
- 5.Wong S-Y, Remington JS. Toxoplasmosis in pregnancy. Clinical Infectious Diseases. 1994;18(6):853–861. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]
- 6.Frenkel JK. Adoptive immunity to intracellular infection. The Journal of Immunology. 1967;98(6):1309–1319. [PubMed] [Google Scholar]
- 7.Aliberti J. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat.Rev.Immunol. 2005;5(2):162–170. doi: 10.1038/nri1547. [DOI] [PubMed] [Google Scholar]
- 8.Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. The Journal of Immunology. 1991;146(1):286–292. [PubMed] [Google Scholar]
- 9.Suzuki Y, Remington JS. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. The Journal of Immunology. 1988;140(11):3943–3946. [PubMed] [Google Scholar]
- 10.Gazzinelli RT, Amichay D, Sharton-Kersten T, Grunwald E, Farber JM, Sher A. Role of macrophage-derived cytokines in the induction and regulation of cell-mediated immunity to Toxoplasma gondii. Current Topics in Microbiology and Immunology. 1996;219:127–139. doi: 10.1007/978-3-642-51014-4_12. [DOI] [PubMed] [Google Scholar]
- 11.Gazzinelli RT, Wysocka M, Hayashi S, et al. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. The Journal of Immunology. 1994;153(6):2533–2543. [PubMed] [Google Scholar]
- 12.Liu C-H, Fan Y-T, Dias A, et al. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. The Journal of Immunology. 2006;177(1):31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Remington JS. The effect of anti-IFN-γ antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. The Journal of Immunology. 1990;144(5):1954–1956. [PubMed] [Google Scholar]
- 15.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clinical Microbiology Reviews. 1998;11(4):569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki Y, Sher A, Yap G, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. The Journal of Immunology. 2000;164(10):5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 17.Sojka DK, Huang Y-H, Fowell DJ. Mechanisms of regulatory T-cell suppression—a diverse arsenal for a moving target. Immunology. 2008;124(1):13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nature Reviews Immunology. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nature Immunology. 2008;9(3):239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL- 2 receptor alpha-chains (CD25): breakdown of a single mechanism of self- tolerance causes various autoimmune diseases. The Journal of Immunology. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 21.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. The Journal of Experimental Medicine. 2000;192(2):303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunology. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 23.Khattri R, Cox T, Yasayko S-A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunology. 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature Immunology. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 26.Rouse BT, Suvas S. Regulatory cells and infectious agents: détentes cordiale and contraire. The Journal of Immunology. 2004;173(4):2211–2215. doi: 10.4049/jimmunol.173.4.2211. [DOI] [PubMed] [Google Scholar]
- 27.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions. Annual Review of Immunology. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 28.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature Immunology. 2005;6(4):353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 29.Mills KHG. Regulatory T cells: friend or foe in immunity to infection? Nature Reviews Immunology. 2004;4(11):841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 30.Cambos M, Bélanger B, Jacques A, Roulet A, Scorza T. Natural regulatory (CD4+CD25+FOXP+) T cells control the production of pro-inflammatory cytokines during Plasmodium chabaudi adami infection and do not contribute to immune evasion. International Journal for Parasitology. 2008;38(2):229–238. doi: 10.1016/j.ijpara.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Guilliams M, Oldenhove G, Noel W, et al. African trypanosomiasis: naturally occurring regulatory T cells favor trypanotolerance by limiting pathology associated with sustained type 1 inflammation. The Journal of Immunology. 2007;179(5):2748–2757. doi: 10.4049/jimmunol.179.5.2748. [DOI] [PubMed] [Google Scholar]
- 32.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. The Journal of Immunology. 2006;176(10):5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 33.Amante FH, Stanley AC, Randall LM, et al. A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. The American Journal of Pathology. 2007;171(2):548–559. doi: 10.2353/ajpath.2007.061033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. The Journal of Immunology. 2005;174(8):4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 35.Piña-Vázquez C, Saavedra R, Hérion P. A quantitative competitive PCR method to determine the parasite load in the brain of Toxoplasma gondii-infected mice. Parasitology International. 2008;57(3):347–353. doi: 10.1016/j.parint.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Quinn KM, McHugh RS, Rich FJ, et al. Inactivation of CD4+CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunology & Cell Biology. 2006;84(5):467–474. doi: 10.1111/j.1440-1711.2006.01460.x. [DOI] [PubMed] [Google Scholar]
- 37.McNeill A, Spittle E, Bäckström BT. Partial depletion of CD69low-expressing natural regulatory T cells with the anti-CD25 monoclonal antibody PC61. Scandinavian Journal of Immunology. 2007;65(1):63–69. doi: 10.1111/j.1365-3083.2006.01870.x. [DOI] [PubMed] [Google Scholar]
- 38.Long TTA, Nakazawa S, Onizuka S, Huaman MC, Kanbara H. Influence of CD4+CD25+ T cells on Plasmodium berghei NK65 infection in BALB/c mice. International Journal for Parasitology. 2003;33(2):175–183. doi: 10.1016/s0020-7519(02)00261-8. [DOI] [PubMed] [Google Scholar]
- 39.Vigário AM, Gorgette O, Dujardin HC, et al. Regulatory CD4+CD25+Foxp3+ T cells expand during experimental Plasmodium infection but do not prevent cerebral malaria. International Journal for Parasitology. 2007;37(8-9):963–973. doi: 10.1016/j.ijpara.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, Riley EM. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. The Journal of Immunology. 2007;178(7):4136–4146. doi: 10.4049/jimmunol.178.7.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Couper KN, Lanthier PA, Perona-Wright G, et al. Anti-CD25 antibody-mediated depletion of effector T cell populations enhances susceptibility of mice to acute but not chronic Toxoplasma gondii infection. The Journal of immunology. 2009;182(7):3985–3994. doi: 10.4049/jimmunol.0803053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nie CQ, Bernard NJ, Schofield L, Hansen DS. CD4+CD25+ regulatory T cells suppress CD4+ T-cell function and inhibit the development of Plasmodium berghei-specific TH1 responses involved in cerebral malaria pathogenesis. Infection and Immunity. 2007;75(5):2275–2282. doi: 10.1128/IAI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge YY, Zhang L, Zhang G, et al. In pregnant mice, the infection of Toxoplasma gondii causes the decrease of CD4+CD25+-regulatory T cells. Parasite Immunology. 2008;30(9):471–481. doi: 10.1111/j.1365-3024.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- 44.Finney CAM, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4+CD25+ regulatory T cells in Heligmosomoides polygyrus infection. European Journal of Immunology. 2007;37(7):1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott-Browne JP, Shafiani S, Tucker-Heard G, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. The Journal of Experimental Medicine. 2007;204(9):2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couper KN, Blount DG, Wilson MS, et al. IL-10 from CD4+CD25+Foxp3−CD127− adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathogens. 2008;4(2) doi: 10.1371/journal.ppat.1000004. Article ID e1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McSorley HJ, Harcus YM, Murray J, Taylor MD, Maizels RM. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. The Journal of Immunology. 2008;181(9):6456–6466. doi: 10.4049/jimmunol.181.9.6456. [DOI] [PubMed] [Google Scholar]
- 48.Taylor MD, van der Werf N, Harris A, et al. Early recruitment of natural CD4+Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. European Journal of Immunology. 2009;39(1):192–206. doi: 10.1002/eji.200838727. [DOI] [PubMed] [Google Scholar]
- 49.Zenner L, Darcy F, Capron A, Cesbron-Delauw M-F. Toxoplasma gondii: kinetics of the dissemination in the host tissues during the acute phase of infection of mice and rats. Experimental Parasitology. 1998;90(1):86–94. doi: 10.1006/expr.1998.4301. [DOI] [PubMed] [Google Scholar]
- 50.Sumyuen MH, Garin YJF, Derouin F. Early kinetics of Toxoplasma gondii infection in mice infected orally with cysts of an avirulent strain. Journal of Parasitology. 1995;81(2):327–329. [PubMed] [Google Scholar]
- 51.Hisaeda H, Maekawa Y, Iwakawa D, et al. Escape of malaria parasites from host immunity requires CD4+CD25+ regulatory T cells. Nature Medicine. 2004;10(1):29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 52.Kohm AP, McMahon JS, Podojil JR, et al. Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. The Journal of Immunology. 2006;176(6):3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 53.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clinical Microbiology Reviews. 1998;11(4):569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]