Abstract
Brain serotonin (5-HT) has been implicated in a number of physiological processes and pathological conditions. These effects are mediated by at least 14 different 5-HT receptors. We have inactivated the gene encoding the 5-HT1A receptor in mice and found that receptor-deficient animals have an increased tendency to avoid a novel and fearful environment and to escape a stressful situation, behaviors consistent with an increased anxiety and stress response. Based on the role of the 5-HT1A receptor in the feedback regulation of the 5-HT system, we hypothesize that an increased serotonergic neurotransmission is responsible for the anxiety-like behavior of receptor-deficient animals. This view is consistent with earlier studies showing that pharmacological activation of the 5-HT system is anxiogenic in animal models and also in humans.
Brain serotonin (5-HT) is implicated in the control of a wide variety of physiological processes such as nociception, cardiovascular function, and thermoregulation, as well as in different behavioral processes including feeding, aggression, and response to stress (reviewed in refs. 1–5). The 5-HT system also appears to be involved in the etiology of neuropsychiatric disorders such as depression and anxiety (reviewed in refs. 6 and 7). In recent years, our understanding of the physiological and pathological aspects of the 5-HT system has benefited from the identification, classification, and more recently the cloning of the 5-HT receptor subtypes (8). Among the receptor subtypes that have received the most attention is the 5-HT1A receptor (5-HT1AR). This was because of the availability of 5-HT1AR agonists and the implication of the 5-HT1A receptor in anxiety (6, 9).
The 5-HT1AR, like most of the 5-HT receptors, belongs to the superfamily of G-protein coupled receptors (10, 11). It is negatively coupled to adenyl cyclase. Brain 5-HT1AR is located both pre- and postsynaptically. Presynaptic 5-HT1AR is found mainly in the dorsal and median raphe nuclei. Activation of these receptors by agonists causes a reduction in the firing rate of serotonergic neurons (12–14) and leads to the suppression of 5-HT synthesis, 5-HT turnover, and 5-HT release in the diverse projection areas (15, 16). Postsynaptic 5-HT1AR is found in limbic regions (such as hippocampus and septum) and in some cortical layers. As in the case of presynaptic receptors, activation of postsynaptic 5-HT1AR is generally believed to induce a decrease in the firing rate of the postsynaptic cell (14).
The 5-HT1AR has been extensively studied by pharmacological methods. Activation of the receptor by agonists results in an anxiolytic effect (17, 18). Correlations were found among the time and dose dependency of the anxiolytic effect, the inhibition of serotonergic firing in the dorsal raphe nuclei, and the inhibition of 5-HT release after systemic administration of agonists (19, 20). The 5-HT1AR partial agonist buspirone and a series of congeners also produce this neurochemical effect and are used clinically for the treatment of anxiety (9). Based on these findings, it has been proposed that 5-HT1AR agonists stimulate presynaptic receptors, which inhibit 5-HT release and consequently reduce 5-HT signaling at a multitude of diverse target receptors (18). These may include 5-HT2A, 5-HT2C, and 5-HT3 receptors, because selective antagonists acting on these receptors have been suggested to be anxiolytics (18, 21). Because of the role of presynaptic 5-HT1AR in regulating the 5-HT system, blockade of the receptor by antagonists is expected to increase central 5-HT function. Indeed, electrophysiological studies showed that the 5-HT1AR antagonist WAY-100635 induced an increased firing of serotonergic neurons (22). However, microdialysis studies showed no detectable increase of 5-HT neurotransmission by 5-HT1AR antagonists under normal conditions (23). When administered with selective 5-HT reuptake inhibitors, 5-HT1AR antagonists augment 5-HT levels in terminal regions (24). At the behavioral level, the effect of 5-HT1AR antagonists is controversial. Antagonists, such as pindolol, exhibit a biphasic dose-response curve with an initial anxiolytic effect that converts to an anxiogenic effect at high doses (25, 26). However, pindolol is also an adrenoreceptor antagonist, and this activity may confound the drug’s effect on the 5-HT1AR (27). The more selective 5-HT1AR antagonist, WAY-100635, induced anxiolytic-like effects in the light/dark box anxiety model with no anxiogenic effect at high doses (28).
Because buspirone is a clinically effective antidepressant, the 5-HT1AR has also been associated with depression (9). Depressed patients show blunted HPA (hypothalamic-pituitary-adrenal)-axis responses following 5-HT1AR agonist challenge, which can be interpreted as downregulation or hyporesponsiveness of postsynaptic 5-HT1AR (29).
Because the 5-HT1AR has been associated with anxiety and depression, receptor-deficient mutant mice could provide important information on predisposition toward these conditions. Here we show that mice lacking the 5-HT1AR avoid a novel and fearful environment and vigorously attempt to escape stressful situations, behavior consistent with an increased anxiety and stress response.
MATERIALS AND METHODS
Generation of 5-HT1AR-Deficient Animals.
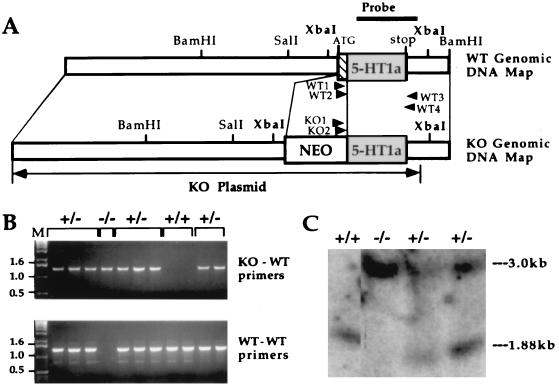
The gene for the 5-HT1AR was cloned from a BALB/c mouse genomic library (30). Construction of the targeting vector (Fig. 1A), electroporation of the targeting vector into E14 embryonic stem (ES) cells, and selection of the targeted clones were carried out by standard procedures (31). Correctly targeted cell clones were identified by a nested PCR assay (32) that could amplify a fragment from the substituted allele but not a fragment from the wild-type allele or targeting vector. The PCR primers were located in the 3′ noncoding regions of the heterologous neomycin resistance gene (KO primers 1 and 2) and the endogenous receptor gene (wild-type primers 3 and 4). The first 20 cycles of PCR were performed with KO primer 1 (5′-GACCGCTATCAGGACATAGCG-3′) and wild-type primer 3 (5′-TCTTAGGTGTTGCTTCCAGGG-3′). KO primer 2 (5′-ACGGTATCGCCGCTCCCGATTC-3) and wild-type primer 4 (5′-CCCTGTAAGCCCTACACTCTT-3′) were used for the next 30 cycles. The wild-type allele was detected by a similar procedure except that KO primers 1 and 2 were replaced by wild-type primers 1 (5′-ATGGATATGTTCAGTCTTGGC-3′) and 2 (5′-CAGGGCAACAACACCACAACG-3′), both corresponding to the 5′ coding region of the receptor gene absent in the knockout allele. The wild-type primers could amplify a fragment only from the wild-type allele. Chimeras were generated by aggregation (33) from two ES cell lines (designated C9W and D8M). Two independent knockout (5-HT1AR−) mouse lines were established from these clones. Because the 129sv genetic background (the standard background for knockouts) is not particularly suitable for behavioral testing, chimeras were backcrossed to Swiss–Webster mice to obtain heterozygotes. These F1 animals were crossbred to produce homozygous F2 mutants. Control nonchimeric littermates were similarly bred to control for a disequilibrium of genes that are linked to the mutation (34). F2 progeny with two wild-type 129sv 5-HT1AR alleles were selected by single-strand length polymorphism. Single-strand length polymorphism analysis was based on the closest marker (D13MIT193, located 5.1 centimorgans from the 5-HT1AR locus) (35) that showed a difference between the two genotypes. D13MIT193-specific primers (Research Genetics, Huntsville, AL) were used in standard PCR reactions and separated on 4% agarose gels. Amplified bands were approximately 130 and 110 bp long in Swiss–Webster and 129sv mice, respectively.
Figure 1.
Genetic inactivation of the 5-HT1AR gene in ES cells and mice. (A) Genomic structure and restriction map of the 5-HT1AR locus (WT Genomic) and the targeting vector (KO Genomic). Primers used for PCR (wild-type 1–4 and KO 1, 2) and a probe employed in Southern experiments are depicted by arrowheads and a bar, respectively. (B) PCR screening of progeny derived from matings of heterozygote animals. (Upper) The presence of an amplified product derived from the substituted allele in heterozygote (+/−) and homozygote (−/−) mice. (Lower) PCR products derived from the wild-type allele in wild-type (+/+) and heterozygote animals. Size markers are in lane M. (C) Southern blot analysis of the wild-type receptor-specific XbaI fragment. The size of this fragment is increased from 1.88 to 3.0 kb by the substitution mutation.
Receptor Autoradiography.
Brains were removed and kept at −80°C until assayed. Coronal sections were cut with a microtome cryostat at four neuroanatomical levels through the raphe, amygdala, septal nuclei, and, most anteriorly, through the nucleus accumbens and prefrontal cortex. Consecutive superimposable sections were used to determine total and nonspecific binding. The 5-HT1AR was labeled in the presence of 2 nM 3H-labeled 8-hydoxy-N,N-dipropyl-2-amino-1,2,3,4-tetrahydronaphthalene ([3H]-8-OH-DPAT; NEN catalog no. NET-929) as described (36). Nonspecific binding was determined in the presence of 1 μM 5-HT. Both C9W and D8M cell-derived mice were investigated by autoradiography. Sections were exposed to Hyperfilm (Amersham) for 2 weeks.
Behavioral Studies.
The open field test (37) used a black box (38.1 cm × 53.3 cm), divided into 12 even-sized rectangles (7.6 cm × 10.2 cm). The total number of crosses in the open field was recorded for 10 min to measure the locomotor activity. The time spent in and number of entries into the two rectangles at the center of the field were recorded to evaluate anxiety. In the forced swim test, mice were forced to swim in a clear, water-filled cylinder (diameter, 20.3 cm; depth, 10 cm), essentially as described by Porsolt et al. (38). In this test, mobility of the mice is measured in blocks of 2 min for a total of 6 min. For the rotarod test, animals were placed on a rotating bar (five turns per minute) and the time spent on the rod without falling was recorded (39). The best time in three trials was used for each mouse. Statistical significance was calculated by the independent t test. In all experiments, data for males and females were analyzed separately. Mice derived from C9W ES cells were used in all behavioral tests, but both 5-HT1AR− mouse lines were evaluated in the open field test. Mice were cared for in accordance with institutional guidelines.
RESULTS
Analysis of the Knockout Genotype.
A gene targeting vector was constructed by substituting a transcriptionally active neomycin resistance gene cassette for the 5′-coding region of the 5-HT1AR gene (Fig. 1A). Homologous recombination between the targeting construct and the receptor allele resulted in a deletion that included the initiation codon and the first 123 bp of the coding sequence, inactivating the 5-HT1AR gene. Initially, a PCR assay that was specific for the knockout allele was used to identify animals carrying the disrupted receptor allele (Fig. 1B Upper). A wild-type-specific PCR assay differentiated between the homozygous and heterozygous mutant animals (Fig. 1B Lower). The disruption of the receptor allele was confirmed by Southern blot (Fig. 1C). In wild-type animals, the receptor-specific probe (Fig. 1A) recognized an XbaI fragment (1.88 kb) that contained the entire coding region (1.27 kb), a section of the 5′ untranslated region (0.12 kb), and a portion of the 3′ untranslated region (0.49 kb). The XbaI fragment in the homozygous 5-HT1AR− animals migrated relative to markers at about 3 kb, the sum of the wild-type allele (1.88 kb) and the neomycin resistance gene cassette (1.10 kb). As expected, heterozygote animals contained both the wild-type and the disrupted alleles.
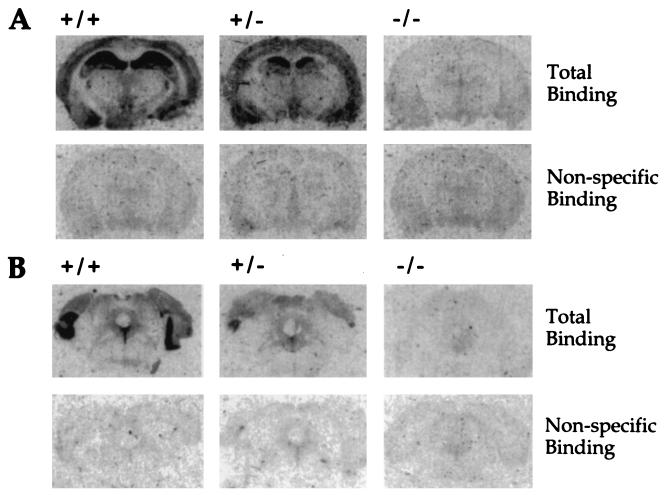
The loss of the receptor at the protein level was demonstrated by receptor autoradiography using [3H]-8-OH-DPAT, a selective 5-HT1AR ligand (Fig. 2). In wild-type animals, a strong signal was detected in the hippocampus, and somewhat less activity was found in the cortex (Fig. 2A Upper Left). The receptor was also detected in the raphe nuclei (Fig. 2B Upper Left). No significant binding was measured in the presence of 1 μM 5-HT in these regions, demonstrating a low nonspecific binding of [3H]-8-OH-DPAT (Fig. 2 A and B Lower Left). Homozygotes showed no specific binding in either region confirming the inactivation of the 5-HT1AR gene (Fig. 2 A and B Upper Right), and heterozygotes showed an intermediate level of binding (Fig. 2 A and B Upper Middle).
Figure 2.
5-HT1AR density of wild-type (+/+), heterozygote (+/−), and homozygote (−/−) animals in coronal sections of the hippocampus and cortex (A) and sections of the dorsal raphe nuclei (B). Receptors were labeled by the binding of [3H]-8-OH-DPAT. Nonspecific binding was determined in the presence of 5-HT. Three mice from each group were studied in this experiment, and sections from one is displayed.
Behavioral Analysis.
The 5-HT1AR gene was disrupted in two 129sv-derived ES cell clones (C9W and D8M). Chimeric animals were generated from each of the two mutant ES cell lines. For behavioral analysis, the chimeras were crossed to Swiss–Webster mice to produce F1 heterozygotes that were then crossbred to produce homozygous F2 mutants. To control for disequilibrium of genes linked to the mutation (34, 40), wild-type 129sv mice were similarly bred to produce F2 animals with the 129sv-derived 5-HT1AR locus at both alleles and a distribution of linked genes similar to that in the mutant F2 population. The two independent lines of homozygous mutant animals exhibited normal weight gain, fertility, and survival (data not shown).
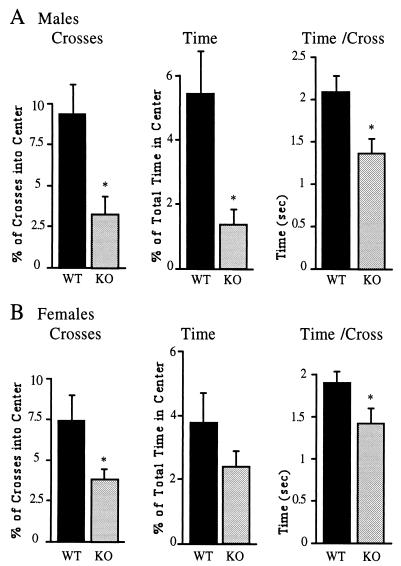
Pharmacological data indicating that the 5-HT1AR plays a role in anxiety (6) prompted us to compare the homozygous mutant and wild-type animals in an anxiety-related behavioral paradigm. Mice fear open space and avoid the center of the open field apparatus. The extent of anxiety can be determined by counting the number of entries by a test animal into the open field and by measuring the time spent in the center of the open field. Fig. 3A shows that 5-HT1AR− males entered into the center approximately three times less frequently (P < 0.05) than wild-type males (Fig. 3A). The overall locomotor activities of mutant and wild-type males were not statistically different in the open field test [143.7 ± 16.9 crosses (mean ± SD) and 162.1 ± 9.6 crosses, respectively]. In addition to the reduced number of entries into the center, receptor-deficient males spent approximately 4-fold less time in the center of the open field (P < 0.05) as compared with wild-type males (Fig. 3A). When normalized to the number of entries into the center of the open field, time spent in the center (time per cross) was still less in the mutant group (Fig. 3A). These changes in open field behavior were also observed in similar experiments with another 5-HT1AR− mouse line derived from an independent ES cell clone (data not shown). Lack of the 5-HT1AR also resulted in anxiety in females, but the effect was less pronounced than in males. 5-HT1AR− females entered into the center of the open field, determined as the percent of total crosses, two times less frequently (P < 0.05) than wild-type females (Fig. 3B). However, this difference was found only after normalization to total crosses, because mutant females showed an increased locomotor activity compared with wild-type females (239.5 ± 28.8 and 151.4 ± 18.4 crosses, respectively). When normalized to the number of entries into the center, the time spent in the center (time per cross) was significantly reduced in the group of mutant females as compared with wild-type female mice (Fig. 3B).
Figure 3.
Open field test of wild-type and 5-HT1AR− males (A) and females (B). Fifteen wild-type and homozygotic males, 10 wild-type females, and 12 heterozygotic females were studied. The number of crosses into the center was normalized to locomotor activity and expressed as the percent of total crosses. Time spent in the center of the test apparatus is expressed as the percent of total time (10 min). Time per cross indicates the average time spent in the center of the open field for each entry. Asterisks designate statistically significant differences (P < 0.05) for the mutant as compared with the wild-type group.
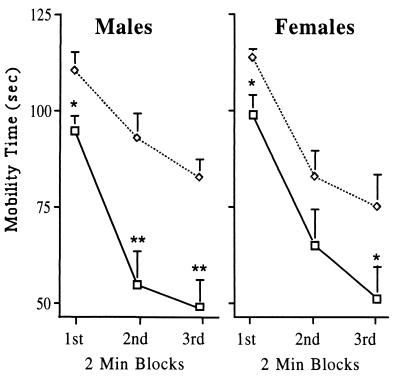
In addition to anxiety, the 5-HT1AR also appears to play a role in the stress response (41). Stress can be elicited by a number of stimuli including forced swim (42, 43). Mice, when placed into a water-filled tank, show a stress-induced increase in mobility that can be quantified by measuring swimming and climbing activity. With habituation, mice become progressively less mobile during the 6 min test (Fig. 4). The stressful nature of forced swim is demonstrated by the activation of the hypothalamic–pituitary–adrenal axis (44) and induction of the immediate–early gene c-fos in subcortical nuclei (lateral septal nucleus, bed nucleus of the stria terminalis, and hypothalamic and thalamic paraventricular nuclei) (45). As shown in Fig. 4, we tested whether receptor-deficient animals exhibit an altered response to swim stress. Interestingly, both 5-HT1AR− males and females were significantly more mobile as compared with wild-type controls. No gender difference was observed in this test.
Figure 4.
Mobility in the forced swim test of wild-type and 5-HT1AR− mice. Each group consisted of 8–10 mice. The mobility of animals was measured in seconds between 0–2 min (1st block), 2–4 min (2nd block), and 4–6 min (3rd block) of the test. (□), Wild-type animals; (◊), 5-HT1AR− mice. Asterisks designate statistically significant differences (∗, P < 0.05 and ∗∗, P < 0.005) for the mutant as compared with wild-type group.
Performance in the rotarod apparatus, a test for motor and spatial coordination (46), showed no difference between mutant and wild-type animals. Fall latencies in wild-type and 5-HT1AR− males were 16.9 ± 5.8 and 16.9 ± 5.0 sec, respectively; fall latencies in wild-type and 5-HT1AR− females were 16.3 ± 3.1 and 19.9 ± 4.3 sec, respectively.
DISCUSSION
The major finding of this study is that mice lacking the 5-HT1AR display enhanced anxiety and an increased response to stress. These mutant animals grow and reproduce normally, so we assume that their somatic and sexual development is normal. The absence of major developmental abnormalities in the receptor-deficient animals suggests that either the receptor does not play an essential role during development or that the receptor loss is compensated for by redundant functions during development. In either case, pharmacological studies support the view that the receptor deficiency in adult animals is responsible for the behavioral abnormalities that we have observed (Figs. 3 and 4). The anxiety level of test subjects can be modified by both 5-HT1AR agonists and antagonists. Agonists are generally anxiolytics, whereas antagonists can have an anxiogenic effect (18, 26). However, there are exceptions, and not all antagonists induce anxiety (25). The difference between antagonists is likely because of variations in selectivity, distribution, and potency. Based on the fact that many antagonists are anxiogenics and that the mutant mice exhibit increased anxiety, we favor the hypothesis that the behavior of the mutant animals is because of the absence of the 5-HT1AR in the adult animals. However, it will be necessary to produce conditionally deficient animals to exclude conclusively the possibility that a developmental defect contributes to the behavioral abnormalities that we have observed.
How can loss of the 5-HT1AR lead to increased anxiety? It is known that the 5-HT1AR is involved in the regulation of 5-HT release, either directly as is the case for presynaptic autoreceptors in the midbrain serotonergic nuclei or indirectly as for postsynaptic receptors in the hippocampus (18). Indeed, stimulation of the presynaptic receptors by an agonist results in an inhibition of the firing rate of the serotonergic neurons and suppression of 5-HT synthesis, as well as a reduction in 5-HT turnover and 5-HT release (12–16). Pharmacological inhibition of the 5-HT1AR, presumably by disrupting the inhibitory feedback, results in an increased activation of serotonergic neurons in the dorsal raphe nuclei that leads to an increased 5-HT turnover (22). Mutant animals are predicted to lack the inhibitory feedback because of the loss of the 5-HT1AR in the dorsal raphe nuclei (Fig. 2B), and the altered behavioral responses of these mice could be caused by the absence of critical presynaptic regulation of 5-HT release, causing 5-HT to be augmented in stressful situations. The absence of postsynaptic 5-HT1AR may also contribute to the altered behavior. Finally, the 5-HT1AR likely interacts with other neurotransmitters, such as dopamine and norepinephrine. The absence of 5-HT1AR could contribute to the altered behavioral responses of mutant mice, especially if compensation develops in the function of these neurotransmitter systems. The hypothesis that a hyperactive serotonergic system causes the anxiety-like phenotype in the mutant animals is supported further by the notion that increased serotonergic neurotransmission is anxiogenic in animal models as well as in humans (47–51). This hypothesis can be tested by measuring 5-HT turnover and extracellular 5-HT levels at baseline and after stress in the receptor-deficient animals.
Although 5-HT1AR− males and females showed a similar phenotype, anxiety was less prominent in female animals (Fig. 3). Also, mutant females but not males showed an increased locomotor activity in the open field test. The cause of this gender difference is not known, but differences in response to serotonergic drugs of males and females have been observed (52).
In addition to anxiety, 5-HT1AR− animals demonstrated an increased mobility in the forced swim test (Fig. 4). This might reflect the involvement of increased anxiety and emotional reactivity when mutant mice are exposed to inescapable stress. Alternatively, the behavior might reflect increased resistance to the development of behaviors associated with depression and antidepressants. Elevated serotonergic neurotransmission in the mutant animals could explain the changes in response in the forced swim test because stimulation of the 5-HT system increases the mobility of mice. For example, injection of the 5-HT precursor tryptophan increased the time that mice spent swimming in the forced swim test (53).
Besides the 5-HT1AR− mice, there are only a few rodent strains that display increased anxiety. The Maudsley reactive and nonreactive inbred rat strains were created as an animal model of anxiety by selective breeding. The Maudsley reactive strain shows a stable and reproducible deficit in an open field compared with the Maudsley nonreactive strain (54). Similar differences in anxiety behavior have been described between recombinant inbred strains of mice (55). Transgenic mice are proving to be useful models for exploration of the pathogenesis of anxiety. For example, the neuronal mechanism involved in anxiety, induced by corticotropin-releasing factor, was studied with corticotropin-releasing factor-overproducing transgenic mice (56). The involvement of enkephalins in the modulation of anxiety has been discovered in a preproenkephalin knockout mouse strain (57). The 5-HT1AR− mice might prove to be useful in studies probing the involvement of the 5-HT system in anxiety-like behavior and anxiety disorders.
Our results raise the possibility that disturbances in the 5-HT1AR and/or its downstream signaling pathway could contribute to the development of certain forms of anxiety disorders. This notion is consistent with the well-established role of the 5-HT1AR in controlling 5-HT levels, the association between high 5-HT levels and anxiety, and the anxiolytic properties of partial receptor agonists. An allelic variation in the human 5-HT1AR has been recently identified (58), but it is not yet known whether this polymorphism is functionally relevant.
Acknowledgments
We thank Irwin Lucki for very helpful comments on the manuscript and Yunbo Chen for technical assistance in preparing the Southern blots. This work was supported by a grant from the National Alliance for Research on Schizophrenia and Depression (M.T.). E.S. is supported by an Advanced Predoctoral Fellowship in Pharmacology/Toxicology from the Pharmaceutical Research and Manufacturers of America. T.S. is an American Cancer Society Professor and an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- 5-HT
serotonin (5-hydroxytryptamine)
- 5-HT1AR
5-HT1A receptor
- ES
embryonic stem
- 8-OH-DPAT
8-hydoxy-N,N-dipropyl-2-amino-1,2,3,4-tetrahydronaphthalene
References
- 1.Jacobs B L, Wilkinson L O, Fornal C A. Neuropsychopharmacology. 1990;3:473–479. [PubMed] [Google Scholar]
- 2.Leysen J E. Neuropsychopharmacology. 1990;3:361–369. [PubMed] [Google Scholar]
- 3.Fuller R W. Neuropsychopharmacology. 1990;3:495–502. [PubMed] [Google Scholar]
- 4.Buhot M C. Curr Opin Neurobiol. 1997;7:243–254. doi: 10.1016/s0959-4388(97)80013-x. [DOI] [PubMed] [Google Scholar]
- 5.Weiger W A. Biol Rev Camb Philos Soc. 1997;72:61–95. doi: 10.1017/s0006323196004975. [DOI] [PubMed] [Google Scholar]
- 6.Murphy D L. Neuropsychopharmacology. 1990;3:457–471. [PubMed] [Google Scholar]
- 7.Maes M, Meltzer H. In: The Serotonin Hypothesis of Depression. Bloom F, Kupfer D, editors. New York: Raven; 1995. pp. 933–944. [Google Scholar]
- 8.Martin G R, Humphrey P P A. Neuropharmacology. 1994;33:261–273. doi: 10.1016/0028-3908(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 9.Coplan J, Wolk S, Klein D. In: Anxiety and the Serotonin1A Receptor. Bloom F, Kupfer D, editors. New York: Raven; 1995. pp. 1301–1310. [Google Scholar]
- 10.Hoyer D, Schoeffter P. J Recept Res. 1991;11:197–214. doi: 10.3109/10799899109066399. [DOI] [PubMed] [Google Scholar]
- 11.Julius D. Annu Rev Neurosci. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- 12.Blier P, de Montigny C, Tardif D. Synapse. 1987;1:225–232. doi: 10.1002/syn.890010302. [DOI] [PubMed] [Google Scholar]
- 13.Jolas T, Haj-Dahmane S, Lanfumey L, Fattaccini C M, Kidd E J, Adrien J, Gozlan H, Guardiola-Lemaitre B, Hamon M. Naunyn-Schmiedebergs Arch Pharmacol. 1993;347:453–463. doi: 10.1007/BF00166735. [DOI] [PubMed] [Google Scholar]
- 14.Sprouse J S, Aghajanian G K. Neuropharmacology. 1988;27:707–715. doi: 10.1016/0028-3908(88)90079-2. [DOI] [PubMed] [Google Scholar]
- 15.Kennett G A, Marcou M, Dourish C T, Curzon G. Eur J Pharmacol. 1987;138:53–60. doi: 10.1016/0014-2999(87)90336-0. [DOI] [PubMed] [Google Scholar]
- 16.Bohmaker K, Eison A S, Yocca F D, Meller E. Neuropharmacology. 1993;32:527–534. doi: 10.1016/0028-3908(93)90048-8. [DOI] [PubMed] [Google Scholar]
- 17.Lucki I, Singh A, Kreiss D S. Neurosci Biobehav Rev. 1994;18:85–95. doi: 10.1016/0149-7634(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 18.De Vry J. Psychopharmacology. 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- 19.Jolas T, Schreiber R, Laporte A M, Chastanet M, De Vry J, Glaser T, Adrien J, Hamon M. J Pharmacol Exp Ther. 1995;272:920–929. [PubMed] [Google Scholar]
- 20.Sommermeyer H, Schreiber R, Greuel J M, De Vry J, Glaser T. Eur J Pharmacol. 1993;240:29–37. doi: 10.1016/0014-2999(93)90541-o. [DOI] [PubMed] [Google Scholar]
- 21.Keane P, Soubrie P. In: Animal Models of Integrated Serotonergic Functions: Their Predictive Value for the Clinical Applicability of Drug Interfering with Serotonergic Transmission. Baumgarten H, Gothert M, editors. New York: Springer; 1997. pp. 707–725. [Google Scholar]
- 22.Fornal C A, Metzler C W, Gallegos R A, Veasey S C, McCreary A C, Jacobs B L. J Pharmacol Exp Ther. 1996;278:752–762. [PubMed] [Google Scholar]
- 23.Kreiss D S, Lucki I. J Pharmacol Exp Ther. 1994;269:1268–1279. [PubMed] [Google Scholar]
- 24.Hjorth S. J Neurochem. 1993;60:776–779. doi: 10.1111/j.1471-4159.1993.tb03217.x. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher A, Forster E A, Bill D J, Brown G, Cliffe I A, Hartley J E, Jones D E, McLenachan A, Stanhope K J, Critchley D J, et al. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 26.Critchley M A, Handley S L. Psychopharmacology. 1987;93:502–506. doi: 10.1007/BF00207243. [DOI] [PubMed] [Google Scholar]
- 27.Sanghera M K, McMillen B A, German D C. Eur J Pharmacol. 1982;86:107–110. doi: 10.1016/0014-2999(82)90406-x. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez C. Pharmacol Toxicol. 1995;77:71–78. doi: 10.1111/j.1600-0773.1995.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 29.Lesch, K., Mayer, S., Disselkamp-Tieze, J., Hoh, A., Wiesmann, M., Osterheider, M. & Schulte, H. (1990) Biol. Psychiatry 620–628. [DOI] [PubMed]
- 30.Parks C L, Shenk T. J Biol Chem. 1996;271:4417–4430. doi: 10.1074/jbc.271.8.4417. [DOI] [PubMed] [Google Scholar]
- 31.Hasty P, Bradley A. In: Gene Targeting Vectors for Mammalian Cells. Joyner A L, editor. New York: Oxford Univ. Press; 1993. pp. 1–31. [Google Scholar]
- 32.Nitschke L, Kopf M, Lamers M C. BioTechniques. 1993;14:914–916. [PubMed] [Google Scholar]
- 33.Wood S A, Pascoe W S, Schmidt C, Kemler R, Evans M J, Allen N D. Proc Natl Acad Sci USA. 1993;90:4582–4585. doi: 10.1073/pnas.90.10.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmer A. Trends Neurosci. 1996;19:470. doi: 10.1016/S0166-2236(96)20053-0. [DOI] [PubMed] [Google Scholar]
- 35.Beckers M C, Ernst E, Diez E, Morissette C, Gervais F, Hunter K, Housman D, Yoshida S, Skamene E, Gros P. Genomics. 1997;39:254–263. doi: 10.1006/geno.1996.4489. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe Y, Sakai R R, McEwen B S, Mendelson S. Brain Res. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]
- 37.Crawley J N. Curr Opin Psychiatry. 1989;2:773–776. [Google Scholar]
- 38.Porsolt R D, Bertin A, Jalfre M. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 39.Baik J H, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Nature (London) 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 40.Gerlai R. Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 41.McKittrick C R, Blanchard D C, Blanchard R J, McEwen B S, Sakai R R. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- 42.Prince C R, Anisman H. Pharmacol Biochem Behav. 1990;37:613–621. doi: 10.1016/0091-3057(90)90535-p. [DOI] [PubMed] [Google Scholar]
- 43.Cullinan W E, Herman J P, Battaglia D F, Akil H, Watson S J. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 44.Kirby L G, Chou-Green J M, Davis K, Lucki I. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- 45.Duncan G E, Knapp D J, Breese G R. Brain Res. 1996;713:79–91. doi: 10.1016/0006-8993(95)01486-1. [DOI] [PubMed] [Google Scholar]
- 46.Hamm R J, Pike B R, O’Dell D M, Lyeth B G, Jenkins L W. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 47.Cloninger C R. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- 48.den Boer J A, Westenberg H G, De Leeuw A S, van Vliet I M. Int Clin Psychopharmacol. 1995;4:47–52. [PubMed] [Google Scholar]
- 49.Griebel G. Pharmacol Ther. 1995;65:319–395. doi: 10.1016/0163-7258(95)98597-j. [DOI] [PubMed] [Google Scholar]
- 50.Handley S L. Pharmacol Ther. 1995;66:103–148. doi: 10.1016/0163-7258(95)00004-z. [DOI] [PubMed] [Google Scholar]
- 51.Plomin R, Owen M J, McGuffin P. Science. 1994;264:1733–1739. doi: 10.1126/science.8209254. [DOI] [PubMed] [Google Scholar]
- 52.Kahn R S, Kling M A, Wetzler S, Asnis G M, van Praag H. Psychopharmacology. 1992;108:225–228. doi: 10.1007/BF02245312. [DOI] [PubMed] [Google Scholar]
- 53.Hilakivi-Clarke L A, Durcan M J, Lister R G, Linnoila M. Pharmacol Biochem Behav. 1990;37:273–276. doi: 10.1016/0091-3057(90)90333-d. [DOI] [PubMed] [Google Scholar]
- 54.Berrettini W H, Harris N, Ferraro T N, Vogel W H. Psychiatr Genet. 1994;4:91–94. doi: 10.1097/00041444-199422000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Mathis C, Neumann P E, Gershenfeld H, Paul S M, Crawley J N. Behav Genet. 1995;25:557–568. doi: 10.1007/BF02327579. [DOI] [PubMed] [Google Scholar]
- 56.Stenzel-Poore M P, Heinrichs S C, Rivest S, Koob G F, Vale W W. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konig M, Zimmer A M, Steiner H, Holmes P V, Crawley J N, Brownstein M J, Zimmer A. Nature (London) 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 58.Bergen A, Wang C Y, Nakhai B, Goldman D. Hum Mutat. 1996;7:135–143. doi: 10.1002/(SICI)1098-1004(1996)7:2<135::AID-HUMU7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]