Abstract
This study is a randomized, prospective, double-blind study to evaluate the effects of the combination of local anesthetics and an intravenous nonsteroidal anti-inflammatory drug (NSAID) vs NSAID alone on quality of recovery following dental rehabilitation under general anesthesia (GA). Twenty-seven healthy children aged 3–5.5 years underwent dental rehabilitation under GA. Fifteen children in the experimental group received oral infiltration of local anesthetic in addition to intravenous ketorolac tromethamine, while 12 children in the control group received intravenous ketorolac tromethamine alone for postoperative pain management. Pain behaviors were evaluated immediately postoperatively using a FLACC scale and 4 hours postoperatively by self-report using various scales. Parents reported perception of child pain and comfort and any occurrences of postoperative cheek biting. The use of intraoral infiltration local anesthesia for complete dental rehabilitation under general anesthesia for children aged 3–5.5 years did not result in improved pain behaviors in the postanesthesia care unit (PACU), nor did it result in improved pain behaviors 4–6 hours postoperatively as measured by the FLACC scale, FACES scale, and subjective reports of parents or a PACU nurse. Those children receiving local anesthesia had a higher incidence of negative symptoms related to local anesthetic administration, including a higher incidence of lip and cheek biting, which was of clinical importance, but not statistically significant. Infiltration of local anesthetic for dental rehabilitation under general anesthesia did not improve quality of recovery in children aged 3–5.5 years.
Keywords: General anesthesia, Pediatric dental general anesthesia, Pain, Local anesthesia, Recovery quality
Dental rehabilitation under general anesthesia is commonly performed in young children because children may be unable to cooperate in a dental clinic setting or because they may require a significant amount of dental work.1 The use of general anesthesia for dental rehabilitation of children, when indicated, is an accepted behavior management technique according to the American Academy of Pediatric Dentistry (AAPD).1 Although benefits of general anesthesia for dentistry include safe and efficient delivery of dental care, the procedure is not without morbidity or mortality.2
A well-documented phenomenon in medicine is the undertreatment of pain in children. Pain has been undertreated across all age groups due to misunderstandings about analgesic use and concerns over addiction, and, in young children, the mistaken belief of no pain perception.3 The American Academy of Pediatrics (AAP) and the American Pain Society (APS) issued joint recommendations in 2001 to eliminate pain-related suffering. Pain is defined as a subjective experience that is the product of both emotional and sensory components interrelated with the context of culture and environment. Suffering occurs when pain is uncontrolled and the patient feels overwhelmed and out of control.4 One goal for all medical and dental practitioners is to vigilantly monitor pain to prevent suffering. Given the fact that, historically, pain has been undertreated in children and measurement tools exist that help quantify pediatric pain, clinical trials should be undertaken to determine best practices in this area.
The topic of morbidity following dental treatment under general anesthesia has been a popular area for research in recent years predominantly in the United Kingdom as a result of a report by the Expert Working Party on General Anesthesia chaired by Professor Poswillo. In response to this sobering report, studies have attempted to measure postoperative morbidity5 and local anesthesia has been looked upon favorably as a potential technique to improve outcomes with varying levels of success.2
Jurgens et al6 could not find a statistically significant difference in self-reported postoperative pain between children treated with local anesthetic compared with those treated with systemic analgesics (intravenous fentanyl and paracetamol [acetaminophen] either alone or in combination) for dental extractions, but subjectively determined that the children appeared “more settled” in recovery. Atan et al2 reported that the odds of experiencing pain at the operation site were reduced by local analgesia by an odds ratio of 0.39, but these children were more likely to report dizziness. Two other studies found a significant benefit to intraligamental injection but only at one isolated time in the immediate postoperative period (5 minutes).7,8
Coulthard et al9 examined postoperative pain following extractions under general anesthesia. In that double-blind study of 142 children aged 4–12 years who received 15 mg/kg of acetaminophen 1 hour preoperatively and acetaminophen elixir for home, infiltration of local anesthetic did not improve postoperative pain reports. McWilliams and Rutherford10 found that local anesthetic decreased postoperative bleeding but had no effect on behavior. Al-Bahlani et al11 agreed that local anesthetic was related to a significant reduction in perioperative bleeding but also found a statistically significant increase in distress. It is important to note that the dentistry in these studies consisted of extractions almost exclusively.
The AAPD guidelines note that local anesthesia has been reported to reduce pain in the postoperative recovery period after general anesthesia; however, the citations used to support this statement provide marginal evidence.12,13 The overall objective of this study was to provide practitioners with more information on how to reduce postoperative pain and improve recovery characteristics in children following dental surgery under general anesthesia. Specifically, this is a randomized, prospective, double-blind study to evaluate the effects of the combination of local anesthetics and an intravenous nonsteroidal anti-inflammatory drug (NSAID) vs the effects of NSAID alone in reducing postoperative pain and improving immediate postoperative recovery and recovery later on the day of surgery. No opioids, such as morphine sulfate, were used to assess more accurately the contribution of local anesthetic per se to postoperative behaviors.
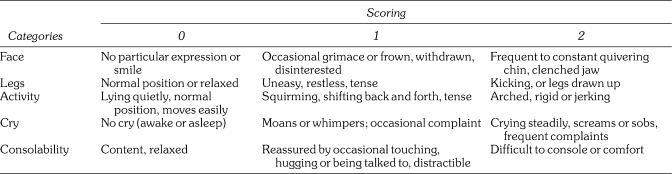
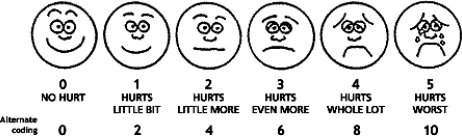
Pain behaviors were evaluated immediately after the surgery using a FLACC (faces, legs, activity, crying, and consolability) scale by a trained examiner (Figure 1). The FLACC score has been validated in children aged 2 months to 7 years14 and requires a brief period of training to achieve high interrater reliability.15 In addition, pain intensity was evaluated immediately postoperatively and the evening of the surgery with the Wong-Baker FACES scale administered to participant children. This scale consists of 6 cartoon faces with varying expressions ranging from very happy to very sad and has been validated for children aged 3–7 years16,17 (Figure 2). Parents also evaluated their perception of the child's pain the evening of surgery using a 1–10 visual analog scale (VAS) and were asked to report any occurrences of postoperative cheek biting as well as subjective comments about their child's behavior at home within the first few postoperative hours.
Figure 1.
FLACC scale.14
Figure 2.
Wong-Baker FACES scale.16
The specific aim of this research was to determine whether local anesthesia administered with an intravenous NSAID improved the quality of recovery for children in the immediate postoperative period and while at home later in the day.
METHODS
The Institutional Review Board of Nationwide Children's Hospital in Columbus, Ohio reviewed and approved this study prior to commencement. A convenience sample of 27 subjects was recruited for this study at the Dental Surgery Center at Nationwide Children's Hospital. Subjects were children between 3 and 5.5 years of age undergoing general anesthesia for dental rehabilitation; they spoke English, had a phone where they could be reached, were ASA I or II, and were free of any developmental delays or psychiatric conditions, including attention deficit hyperactivity disorder. Inclusion criteria required that the dental surgical procedures include a minimum of either 2 anterior preveneered crowns or 2 anterior extractions (in order for lip anesthesia to be experienced in the local anesthesia group) and a minimum of 4 stainless steel crowns with at least 1 in the maxillary arch and 1 in the mandibular arch (in order that teeth in both the maxillary and mandibular posterior arches would also be anesthetized). Patients with a history of adverse drug reactions or medical contraindications to any analgesic or anesthetic agents used in the study were excluded. If the total amount of local anesthetic the child required exceeded 7 mg/kg of lidocaine (with epinephrine 1 ∶ 100,000), then the child was also excluded.
Prior to inclusion, informed consent was obtained by the study investigator from a legal guardian for each child. Demographic information, such as age, weight, and sex, was recorded about the patient.
Patients underwent general anesthesia according to the long-standing protocols. General anesthesia was induced with sevoflurane in oxygen; nasotracheal intubation was accomplished with propofol, 1–2 mg/kg intravenously, without neuromuscular blockade, followed by anesthetic maintenance with isoflurane in 50% nitrous oxide with oxygen. Dexamethasone, 0.1 mg/kg intravenously, was administered at induction or very early in the case prior to surgical intervention. Approximately 10–20 minutes prior to case completion, isoflurane was discontinued targeting 0% expired isoflurane at case termination, and general anesthesia was maintained during this interval with propofol boluses and 66% nitrous oxide in oxygen. Subjects were extubated unconsciously after several breaths of 100% oxygen and transferred to the postanesthesia care unit (PACU) when nearing wakefulness. The dental surgeons were pediatric dental faculty, private practitioner pediatric dentists, and second year pediatric dental residents.
Subjects were assigned by a random number generator to either a control or experimental group. The control group received 1 mg/kg ketorolac intravenously within 15 minutes of case completion. The experimental group received 1 mg/kg ketorolac within 15 minutes of case completion as well as local anesthetic infiltration. Two percent lidocaine with 1 ∶ 100,000 epinephrine was infiltrated on the buccal and lingual mucosa of each tooth treated. No inferior alveolar block anesthesia was provided. A standardized amount of 0.3 mL was administered for each tooth with stainless steel crowns or extractions (not for composite or amalgam restorations) utilizing premarked dental cartridges, not to exceed 7.0 mg/kg based on lidocaine dosage. The anesthetic infiltration took place prior to the commencement of the last sextant of operative dentistry. The surgeon and anesthesiologist were not blinded to local anesthetic administration in this study to allow for proper emergency care and record-keeping.
After transfer to PACU, subjects were monitored by a registered nurse. The nurse, who was blinded to local anesthetic status, evaluated the patient at 5-minute intervals using the FLACC assessment tool. The nurse also attempted to record patient self-assessment of pain using the Wong-Baker FACES scale and recorded subjective comments about patient behavior. All pertinent surgical and PACU times were recorded. Parents were present in the PACU when subjects were transferred there, and parents were also blinded as to local anesthetic administration. A standard discharge script with postoperative instructions that advised parents to watch for signs of self-mutilation, such as cheek biting, was given to each caregiver. The instruction sheet also advised the parents to give acetaminophen (15 mg/kg) 4 to 6 hours after discharge, and a bottle of Children's Tylenol was dispensed. A phone number where the parents could be reached 4 to 6 hours after discharge was recorded.
Four to six hours after discharge from the PACU, parents were contacted by phone by a research investigator blinded as to the experimental group. The parents were asked to evaluate the patient's pain level on a provided 10-point VAS as well as to obtain a self-report of the child's pain using the FACES assessment tool. Parents were also asked to report analgesic use, cheek or lip biting, and subjective reports of behavior.
Statistical analysis of the data was performed using JMP (SAS Institute, Cary, NC) and GraphPad Prism Statistical Software (Graph Software, San Diego, Calif). An unpaired t test was used to analyze weight, restorations placed, and various operative times. A Fisher exact test was used to analyze sex differences and reporting of negative comments. A Wilcoxon rank sum test was used to analyze the postoperative FLACC score, the parent VAS score, and the child FACES score. All statistical analyses assumed a P level ≤ .05 to be significant. A priori, we determined that the following were clinically significant differences on testing: 2 for the FLACC score, 2 for the FACES score, and 3 for the VAS score.
RESULTS
Consent was obtained from parents prior to surgery for 48 children whose preoperative dental exam indicated they would meet the inclusion criteria. After examination and radiographs, 27 children met the inclusion criteria, with 15 in the lidocaine group and 12 in the control group. A number of children assigned to the lidocaine group were excluded from the study because the amount of local anesthesia would have exceeded 7 mg/kg due to a large number of teeth requiring treatment, and the protocol prevented reassigning children to the control group.
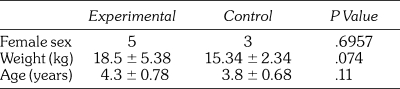
Eight female and 19 male participants completed the study. There were no statistically significant differences in sex, age, or weight between groups, although weight approached significance (control [C], 15.34 ± 2.34 kg vs lidocaine [L], 18.50 ± 5.38 kg; P < .07) (Table 1).
Table 1.
Participant Demographics
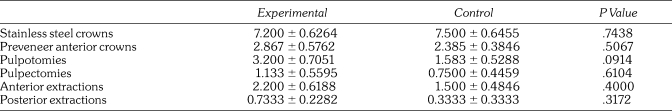
When analyzing the number and type of procedures in the 2 groups, none of the individual procedure totals per case reached significance with an unpaired t test between the lidocaine and control groups (Table 2). Length of the case (C, 91 ± 34 minutes vs L, 73 ± 12 minutes; P .10), total PACU time (C, 20 ± 4 minutes vs L, 20 ± 6 minutes; P < .96), and time to eye opening (C, 12 ± 14 minutes vs L, 9 ± 5 minutes; P < .47) did not differ significantly between groups.
Table 2.
Average Number of Restorations Per Group
FLACC scores were recorded by a registered nurse in the PACU at 5-minute intervals and interpreted using a Wilcoxon rank sum test. The times of awakening, degree of recovery at any time period, and discharge time in patients were variable, and therefore a comparison of all subjects at a set time period would not yield a valid comparison. The FLACC score at 15 minutes in the PACU captured the most children awake and yet not discharged (N = 22). Almost all participants were discharged by 30 minutes and FLACC scores were not noted to be very different from 15 minutes to discharge. For all but 3 participants, this was the same as the 15-minute postoperative FLACC score. For the 3 participants who had different discharge FLACC scores from the 15-minute FLACC score, the difference was only 1 unit for all participants (0 → 1; 5 → 4; 1 → 0) and not clinically significant. A priori, it was determined that a difference of greater than 2 would be considered clinically significant. Only 1 patient was discharged prior to 15 minutes and that FLACC score was zero at 10 minutes, so no change was anticipated in that participant. Therefore, the FLACC score closest to discharge was used for comparison. The mean FLACC score at discharge did not differ between the experimental or control groups (L, 2.47 ± 2.69 vs C, 2.58 ± 2.54; P < .88). The highest FLACC score provides another means for comparison. There was no significant difference between groups based on highest FLACC score (P < .84).
The immediate postoperative FACES score was difficult to obtain due to variable cooperation of subjects in the immediate postoperative period and therefore yielded insufficient data for analysis.
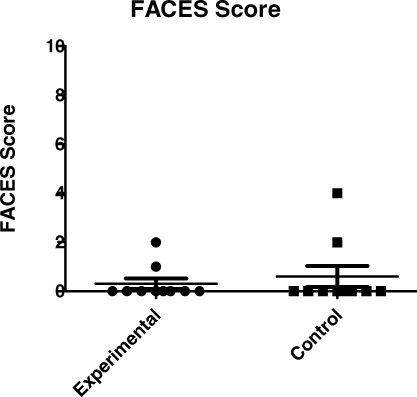
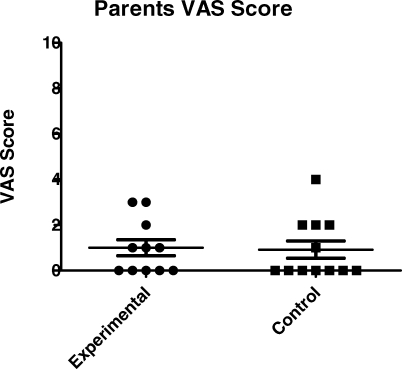
Twenty-three of the 27 subjects were reached for a postoperative phone call. There was no significant difference in time of parent contact for postoperative phone call from PACU discharge between groups (L, 277 ± 34 minutes vs C, 311 ± 96 minutes; P < .28). Of these, 23 subjects whose parents were contacted, 20 were able to self-report pain using the FACES scale, 10 in the experimental and 10 in the control group. These scores were similar between groups (L, 0.30 ± 0.21 vs C, 0.60 ± 1.35; P < .92), using a Wilcoxon rank sum analysis as shown in Figure 3. The VAS scores reported by the parents were also similar (P < .74) as shown in Figure 4, but clearly, this is a subjective measure without internal validity.
Figure 3.
Postoperative phone call FACES scores.
Figure 4.
Postoperative phone call VAS scores.
The number of children requiring oral analgesics at home, as reported by parents, was not different, based on the Fisher exact test, between the two groups (L, 2 of 11 vs C, 4 of 12; P < .70) with the majority (17 of 23 subjects; 74%) not needing additional pain medication beyond the intraoperative intravenous ketorolac with or without local anesthesia.
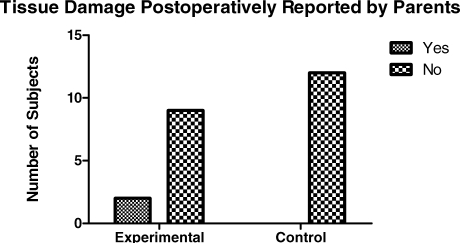
When asked if subjects had been observed biting their lips or cheeks, more children from the local anesthetic group (4 of 11; 36%) reported biting than from the control group (1 of 12; 8%), although the results were not significant with a Fisher exact test (P < .155). When asked if physical damage to the oral tissues was observed, only 2 children from the local anesthetic group (2 of 15; 13%) reported damage to oral tissues vs the control group (0 of 18; 0%). The report of visible damage to the oral structures was not significant with the Fisher exact test (P < .22) as shown in Figure 5.
Figure 5.
Tissue damage postoperatively reported by parents.
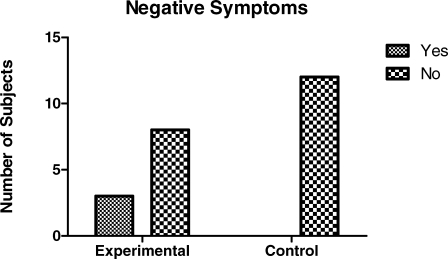
Parents were asked for subjective comments about their child's behavior postoperatively. A category called “Negative Comments” was added for children who demonstrated distressed behavior. Only the parents of children who received local anesthetic in addition to ketorolac (3 of 15 children; 20%), reported negative comments. None of the parents of children who received ketorolac alone reported negative comments. The presence of negative symptoms was, however, not statistically significant with a Fisher exact test (P < .09) as shown in Figure 6. Examples of negative comments included: “He cried for a half hour. He didn't like the numb feeling and couldn't eat”; “He was pulling on his lips. He couldn't eat or drink”; “She was biting her lip when she first woke up”; “He was biting and pulling at his lips on the way home”; and “She didn't like the feeling of her front teeth and complained they felt tingly.”
Figure 6.
Negative symptoms postoperatively reported by parents.
DISCUSSION
Pediatric dental rehabilitation is performed under general anesthesia to treat children unable to cooperate in a dental setting to provide high-quality dental surgical care in an environment that causes minimal distress to patients and parents. The findings of this study suggest that local anesthetic has no beneficial effect on pain behaviors postoperatively following comprehensive pediatric dentistry under general anesthesia.
Our findings support Al-Bahlani's previous finding of increased distress identified with local anesthetic use.11 Parents were able to identify negative behaviors secondary to the sensation of numbness, in spite of being blinded to local anesthetic usage. Examples of parent's comments were: “She was fussy until the numbing wore off”; “She was clawing at her mouth and scratching her lips”; and “She had a hard time eating or drinking.” These responses were grouped in the results as “Negative Comments,” and although the value was not statistically significant, the number of cases was clinically significant (3 of 15 for the local anesthesia group vs 0 of 18 for the ketorolac only group). We consider this clinically significant because it occurred with 20% of the children in the experimental group whose caregivers were contacted. When 1 out of 5 children treated under general anesthesia perceive discomfort due to the sensation of numbness, then quality of recovery is not optimal. The parents of children with negative symptoms secondary to local anesthetics reported their children were in distress until the local anesthetic sensations wore off. Though one of the aims of this study was to determine improvement in the postoperative experience with local anesthetics, the outcome seems to show the opposite to be true.
The incidence of cheek and lip biting did not reach statistical significance but, again, the incidence reported is clinically significant due to the discomfort associated with oral trauma (L, 2 of 15 vs C, 0 of 18). Again, this is deemed clinically significant because approximately 13% of children were affected. The small sample size may also have not allowed statistical significance to be demonstrated. None of the patients required a follow-up visit because the degree of oral trauma was not concerning enough for parents, even with a follow-up phone call on postoperative day 1. Our finding of 13% postoperative cheek biting is not dissimilar to the 18% incidence of trauma following mandibular block anesthesia by College et al18 in sedated and nonsedated children. Postoperative lip and cheek biting injuries were also found by Coulthard et al9 who found injuries in 1 of 69 control patients and 3 of 70 local anesthesia patients. The authors conjectured that in children the alteration of sensation is more distressing when it occurs in the orofacial area as opposed to other surgical areas because the face is more highly innervated, and therefore they are more aware of it.9 Other reasons may include the inability to view the area of anatomy with altered sensation as is possible with any other area of anatomy as well as the unconscious importance of the mouth to children.
Another interesting result was found when reviewing the pattern of FLACC scores. There was minimal change in FLACC scores for any individual subject during the overall PACU stay. In other words, those participants who were comfortable on awakening were likely to be so at discharge, and those who awoke exhibiting discomforting behaviors, continued to do so during the short PACU stay, which averaged 20 minutes. Regardless, the majority of children whose parents were contacted by phone, 17 of 23, did not require additional analgesics later in the day (L, 2 of 11 vs C, 4 of 12; P < .695). This is interesting considering that a significant amount of dental treatment was provided in both groups.
This is one of the very few studies that have been conducted on pain perception following dental rehabilitation, under fully intubated general anesthesia in children, especially with a double-blind design. Previous studies have exclusively studied pain perception after extractions only. The presence of tooth preparation and restoration in addition to extractions may have different effects on the perception of postoperative pain, and their inclusion is a major strength of this study.
The primary study weakness is the small sample size. A larger cohort might have uncovered statistically significant differences in those parameters that approached clinical significance. Since this is a novel area of study, attempts at a pre-study power analysis proved arbitrary. Therefore, a convenience sample was recruited using rigid inclusion criteria to ensure similarity between the 2 groups. As a result, sample sizes were low. However, this research provides a foundation for future studies in this area with a more appropriate sample size.
Other changes in methodology would strengthen future study designs. Preoperative FLACC and FACES scores would control for the presence or absence of preoperative pain. Reliability testing for the nurse evaluator was not conducted in this study since one nurse was used throughout. However, intrarater reliability tests could have ensured more consistent evaluation of postoperative FLACC scores. Previous research demonstrates that parents can be trained in the FLACC assessment tool in a brief period of time. In future studies, a parental FLACC assessment at home at more frequent intervals could strengthen the postoperative evaluation process. Additionally, the study design that limited lidocaine administration to 7 mg/kg resulted in older children, possibly those with more dental work required and those weighing more, being in the experimental local anesthetic group. The older group may have skewed results, especially with this small sample size.
In conducting the study, it was interesting to note that some practitioners, both dentists and 1 dentist anesthesiologist, felt so strongly about their preferred technique of practicing, that they were either unwilling to participate in the study or expressed discomfort at being assigned to a group other than their preferred method. Despite the lack of research in the area, both practitioners who routinely administered local anesthetic and those who do not were confident that switching techniques would affect patient recovery. This validates the need for the profession to continually challenge our practices to avoid continuation of ineffective techniques. It should also be noted that 7.0 mg/kg of lidocaine, the maximum dose for the monitored patient with full emergency capability immediately available, was used in this study. This is more than the AAPD recommended maximum of 4.4 mg/kg, which assumes an unmonitored patient and possibly the addition of inhalation and oral sedatives.
In light of the absence of beneficial effects of the use of local anesthetic and the presence of negative symptoms, this study does not support use of local anesthetics during dental rehabilitation under general anesthesia to decrease postoperative pain. Alternative methods of pain control should be investigated that avoid altered orofacial sensation. The use of intraoperative opioids, such as morphine sulfate, with or without intravenous NSAIDs, appears to result in a greater incidence of calm children in the PACU as reported anecdotally by our PACU nurse.
REFERENCES
- American Academy of Pediatric Dentistry. Guideline on behavior guidance for the pediatric dental patient. Pediatr Dent. 2006–2007;28(suppl):97–105. [Google Scholar]
- Atan S, Ashley P, Gilthorpe M.S, Scheer B, Mason C, Roberts G. Morbidity following dental treatment of children under intubation general anaesthesia in a day-stay unit. Int J Paediatr Dent. 2004;14:9–16. doi: 10.1111/j.1365-263x.2004.00520.x. [DOI] [PubMed] [Google Scholar]
- Schechter N. The undertreatment of pain in children: an overview. Pediatr Clin North Am. 1989;36:781–794. doi: 10.1016/s0031-3955(16)36721-9. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics and American Pain Society. The assessment and management of acute pain in infants, children, and adolescents. Pediatrics. 2001;108:793–797. doi: 10.1542/peds.108.3.793. [DOI] [PubMed] [Google Scholar]
- Bridgman C.M, Ashby D, Holloway P.J. An investigation of the effects on children of tooth extraction under general anaesthesia in general dental practice. Br Dent J. 1999;186:245–247. doi: 10.1038/sj.bdj.4800076. [DOI] [PubMed] [Google Scholar]
- Jurgens S, Warwick R.S, Inglehearn P.J, Gooneratne D.S. Pain relief for paediatric dental chair anaesthesia: current practice in a community dental clinic. Int J Paediatr Dent. 2003;13:93–97. doi: 10.1046/j.1365-263x.2003.00430.x. [DOI] [PubMed] [Google Scholar]
- Sammons H.M, Unsworth V, Gray C, Choonara I, Cherrill J, Quirke W. Randomized controlled trial of the intraligamental use of a local anaesthetic (lignocaine 2%) versus controls in paediatric tooth extraction. Int J Paediatr Dent. 2007;17:297–303. doi: 10.1111/j.1365-263X.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- Leong K.J, Roberts G.J, Ashley P.F. Perioperative local anaesthetic in young paediatric patients undergoing extractions under outpatient ‘short-case’ general anaesthesia. A double-blind randomized controlled trial. Br Dent J. 2007;203:334–335. doi: 10.1038/bdj.2007.724. [DOI] [PubMed] [Google Scholar]
- Coulthard P, Rolfe S, Mackie I.C, Gazal G, Morton M, Jackson-Leech D. Intraoperative local anaesthesia for paediatric postoperative oral surgery pain—a randomized controlled trial. Int J Oral Maxillofac Surg. 2006;35:1114–1119. doi: 10.1016/j.ijom.2006.07.007. [DOI] [PubMed] [Google Scholar]
- McWilliams P.A, Rutherford J.S. Assessment of early postoperative pain and haemorrhage in young children undergoing dental extractions under general anaesthesia. Int J Paediatr Dent. 2007;17:352–357. doi: 10.1111/j.1365-263X.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- Al-Bahlani, Sherriff A, Crawford P.J.M. Tooth extraction, bleeding, and pain control. J R Coll Surg Edinb. 2001;46:261–264. [PubMed] [Google Scholar]
- American Academy of Pediatric Dentistry. Guideline on appropriate use of local anesthesia for pediatric dental patients. Pediatr Dent. 2006–2007;28(suppl):106–111. [Google Scholar]
- Nick D, Thompson L, Anderson D, Trapp L. The use of general anesthesia to facilitate dental treatment. Gen Dent. 2003;51:464–468. [PubMed] [Google Scholar]
- Merkel S.I, Voepel-Lewis T, Shayevitz J.R, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–297. [PubMed] [Google Scholar]
- Manworren R.C, Hynan L.S. Clinical validation of FLACC: preverbal patient pain scale. Pediatr Nurs. 2003;29:140–146. [PubMed] [Google Scholar]
- Wong D.L, Baker C.M. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9–17. [PubMed] [Google Scholar]
- Bosenberg A, Thomas J, Lopez T, Kokinsky E, Larsson L.E. Validation of a six-graded faces scale for evaluation of postoperative pain in children. Paediatr Anaesth. 2003;13:708–713. doi: 10.1046/j.1460-9592.2003.01142.x. [DOI] [PubMed] [Google Scholar]
- College C, Feigal R, Wandera A, Strange M. Bilateral versus unilateral mandibular block anesthesia in a pediatric population. Pediatr Dent. 2000;22:453–457. [PubMed] [Google Scholar]