Abstract
Reduced nitric oxide (NO) production and bioactivity is a major contributor to endothelial dysfunction. Animal data suggests that improvements in endothelial function in response to aerobic exercise training may depend on the duration of the training program. However, no studies have examined changes in NO (as assessed by the major NO metabolites, nitrate and nitrite, NOx) after long-term training in humans. In addition, aging may impair the ability of the vasculature to increase NO with exercise. Thus, we determined whether 24 weeks of aerobic exercise training increases plasma NOx levels in sedentary older adults. We also examined changes in forearm blood flow (FBF) at rest and during reactive hyperemia as a measure of vasomotor function. Plasma NOx levels were measured in 82 men and women using a modified Griess assay. FBF was assessed in a subset of individuals (n=15) using venous occlusion plethysmography. After 24 weeks of exercise training, there were significant improvements in maximum oxygen consumption, HDL cholesterol, triglycerides, and body fat. Changes in plasma NOx levels ranged from −14.83 to +16.69 μmol/L; however, the mean change overall was not significant (−0.33±6.30 μmol/L, p=0.64). Changes in plasma NOx levels were not associated with age, gender, race, HDL cholesterol, triglycerides, body weight, body fat, or maximal oxygen consumption. There were also no significant changes in basal FBF, peak FBF, hyperemic response, total hyperemic flow, or minimum forearm vascular resistance with exercise training. In conclusion, improvements in plasma NOx levels and FBF are not evident after long-term training in older adults.
Keywords: exercise training, nitric oxide, forearm blood flow, aging
INTRODUCTION
The endothelium plays a major role in vascular homeostasis through the release of various vasoactive and thromboregulatory substances, signaling molecules, and growth factors [1]. Owing to the seminal studies of Robert Furchgott [2], it is now well established that nitric oxide (NO) is one of the most important endothelium-derived substances, as this free radical signaling molecule mediates many of the protective effects of the vascular wall [3]. Reduced NO bioactivity is a major mechanism for endothelial dysfunction and commonly results from increased oxidative stress, which ultimately can lead to decreased NO production and/or increased NO degradation [3]. Endothelial dysfunction is considered an early marker of atherosclerosis, often preceding clinical evidence of atherosclerotic plaques [3]. In general, endothelial dysfunction is characterized by reduced plasma concentrations of the major NO metabolites, nitrate and nitrite (NOx), and/or impaired endothelium-dependent vasodilation in response to physiological or pharmacological stimuli [1].
Moderate-intensity exercise can not only help to slow, halt, and even reverse the progression of atherosclerosis, but may also reduce the incidence of all-cause mortality, particularly deaths due to cardiovascular disease (CVD) [4]. The beneficial effects of exercise training on CVD risk are partly mediated through improvements in endothelial function. Studies have consistently shown that short-term training (<12 weeks) increases endothelium-dependent vasodilation across multiple patient populations, as reviewed previously by Green et al [5]. Similarly, a few studies have reported increases in plasma NOx levels after short-term exercise training [6–8]. On the other hand, long-term training studies (≥16 weeks) have shown variable changes in NO-related vasomotor function [5; 9–11]. In addition, one study found that 18 weeks of graduated swimming significantly increased plasma NOx levels in hypercholesterolemic mice [12]. However, to our knowledge, no studies have determined whether plasma NOx levels are changed after long-term training in humans. It has also recently been shown that older persons may have an impaired ability to increase plasma nitrite levels in response to physiological stimuli such as exercise [13]. Thus, the primary aim of this study was to examine the effects of a 24-week exercise training intervention on plasma NOx levels in older men and women. We also examined changes in forearm blood flow (FBF) at rest and during reactive hyperemia in a subset of individuals as a measure of vasomotor function.
METHODS
Subjects
The study population included 41 men and 41 women who were participants of a larger study designed to examine the influence of genetic polymorphisms on changes in CVD risk factors in response to an exercise training intervention. The training-induced changes in plasma lipid levels and body fat have been reported previously [14]. All subjects were 50–75 yrs old, sedentary, non-smokers, non-diabetic, and free of cardiovascular, liver, kidney, and lung disease. Subjects also had at least one National Cholesterol Education Program lipid abnormality (total cholesterol > 200 mg/dL, LDL cholesterol > 130 mg/dL, HDL cholesterol < 40 mg/dL, or triglycerides > 200 mg/dL). No subjects were on lipid-lowering medications and subjects using anti-hypertensive medications underwent a medication tapering process recommended by their personal physician and supervised by the study physician. All women were postmenopausal (>2 years) and agreed to maintain their hormone replacement therapy (HRT) regimen, either on or not on HRT, for the duration of the study. Subjects were informed of the study requirements and provided their written consent. This study was approved by the University of Maryland at College Park Institutional Review Board.
Screening
Subjects reported to the laboratory in the morning after a 12-hour overnight fast. During this visit height and weight were measured and blood was drawn for various blood chemistries. Subjects with a body mass index (BMI) > 37 kg/m2, fasting triglycerides > 400 mg/dL, and fasting or postprandial glucose levels > 126 mg/dL or > 200 mg/dL, respectively, were excluded from the study. Qualified subjects then underwent a maximal graded exercise test to detect any cardiovascular, pulmonary, or other chronic diseases that would preclude exercise testing or training [15]. Subjects with tests indicating signs or symptoms of CVD were excluded from the study.
Dietary Stabilization
Subjects completed 6 weeks of dietary instruction with a registered dietitian, which consisted of two 1-hour classes per week on the American Heart Association Dietary Guidelines for the General Population [16]. Subjects were required to follow the diet and remain weight stable (maintain ±5% of initial body weight) for more than 3 weeks before undergoing baseline testing. The diet and body weight requirements were enforced throughout the study to eliminate the potentially confounding effects of diet and weight loss on the outcome variables. To monitor adherence, body weight was measured weekly and dietary intake was assessed every 8 weeks via 7-day food records and food frequency questionnaires. Caloric intake was increased as necessary to maintain body weight.
Baseline Testing
After the dietary stabilization period, subjects underwent baseline testing to assess the main outcome variables before exercise training. Blood was drawn into EDTA tubes in the morning after a 12-hour overnight fast and centrifuged at 3000 rpm at 4°C for 20 min. Frozen plasma aliquots were stored at −80°C until further analysis. Blood samples for plasma lipoprotein-lipid levels were analyzed on at least 2 separate days and averaged. Total cholesterol and triglyceride levels were measured enzymatically on a Hitachi 717 Autoanalyzer. HDL cholesterol was measured after precipitation with dextran sulfate [17] and LDL cholesterol was calculated using the Friedewald equation [18]. Total body fat was measured using dual energy x-ray absorptiometry (DPX-L, Lunar Corp, Madison, WI). Maximum oxygen consumption (VO2 max) was determined during a graded exercise treadmill test, and standard criteria were used to verify that VO2 max was achieved [19].
Plasma NOx Levels
Plasma samples were deproteinized using centrifugal filter units (Ultrafree-MC, Millipore Corporation, Billerica, MA) at 9000 rpm at 4°C for 40–50 min, and NOx levels were quantified via a modified Griess assay [20]. The intra-assay and inter-assay coefficients of variation (CVs) were 3.3% and 4.9%, respectively.
Forearm Blood Flow
FBF was measured in the non-dominant arm using venous occlusion plethysmography, as previously described [21]. All studies were conducted between 7:00 a.m. and 9:00 a.m. in the morning after a 12-hour overnight fast. Basal FBF was measured 3 times and the average of these values was used for analysis. Reactive hyperemia was induced by inflating an upper arm cuff to 50 mmHg above the systolic blood pressure for 5 minutes. After the cuff was released, FBF was measured every 15 seconds for 3 minutes. Three parameters were obtained from the FBF curve: peak FBF; minimum forearm vascular resistance (FVR) calculated from peak FBF and mean blood pressure measured just after cuff release; and total hyperemic flow, determined from the area under the curve.
Exercise Training Intervention
Subjects attended supervised exercise sessions 3 days/week for 24 weeks. In the initial sessions, subjects exercised for 20 minutes at 50% VO2 max. Exercise duration and intensity were gradually increased such that by week 10, subjects were exercising for 40 min per day at 70% VO2 max. A fourth unsupervised exercise session was added during the last 14 weeks of training. Exercise intensity was assessed using heart rate monitors (Polar, Brooklyn, NY). The average compliance over the 24-week training period was 91 ± 4%.
Final Testing
At the completion of the exercise training intervention, subjects underwent the same tests as at baseline. Subjects continued training until all final tests were complete. All final tests were completed 24–36 hours after exercise.
Statistical Analyses
All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). Data are presented as mean ± standard deviation (SD). Paired t-tests were used to determine changes with exercise training. Pearson correlation coefficients were examined to determine whether changes in plasma NOx levels and FBF variables are related to changes in CVD risk factors, including lipid levels, body weight, body fat, and VO2 max. Analysis of variance was used to determine the effect of gender, race, and HRT status on changes in plasma NOx with training. Significance was set at p ≤ 0.05.
RESULTS
On average the study population was 58.3 ± 6.0 yrs old and had a mean BMI of 27.8 ± 4.1 kg/m2. Approximately 70% of subjects were White, and 39% of women were on HRT. Body weight, body fat, VO2 max, lipids, and NOx levels before and after training are shown in Table 1. After 24 weeks of moderate-to-vigorous intensity aerobic exercise training, there was a 15% increase in VO2 max (p<0.0001). All participants remained within ±5% of their baseline weight, with an average change of −1.2 ± 2.4% (range: −4.9% to +4.7%). Despite remaining weight stable, there was a significant decrease in total body fat with training (p<0.0001). Aerobic exercise training also reduced triglyceride levels by 12% (p<0.0001) and increased HDL cholesterol by 9% (p<0.0001). There was no significant change in total or LDL cholesterol.
Table 1.
Subject characteristics before and after exercise training (n=82)
| Before Training | After Training | Change | P-value | |
|---|---|---|---|---|
| Weight (kg) | 81.7 ± 15.8 | 80.6 ± 15.2 | −1.1 ± 1.9 | <0.0001 |
| Total body fat (%) | 34.7 ± 9.3 | 33.1 ± 9.4 | −1.5 ± 2.1 | <0.0001 |
| VO2 max (mL/kg/min) | 25.5 ± 4.7 | 29.3 ± 6.3 | 3.8 ± 3.3 | <0.0001 |
| Total Cholesterol (mg/dL) | 196.6 ± 32.9 | 197.0 ± 35.3 | 0.4 ± 20.0 | 0.87 |
| HDL cholesterol (mg/dL) | 45.3 ± 13.0 | 49.4 ± 13.4 | 4.2 ± 5.5 | <0.0001 |
| LDL cholesterol (mg/dL) | 122.0 ± 28.5 | 121.6 ± 29.7 | −0.3 ± 18.8 | 0.88 |
| Triglycerides (mg/dL) | 139.8 ± 69.7 | 123.0 ± 60.4 | −16.8 ± 32.5 | <0.0001 |
| NOx (μmol/L) | 18.67 ± 4.83 | 17.71 ± 4.91 | −0.33 ± 6.30 | 0.64 |
Table values are means ± SD. VO2 max, maximum oxygen consumption; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NOx, nitrates/nitrites
Changes in plasma NOx levels with exercise training ranged from −14.83 μmol/L to +16.69 μmol/L. However, the overall mean change was not significant (−0.33 ± 6.30 μmol/L, p=0.64), and did not differ by gender (p=0.36), race (p=0.58), or HRT status in women (p=0.91). Changes in plasma NOx levels were not associated with changes in body weight (r=−0.16, p=0.16), total body fat (r=0.08, p=0.46), VO2 max (r=−0.03, p=0.81), HDL cholesterol (r=−0.01, p=0.90), or triglycerides (r=0.14, p=0.21). Changes in plasma NOx levels were also not associated with age (r=0.02, p=0.85). There was, however, a positive correlation between changes in plasma NOx levels and changes in both total cholesterol (r=0.30, p=0.007) and LDL cholesterol (r=0.26, p=0.02).
Among the 15 participants with FBF measurements, there were no significant changes in any of the FBF variables in response to exercise training (Table 2). In addition, the hyperemic FBF response curves before and after training were virtually identical (Figure). In correlation analyses, changes in FBF variables were unrelated to changes in plasma NOx levels. In addition, there were no significant associations with any of the CVD risk factors.
Table 2.
Forearm blood flow variables before and after exercise training (n=15)
| Before Training | After Training | P-value | |
|---|---|---|---|
| Basal FBF (mL/100 mL/min) | 2.08 ± 0.81 | 1.87 ± 0.72 | 0.25 |
| Peak FBF (mL/100 mL/min) | 11.34 ± 4.18 | 10.61 ± 3.03 | 0.48 |
| Hyperemic response (%) | 532.0 ± 357.7 | 570.0 ± 372.1 | 0.75 |
| Total hyperemic flow (ml/100 mL) | 5.98 ± 1.83 | 5.62 ± 1.94 | 0.57 |
| Minimum FVR (mmHg/mL/min/100 mL) | 10.09 ± 4.01 | 9.88 ± 3.12 | 0.85 |
Table values are mean ± SD. FBF, forearm blood flow; FVR, forearm vascular resistance
Figure.
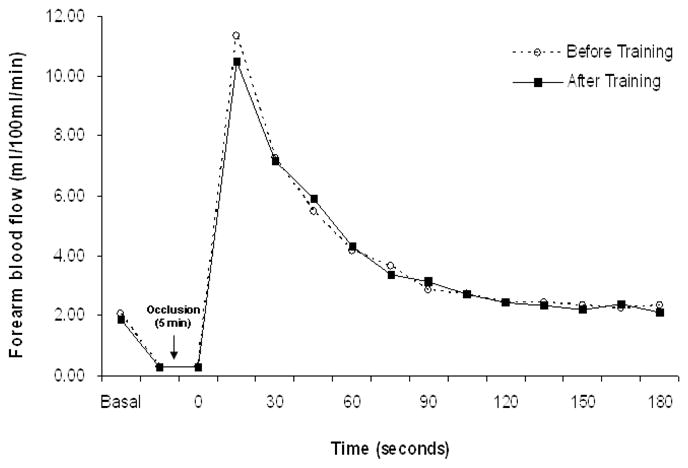
Time course of forearm blood flow at rest and during reactive hyperemia measure before and after aerobic exercise training
DISCUSSION
There is strong evidence to support a beneficial effect of exercise training on endothelial function, as measured by endothelium-dependent vasodilation [5; 22; 23]. On the other hand, although reduced NO production and bioactivity is a major contributor to endothelial dysfunction, less is known regarding the effects of exercise training on NOx levels. Cross-sectional studies generally show that urinary and plasma NOx levels are higher in trained vs. untrained persons [24; 25]. In the few intervention studies that exist, NOx levels were increased after 3 to 12 weeks of exercise training [6–8; 26]. In contrast, the major finding of the present study is that plasma NOx levels are unchanged after 24 weeks of aerobic exercise training in older adults. In addition, long-term exercise training had no effect on vasomotor function, as assessed by changes in the FBF hyperemic response.
Endothelial dysfunction can be assessed in plasma and urine samples by measuring circulating markers of NO metabolism (i.e. NOx) or markers of endothelial injury, such as soluble adhesion molecules [1]. Alternatively, functional methods can be used to measure NO-related vasomotion in response to pharmacological or physiological stimuli. NO regulates various functions of the cardiovascular system, including vascular tone, platelet activation, and cell proliferation. As such, decreased NO bioactivity may be one of the earliest detectable findings during the development of cardiovascular diseases [1]. It has been suggested that because plasma NOx levels may not reflect biologically active NO and are not solely endothelium-dependent, these measurements should be made in combination with functional assessments. In the present study we examined both plasma NOx levels and FBF at rest and during reactive hyperemia, and data from both measures indicate that long-term training does not improve endothelial function in older adults.
Our study examined 82 older (mean age = 58 yrs), overweight (mean BMI = 28 kg/m2) men and women with CVD risk factors including dyslipidemia and hypertension. Mean baseline plasma NOx levels were ~19 μmol/L and were unchanged after dietary stabilization and a 24-week exercise intervention consisting of aerobic exercise at 70% VO2 max for 40 min/day, 3–4 days/wk. In a previous study, Roberts et al. reported that a 3-week diet and exercise intervention increased urinary NOx levels in 11 men with CVD risk factors [26]. The study population, which included 7 hypertensives and 2 diabetics, were 38 to 72 yrs old and predominately obese (mean BMI = 37.6 kg/m2). The subjects followed a low-fat (~10% of calories), high-carbohydrate (~70–75% of calories) diet and walked daily for 45–60 min at 70–85% VO2 max. With such an intensive dietary intervention, it is not clear if the improvement in NOx levels was due primarily to the dietary intake, the exercise training, or both. It is also not clear if age had any effect on the training-induced changes in NOx levels. Moreover, the measurement of NOx in plasma vs. urine may reflect different aspects of NO metabolism [27]. Another study reported that 8 weeks of aerobic exercise training increased plasma NOx levels by 58% in 8 young healthy subjects (mean age = 20 yrs) who exercised at 70% VO2 max for 1 hr/day, 3–4 days/week [7]. Differences in the age and health status of the study population may account for some of the varying results. Thus, while previous studies examined only a small number of subjects after a short-term training intervention, this study provides the first examination of changes in plasma NOx levels after long-term training in an older population.
Changes in NO levels after exercise not only depend on the duration of the training program, but may also be influenced by age. Recently, Lauer et al. reported that among healthy subjects with no CVD risk factors, older persons (mean age = 58 yrs) had an impaired capacity to increase plasma nitrite levels after acute exercise compared to younger persons (mean age = 25 yrs) [13]. Consistent with this, Gomes et al. found that cycling for 45 min/day, 3 days/week, for 12 weeks at a heart rate corresponding to the anaerobic threshold did not improve plasma nitrite levels in 18 patients with metabolic syndrome (mean age = 46 yrs) [28]. On the other hand, 12 weeks of cycling at ~50% VO2 max for 30 min/day, 5 days/week increased plasma NOx levels by 54% (from ~28 to 43 μmol/L) in 10 elderly women (mean age = 63 yrs) who were normotensive, non-obese, and not on any medications including HRT. Besides the difference in health status, differences in the measurement of NO (nitrite only vs. nitrite + nitrate) make it difficult to compare these results. However, in the present study subjects exercised not only for a longer duration, but also at a higher intensity. It is possible that the higher exercise intensity in our study negatively affected the plasma NOx response to exercise, as 12 weeks of aerobic exercise training at 75% VO2 max has been shown to impair training-induced improvements in endothelial function in young healthy men (mean age = 25 yrs), most likely due to increased oxidative stress [29]. In contrast, training at 50% VO2 max augmented endothelial function through increased NO production. Older individuals may be particularly vulnerable to the adverse effects of high-intensity training. Taken together, these studies highlight the need to clarify the effect of aerobic exercise training on NO levels in older adults. Although in our study age was not associated with changes in plasma NOx levels with training, it is likely that the aging process per se alters NO-related adaptations to long-term exercise training.
To our knowledge, only 3 other studies have examined endothelial vasomotor function after long-term training in humans. Hambrecht et al. found that in chronic heart failure patients (mean age = 56 yrs), 24 weeks of exercise training at 70% VO2 max for 40–60 min/day, 5–6 days/week significantly increased acetylcholine-induced blood flow in the femoral artery [11]. In addition, 16 weeks of aerobic exercise training enhanced flow-mediated dilation in the brachial artery and blood flow in ocular resistance vessels in diabetics (mean age = 42 yrs) [9]. In the third study, 4 weeks of a hospital-based exercise training program (at 80% VO2 max for 60 min/day, 6 days/week), followed by 20 weeks of a home-based program (at 80% VO2 max for 20 min/day, 7 days/week), significantly improved coronary blood flow in patients with coronary artery disease (mean age = 60 yrs) [10]. In our population of older men and women free of clinical CVD and diabetes, there was no improvement in blood flow in forearm resistance vessels with exercise training. Differences in the study population, the vascular bed examined, and the measurement of endothelial function may account for the differences in our results.
As mentioned previously, most studies demonstrate that training programs of 12 weeks or less elicit significant improvements in NO-related vasomotor function. However, these adaptations appear to be transient and may disappear in the longer term [5]. Improvements in endothelial function in the short term are largely due to shear stress-induced changes in endothelial NO synthase expression, phosphorylation status, and enzyme activity [5; 22; 23]. In contrast, prolonged exercise training may lead to structural enlargement of blood vessels [30–32], and over time an increase in arterial diameter may occur that reduces the shear stress signal associated with a given exercise-induced elevation in blood flow and allows NO bioactivity to return towards pre-training levels [33–35]. These structural changes are consistent with the restoration of endothelial NO synthase expression, NO production, and endothelial-dependent vasodilation to control levels in the fully trained state [11; 35–37]. While these findings are supported by both animal and human data, more studies are needed to identify factors that may influence long-term training adaptations, such as characteristics related to the study population (i.e. age, gender, and health status) and the training program (i.e. exercise mode, frequency, intensity, and duration).
Limitations
Although the number of subjects in this study with plasma NOx levels was larger than in previous studies, we had limited power to detect changes with training for the FBF variables. In addition, because we did not have a non-exercise control group, we cannot rule out the possibility that exercise training simply prevented a decline in NOx levels and FBF. Similarly, because this study only included 50–75 yr-olds, we do not know if our exercise intervention would have improved plasma NOx levels and FBF in younger persons. We also cannot determine whether an increase in plasma NOx levels occurred earlier in the intervention (i.e. within the first 12 weeks of training). Finally, because we did not distinguish between endothelial-dependent and endothelial-independent vasodilation, we cannot determine whether the role of NO in the reactive hyperemic response is altered by exercise training. Nevertheless, measuring reactive hyperemia using venous occlusion plethysmography is a commonly used method to assess endothelial function in resistance arteries and correlates well with more invasive measures using intra-arterial drug infusions [38].
Summary and Conclusion
This is the first study to demonstrate that 24 weeks of aerobic exercise training does not increase plasma NOx levels or augment FBF in older men and women. Despite the limitations mentioned above, participants in our study were carefully monitored to keep their diet, medications, and body weight constant during the progressive, well-standardized exercise training program. We also followed standard procedures to ensure that blood samples for the measurement of NOx were drawn in the morning after an overnight fast and within 24–36 hrs after exercise. In addition, plasma samples were deproteinized before analysis to minimize experimental artifacts. While our results are consistent with the idea that improvements in NO production and endothelial function with exercise are transient and may be lost over time, more long-term training studies with repeated assessments of NO production and endothelial function are needed to better characterize the time course of vascular adaptations to exercise training. In addition, future studies will need to clarify the effect of aging on pathways involved in these adaptive responses.
Acknowledgments
We would like to thank all the staff members of Team Gene and the participants of the Gene Exercise Research Study.
FUNDING
This research was supported by NIH Grants AG-17474 (JMH), AG-15389 (JMH) and AG-00268 (TEB, NMF).
Footnotes
CONFLICT OF INTEREST
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raitakari OT, Celermajer DS. Testing for endothelial dysfunction. Ann Med. 2000;32:293–304. doi: 10.3109/07853890008995931. [DOI] [PubMed] [Google Scholar]
- 2.Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep. 1999;19:235–251. doi: 10.1023/a:1020537506008. [DOI] [PubMed] [Google Scholar]
- 3.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 4.Smith JK. Exercise and atherogenesis. Exerc Sport Sci Rev. 2001;29:49–53. doi: 10.1097/00003677-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis TV, Dart AM, Chin-Dusting JP, Kingwell BA. Exercise training increases basal nitric oxide production from the forearm in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 1999;19:2782–2787. doi: 10.1161/01.atv.19.11.2782. [DOI] [PubMed] [Google Scholar]
- 7.Maeda S, Miyauchi T, Kakiyama T, Sugawara J, Iemitsu M, Irukayama-Tomobe Y, Murakami H, Kumagai Y, Kuno S, Matsuda M. Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci. 2001;69:1005–1016. doi: 10.1016/s0024-3205(01)01192-4. [DOI] [PubMed] [Google Scholar]
- 8.Maeda S, Tanabe T, Otsuki T, Sugawara J, Iemitsu M, Miyauchi T, Kuno S, Ajisaka R, Matsuda M. Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens Res. 2004;27:947–953. doi: 10.1291/hypres.27.947. [DOI] [PubMed] [Google Scholar]
- 9.Fuchsjager-Mayrl G, Pleiner J, Wiesinger GF, Sieder AE, Quittan M, Nuhr MJ, Francesconi C, Seit HP, Francesconi M, Schmetterer L, Wolzt M. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care. 2002;25:1795–1801. doi: 10.2337/diacare.25.10.1795. [DOI] [PubMed] [Google Scholar]
- 10.Gielen S, Erbs S, Linke A, Mobius-Winkler S, Schuler G, Hambrecht R. Home-based versus hospital-based exercise programs in patients with coronary artery disease: effects on coronary vasomotion. Am Heart J. 2003;145:E3. doi: 10.1067/mhj.2003.58a. [DOI] [PubMed] [Google Scholar]
- 11.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 12.Napoli C, Williams-Ignarro S, de Nigris F, Lerman LO, Rossi L, Guarino C, Mansueto G, Di Tuoro F, Pignalosa O, De Rosa G, Sica V, Ignarro LJ. Long-term combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2004;101:8797–8802. doi: 10.1073/pnas.0402734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauer T, Heiss C, Balzer J, Kehmeier E, Mangold S, Leyendecker T, Rottler J, Meyer C, Merx MW, Kelm M, Rassaf T. Age-dependent endothelial dysfunction is associated with failure to increase plasma nitrite in response to exercise. Basic Res Cardiol. 2008;103:291–297. doi: 10.1007/s00395-008-0714-3. [DOI] [PubMed] [Google Scholar]
- 14.Halverstadt A, Phares DA, Wilund KR, Goldberg AP, Hagberg JM. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism. 2007;56:444–450. doi: 10.1016/j.metabol.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 15.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; Baltimore: 2000. [Google Scholar]
- 16.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St JS, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 17.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Endurance training in older men and women. I. Cardiovascular responses to exercise. J Appl Physiol. 1984;57:1024–1029. doi: 10.1152/jappl.1984.57.4.1024. [DOI] [PubMed] [Google Scholar]
- 20.Fryburg DA. NG-monomethyl-L-arginine inhibits the blood flow but not the insulin-like response of forearm muscle to IGF- I: possible role of nitric oxide in muscle protein synthesis. J Clin Invest. 1996;97:1319–1328. doi: 10.1172/JCI118548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JY, Farrance IK, Fenty NM, Hagberg JM, Roth SM, Mosser DM, Wang MQ, Jo H, Okazaki T, Brant SR, Brown MD. NFKB1 promoter variation implicates shear-induced NOS3 gene expression and endothelial function in prehypertensives and stage I hypertensives. Am J Physiol Heart Circ Physiol. 2007;293:H2320–H2327. doi: 10.1152/ajpheart.00186.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashi Y, Yoshizumi M. Exercise and endothelial function: role of endothelium-derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol Ther. 2004;102:87–96. doi: 10.1016/j.pharmthera.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res. 2005;67:187–197. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 24.Banfi G, Malavazos A, Iorio E, Dolci A, Doneda L, Verna R, Corsi MM. Plasma oxidative stress biomarkers, nitric oxide and heat shock protein 70 in trained elite soccer players. Eur J Appl Physiol. 2006;96:483–486. doi: 10.1007/s00421-005-0104-6. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Plaza LG, Alfieri AB, Cubeddu LX. Urinary excretion of nitric oxide metabolites in runners, sedentary individuals and patients with coronary artery disease: effects of 42 km marathon, 15 km race and a cardiac rehabilitation program. J Cardiovasc Risk. 1997;4:367–372. [PubMed] [Google Scholar]
- 26.Roberts CK, Vaziri ND, Barnard RJ. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation. 2002;106:2530–2532. doi: 10.1161/01.cir.0000040584.91836.0d. [DOI] [PubMed] [Google Scholar]
- 27.Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res. 2005;39:797–815. doi: 10.1080/10715760500053651. [DOI] [PubMed] [Google Scholar]
- 28.Gomes VA, Casella-Filho A, Chagas AC, Tanus-Santos JE. Enhanced concentrations of relevant markers of nitric oxide formation after exercise training in patients with metabolic syndrome. Nitric Oxide. 2008;19:345–350. doi: 10.1016/j.niox.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530–535. doi: 10.1161/01.CIR.0000080893.55729.28. [DOI] [PubMed] [Google Scholar]
- 30.Kramsch DM, Aspen AJ, Abramowitz BM, Kreimendahl T, Hood WB., Jr Reduction of coronary atherosclerosis by moderate conditioning exercise in monkeys on an atherogenic diet. N Engl J Med. 1981;305:1483–1489. doi: 10.1056/NEJM198112173052501. [DOI] [PubMed] [Google Scholar]
- 31.Miyachi M, Iemitsu M, Okutsu M, Onodera S. Effects of endurance training on the size and blood flow of the arterial conductance vessels in humans. Acta Physiol Scand. 1998;163:13–16. doi: 10.1046/j.1365-201x.1998.0337f.x. [DOI] [PubMed] [Google Scholar]
- 32.Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S. Effects of one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol. 2001;90:2439–2444. doi: 10.1152/jappl.2001.90.6.2439. [DOI] [PubMed] [Google Scholar]
- 33.Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc. 1995;27:1135–1144. [PubMed] [Google Scholar]
- 34.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 35.Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol. 2004;96:1114–1126. doi: 10.1152/japplphysiol.00768.2003. [DOI] [PubMed] [Google Scholar]
- 36.Oltman CL, Parker JL, Laughlin MH. Endothelium-dependent vasodilation of proximal coronary arteries from exercise-trained pigs. J Appl Physiol. 1995;79:33–40. doi: 10.1152/jappl.1995.79.1.33. [DOI] [PubMed] [Google Scholar]
- 37.Woodman CR, Turk JR, Rush JW, Laughlin MH. Exercise attenuates the effects of hypercholesterolemia on endothelium-dependent relaxation in coronary arteries from adult female pigs. J Appl Physiol. 2004;96:1105–1113. doi: 10.1152/japplphysiol.00767.2003. [DOI] [PubMed] [Google Scholar]
- 38.Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Kajiyama G, Oshima T. A noninvasive measurement of reactive hyperemia that can be used to assess resistance artery endothelial function in humans. Am J Cardiol. 2001;87:121–5. A9. doi: 10.1016/s0002-9149(00)01288-1. [DOI] [PubMed] [Google Scholar]


