Abstract
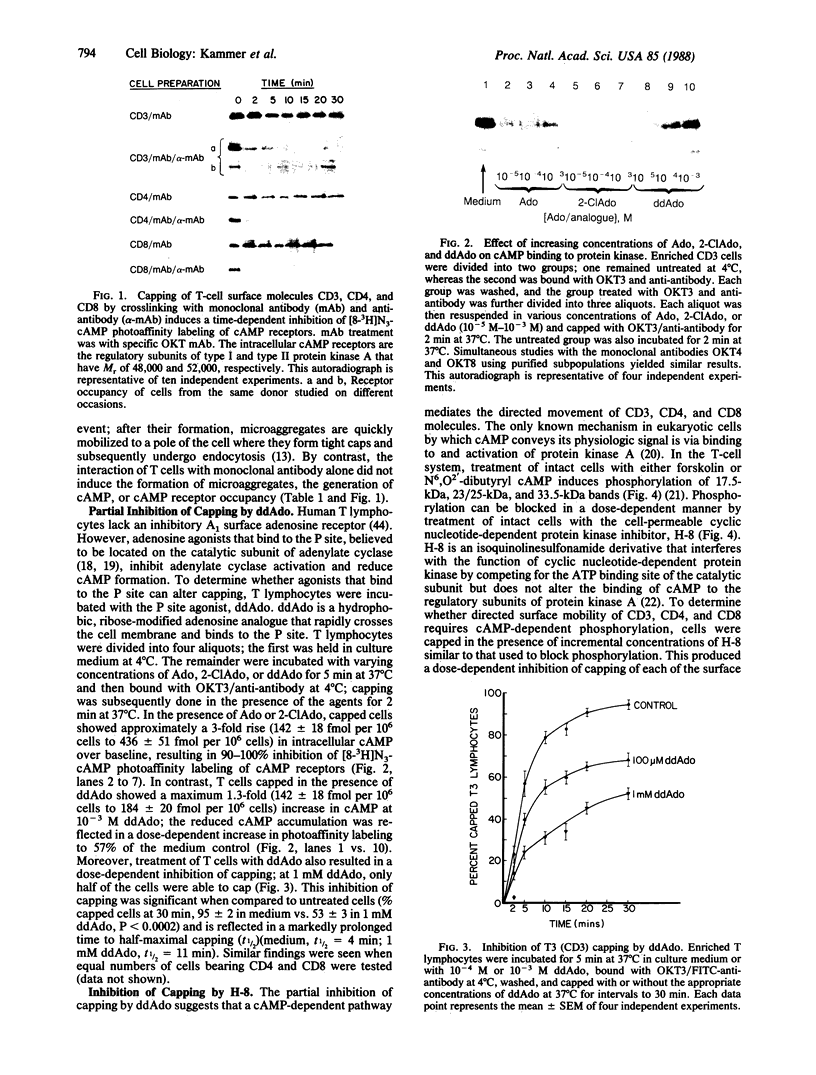
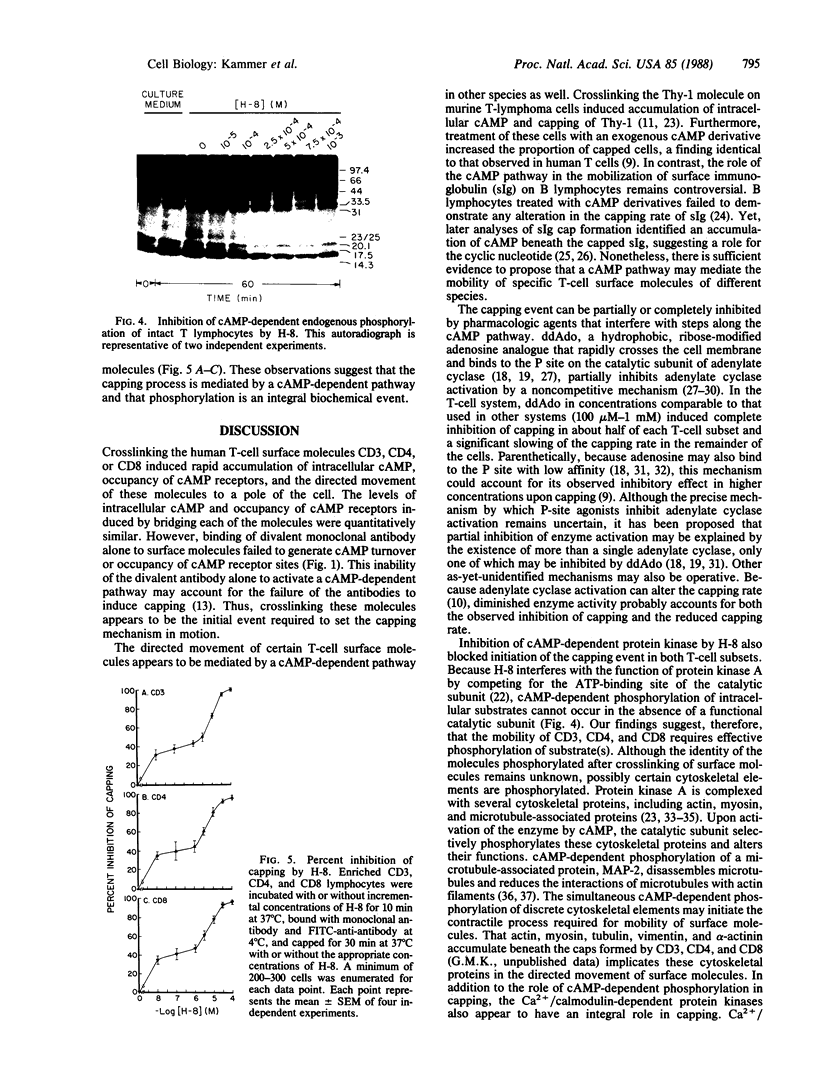
The present study was undertaken to determine whether a cAMP pathway mediates the mobility of CD3, CD4, and CD8 within the membrane. Crosslinking CD3, CD4, and CD8 with monoclonal antibody and anti-antibody induced rapid accumulation of intracellular cAMP, occupancy of cAMP receptors, and was temporally associated with the mobilization and directed movement of these molecules to a pole of the cell. This capping process could be partially inhibited in a dose-dependent manner by treatment of T cells with 2',5'-dideoxyadenosine, a ribose-modified adenosine analogue that binds to the P site of the catalytic subunit of adenylate cyclase and reduces adenylate cyclase activity. Furthermore, inhibition of cAMP-dependent endogenous phosphorylation of 17.5-kDa, 23/25-kDa, and 33.5-kDa bands in intact T cells by N-[2-(methylamino)ethyl]-5-isoquinoline-sulfonamide, a cell-permeable inhibitor of cyclic nucleotide-dependent protein kinase, blocked the capping event. Data support the conclusion that crosslinking of CD3, CD4, and CD8 activates a cAMP-dependent pathway that mediates the mobilization and directed movement of these molecules. cAMP-dependent protein phosphorylation is an integral step leading to the capping process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourguignon L. Y., Bourguignon G. J. Capping and the cytoskeleton. Int Rev Cytol. 1984;87:195–224. doi: 10.1016/s0074-7696(08)62443-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun J., Unanue E. R. The lymphocyte cytoskeleton and its control of surface receptor functions. Semin Hematol. 1983 Oct;20(4):322–333. [PubMed] [Google Scholar]
- Bruns R. F. Adenosine receptor activation in human fibroblasts: nucleoside agonists and antagonists. Can J Physiol Pharmacol. 1980 Jun;58(6):673–691. doi: 10.1139/y80-110. [DOI] [PubMed] [Google Scholar]
- Butman B. T., Jacobsen T., Cabatu O. G., Bourguignon L. Y. The involvement of cAMP in lymphocyte capping. Cell Immunol. 1981 Jul 1;61(2):397–403. doi: 10.1016/0008-8749(81)90387-7. [DOI] [PubMed] [Google Scholar]
- Chatenoud L., Baudrihaye M. F., Kreis H., Goldstein G., Schindler J., Bach J. F. Human in vivo antigenic modulation induced by the anti-T cell OKT3 monoclonal antibody. Eur J Immunol. 1982 Nov;12(11):979–982. doi: 10.1002/eji.1830121116. [DOI] [PubMed] [Google Scholar]
- Cosimi A. B., Colvin R. B., Burton R. C., Rubin R. H., Goldstein G., Kung P. C., Hansen W. P., Delmonico F. L., Russell P. S. Use of monoclonal antibodies to T-cell subsets for immunologic monitoring and treatment in recipients of renal allografts. N Engl J Med. 1981 Aug 6;305(6):308–314. doi: 10.1056/NEJM198108063050603. [DOI] [PubMed] [Google Scholar]
- Curtain C. C. Lymphocyte surface modulation and glycosphingolipids. Immunology. 1979 Apr;36(4):805–810. [PMC free article] [PubMed] [Google Scholar]
- Earp H. S., Utsinger P. D., Yount W. J., Logue M., Steiner A. L. Lymphocyte surface modulation and cyclic nucleotides I. Topographic correlation of cyclic adenosine 3':5'-monophosphate and immunoglobulin immunofluorescence during lymphocyte capping. J Exp Med. 1977 Apr 1;145(4):1087–1092. doi: 10.1084/jem.145.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt L. P., Kresina T. F., Kammer G. M. Effect of anti-T cell autoantibodies from systemic lupus erythematosus sera upon T lymphocyte functions. Arthritis Rheum. 1986 May;29(5):646–654. doi: 10.1002/art.1780290509. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hirata F., Strittmatter W. J., Axelrod J. beta-Adrenergic receptor agonists increase phospholipid methylation, membrane fluidity, and beta-adrenergic receptor-adenylate cyclase coupling. Proc Natl Acad Sci U S A. 1979 Jan;76(1):368–372. doi: 10.1073/pnas.76.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S. T., Lewis R. A., Austen K. F. Role of adenylate cyclase in immunologic release of mediators from rat mast cells: agonist and antagonist effects of purine- and ribose-modified adenosine analogs. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6800–6804. doi: 10.1073/pnas.77.11.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. L., Dawidowicz E. A., Robinson J. M., Karnovsky M. J. Role of cholesterol in the capping of surface immunoglobulin receptors on murine lymphocytes. J Cell Biol. 1983 Jul;97(1):73–80. doi: 10.1083/jcb.97.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson L., Caplow M. Modification of microtubule steady-state dynamics by phosphorylation of the microtubule-associated proteins. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3413–3417. doi: 10.1073/pnas.78.6.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer G. M., Boehm C. A., Rudolph S. A. Role of adenylate cyclase in human T-lymphocyte surface antigen capping. Cell Immunol. 1986 Aug;101(1):251–258. doi: 10.1016/0008-8749(86)90202-9. [DOI] [PubMed] [Google Scholar]
- Kammer G. M., Rudolph S. A. Regulation of human T lymphocyte surface antigen mobility by purinergic receptors. J Immunol. 1984 Dec;133(6):3298–3302. [PubMed] [Google Scholar]
- Kammer G. M., Smith J. A., Mitchell R. Capping of human T cell specific determinants: kinetics of capping and receptor re-expression and regulation by the cytoskeleton. J Immunol. 1983 Jan;130(1):38–44. [PubMed] [Google Scholar]
- Karnovsky M. J., Kleinfeld A. M., Hoover R. L., Klausner R. D. The concept of lipid domains in membranes. J Cell Biol. 1982 Jul;94(1):1–6. doi: 10.1083/jcb.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Bhalla D. K., Dragsten P., Hoover R. L., Karnovsky M. J. Model for capping derived from inhibition of surface receptor capping by free fatty acids. Proc Natl Acad Sci U S A. 1980 Jan;77(1):437–441. doi: 10.1073/pnas.77.1.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. A., Levy R. Response of cutaneous T cell lymphoma to therapy with hybridoma monoclonal antibody. Lancet. 1981 Aug 1;2(8240):226–230. doi: 10.1016/s0140-6736(81)90475-x. [DOI] [PubMed] [Google Scholar]
- Pomerantz A. H., Rudolph S. A., Haley B. E., Greengard P. Photoaffinity labeling of a protein kinase from bovine brain with 8-azidoadenosine 3',5'-monophosphate. Biochemistry. 1975 Aug 26;14(17):3858–3862. doi: 10.1021/bi00688a019. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. Inhibition of Xenopus oocyte adenylate cyclase by progesterone and 2',5'-dideoxyadenosine is associated with slowing of guanine nucleotide exchange. J Biol Chem. 1983 Jul 10;258(13):7935–7941. [PubMed] [Google Scholar]
- Salesse R., Garnier J. Adenylate cyclase and membrane fluidity. The repressor hypothesis. Mol Cell Biochem. 1984;60(1):17–31. doi: 10.1007/BF00226298. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. The modulation of spontaneous and anti-Ig-stimulated motility of lymphocytes by cyclic nucleotides and adrenergic and cholinergic agents. J Immunol. 1975 Feb;114(2 Pt 2):802–808. [PubMed] [Google Scholar]
- Schulman H. Phosphorylation of microtubule-associated proteins by a Ca2+/calmodulin-dependent protein kinase. J Cell Biol. 1984 Jul;99(1 Pt 1):11–19. doi: 10.1083/jcb.99.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden S. C., Pollard T. D. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983 Jun 10;258(11):7064–7071. [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. A. Actin nascent chains are substrates for cyclic AMP-dependent phosphorylation in vivo. Proc Natl Acad Sci U S A. 1980 Feb;77(2):910–914. doi: 10.1073/pnas.77.2.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles G. L., Lefkowitz R. J. Hormone-sensitive adenylate cyclase. Delineation of a trypsin-sensitive site in the pathway of receptor-mediated inhibition. J Biol Chem. 1982 Jun 10;257(11):6287–6291. [PubMed] [Google Scholar]
- Vallee R. B., DiBartolomeis M. J., Theurkauf W. E. A protein kinase bound to the projection portion of MAP 2 (microtubule-associated protein 2). J Cell Biol. 1981 Sep;90(3):568–576. doi: 10.1083/jcb.90.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Londos C., Cooper D. M. Adenosine receptors and the regulation of adenylate cyclase. Adv Cyclic Nucleotide Res. 1981;14:199–214. [PubMed] [Google Scholar]