Abstract
Biomonitoring is a valuable tool for identifying exposures to chemicals that pose potential harm to human health. However, to date there has been little published on ways to evaluate the relative public health significance of biomonitoring data for different chemicals, and even less on cumulative assessment of multiple chemicals. The objectives of our study are to develop a methodology for a health risk interpretation of biomonitoring data, and to apply it using NHANES 1999–2002 body burden data for organophosphorus (OP) pesticides. OP pesticides present a particularly challenging case given the non-specificity of many metabolites monitored through NHANES. We back-calculate OP pesticide exposures from urinary metabolite data, and compare cumulative dose estimates with available toxicity information for a common mechanism of action (brain cholinesterase inhibition) using data from U.S. EPA. Our results suggest that approximately 40% of children in the United States may have had insufficient margins of exposure (MOEs) for neurological impacts from cumulative exposures to OP pesticides (MOE less than 1,000). Limitations include uncertainty related to assumptions about likely precursor pesticide compounds of the urinary metabolites, sources of exposure, and intra-individual and temporal variability.
INTRODUCTION
Biomonitoring data of human exposure to environmental contaminants, which have become increasingly available over the last several years, complement the monitoring of pollutants in air, food, and drinking water (1, 2). They reflect the aggregate of exposure to chemicals from multiple sources. They also serve as an important component of environmental public health tracking, as they can be used to monitor body burdens of environmental contaminants in the population as part of tracking the continuum from sources to exposures to health status. Biomonitoring data can be used to identify where policies should be directed to reduce important exposures, and to document cases in which policies have successfully reduced exposures. For example, biomonitoring confirmed that removing lead in gasoline resulted in reduced lead body burden in children and extensive public health benefits (3, 4).
However, using biomonitoring data in tracking or other policy-oriented contexts poses a number of challenges. Key questions include how to determine exposures from body burden measurements, which methods should be used to identify chemicals of highest health importance, and how researchers should aggregate multiple contaminants measured in an individual. To date, there has been little published on methods to assess risk implications of body burdens of individual chemicals or of multiple chemicals cumulatively.
This paper takes a first step toward assessing the importance of multiple organophosphorus (OP) pesticide exposures for children. Approximately 40 OP insecticides are registered for use in the United States by the U.S. Environmental Protection Agency (5), and about 73 million pounds of OP pesticides were used in the United States in 2001 (70% of all insecticides), mainly for insect control on food crops (1, 5). OP pesticides represent an important and interesting class of chemicals to evaluate for several reasons: 1) OP pesticides are important neurodevelopmental toxicants (6); 2) OP pesticides were the first class of pesticides whose cumulative exposure and risks were evaluated by EPA as required by the Food Quality Protection Act of 1996; 3) nationally representative body burden data are available; 4) toxicity information is available to assist in a health-based interpretation of OP biomonitoring; and 5) one OP metabolite can have multiple precursor compounds, so improved methods are needed to relate body burdens to intakes. Non-specificity of metabolites and environmental exposure to metabolites may be characteristics of other classes of chemicals. Therefore our approach for OPs may be helpful for other chemical exposure concerns with similar characteristics.
We focus on children because they have been recognized as having potentially higher exposures and increased susceptibility to health risks from exposure to OPs. The objectives of our study are to develop a methodology for a health risk interpretation of biomonitoring data, and then to apply it using body burden data for OP pesticides from the National Health and Nutrition Examination Survey (NHANES) 1999–2002. This analysis illustrates the opportunities for and limitations of using these data to understand health implications of cumulative intake doses of multiple environmental contaminants.
METHODS
NHANES 1999–2002 Datasets
As part of NHANES, the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) conducts biomonitoring of pesticides from a subset of survey participants ≥6 years of age (1). NHANES is designed to measure the health and nutrition status of the U.S. population based on a representative sample covering all ages of the civilian noninstitutionalized population (for additional information on NHANES see http://www.cdc.gov/nchs/nhanes.htm). NCHS/CDC reports urinary concentrations of six dialkyl phosphate (DAP) metabolites (dimethylphosphate (DMP), dimethylthiophosphate (DMTP), dimethyldithiophosphate (DMDTP), diethylphosphate (DEP), diethylthiophosphate (DETP) and diethyldithiophosphate (DEDTP)); these are referred to as nonspecific metabolites because each is a metabolic product of multiple parent OP compounds. NCHS/CDC also reports urinary concentrations of several OP-specific pesticide metabolites (e.g. 3,5,6-Trichloro-2-pyridinol (TCPy), 2-Isopropyl-4-methyl-6-hydroxypyrimidine (IMPY), Malathion dicarboxylic acid (MDA), para -Nitrophenol (PNP)). The Supporting Information presents common OP pesticides and their urinary DAP and pesticide specific metabolites (see Figure S1, Table S1 and Table S2).
OP Pesticide Use Profiles
We used national pesticide usage estimates for agricultural applications based on market survey research for the years 1999 and 2000 (to approximate the same period as the NHANES data sets) to allocate non-specific OP metabolites proportionally to their parent OP compounds (see Table 1). On average 58,500,000 lbs of OP pesticides were used annually in 1999 and 2000 for agricultural applications (field, fruit and vegetable crops) (EPA Proprietary Data summarized, unpublished). Malathion and chlorpyrifos are the most common dimethyl and diethyl OP pesticides applied to field, fruit and vegetable crops in the U.S.
Table 1.
Commonly used dimethyl and diethyl OP pesticides by use profile and associated BMD10s and their urinary dialkyl phosphate metabolites
| Use Profiles in 1000Poundsc (% of total dimethyls or diethyls) |
|||||
|---|---|---|---|---|---|
| OP Pesticide | BMD10 (mg/kg/day)d |
Urinary Metabolites |
Nationala | Californiab | Monterey Co.b |
| Dimethyls | |||||
| Azinphos Methyl | 0.86 | DMP,DMTP, DMDTP |
1,610 (4%) | 200 (8%) | 0 (0.4%) |
| Dicrotophos | 0.04 | DMP | 485 (1%) | 0 (0%) | 0 (0%) |
| Dimethoate | 0.25 | DMP,DMTP, DMDTP |
970 (2%) | 441(18%) | 41 (19%) |
| Fenthion | 0.24 | DMP, DMTP | 0 (0%) | 0 (0%) | 0 (0.01%) |
| Malathion | 313.91 | DMP,DMTP, DMDTP |
31,793(80%) | 583 (23%) | 74 (33%) |
| Methidation | 0.25 | DMP,DMTP, DMDTP |
200(1%) | 138 (6%) | 15 (7%) |
| Methyl parathion | 0.67 | DMP, DMTP | 2,600 (7%) | 116 (5%) | 0 (0.01%) |
| Naled | 1.00 | DMP | 83 (0.2%) | 277 (11%) | 25 (11%) |
| Oxydemeton- methyl |
0.09 | DMP, DMTP | 146 (0.4%) | 117 (5%) | 66 (30%) |
| Phosmet | 3.56 | DMP,DMTP, DMDTP |
1,633 (4%) | 611(25%) | 0 (0.02%) |
| Tetrachlorvin phos |
60.69 | DMP | 0 (0%) | 4 (0.2%) | 0 (0%) |
| Trichlorfon | 31.74 | DMP | 0 (0%) | 3 (0.1%) | 0 (0%) |
|
Total Dimethyls |
39,518(100%) | 2,492 (100%) | 223 (100%) | ||
| Diethyls | |||||
| Chlorethoxyphos | 0.65 | DEP, DETP | 83(0.4%) | 0 (0%) | 0 (0%) |
| Chlorpyrifos | 1.48 | DEP, DETP | 9,800 (52%) | 2,119 (64%) | 57 (31%) |
| Diazinon | 6.24 | DEP, DETP | 938 (5%) | 1,006 (30%) | 113 (61%) |
| Dichlorvos (DDVP) |
2.35 | DEP, DETP | 0 (0%) | 12 (0.4%) | 30(0.02%) |
| Disulfoton | 0.07 | DEP, DETP,DEDTP |
418 (2%) | 86 (3%) | 15 (8%) |
| Ethion | N/Ae | DEP, DETP,DEDTP |
530 (3%) | 0 (0%) | 0 (0%) |
| Parathion | N/Ae | DEP, DETP | 10 (0.05%) | 0 (0%) | 0 (0%) |
| Phorate | 0.21 | DEP, DETP,DEDTP |
2,608 (14%) | 101 (3%) | 0 (0%) |
| Sulfotepp | N/Ae | DEP, DETP | 0 (0%) | 0 (0.01%) | 0 (0.05%) |
| Terbufos | 0.1 | DEP, DETP,DEDTP |
4,600 (24%) | 0 (0%) | 0 (0%) |
| Total Diethyls | 18,985 (100%) | 3,324 (100%) | 185 (100%) | ||
| Total OPs | 58,503 | 5,816 | 408 | ||
Average total pounds of OP pesticides used for agricultural (field, fruit and vegetable crops) applications between 1999–2000 based on pesticide purchase by individual farms (EPA Proprietary Data summarized).
Average total pounds of OP pesticides used for agricultural, landscape maintenance, structural pesticide control and right of way between 1999–2000 (DPR 1999, 2000).
Pesticide use is reported in pounds of active ingredient.
BMD10: the benchmark dose with 10% inhibition in brain cholinesterase compared to background response (13).
N/A : U.S. EPA has not established a BMD10. We assume 0 RPF.
Agricultural use of pesticides, however, varies by state and within states. Fruits and vegetables grown in California reach markets across the U.S. with Monterey County ranked first in California in application of commonly used OP pesticides (7). For comparison with our National OP pesticide use profile, we also constructed profiles for California and Monterey County based on California’s Pesticide Use Reporting (PUR) program. In 1999–2000, 408,000 lbs of OP pesticides were used annually in Monterey County and 5,816,000 lbs in California for both agricultural and non-agricultural applications (7).
Cumulative Dose Equivalent Calculations
Similar to prior analyses (8–11), we use a deterministic, steady-state model to calculate cumulative OP dose estimates based upon both non-specific DAP urinary metabolites and chemical-specific OP pesticide metabolites. We apply several assumptions in our dose calculations based on urinary metabolites:
(1) Urinary concentrations are representative of steady-state conditions over a 24-hour period. Under this steady-state assumption, a full day's urinary excretion of metabolites may be estimated based on a spot urine sample using creatinine as an index of total daily urinary output volume. The relationship between 24-hour urine output volume and urinary creatinine is given by the following formula:
| Eq(1) |
where, Vi = expected 24-hour urine output volume for ith individual (L/day); CrExi=estimate for ith individual’s daily creatinine excretion (mg/day); Cci = creatinine concentration in ith individual’s urine sample (mg/L).
For our values of CrEx we apply a gender-specific approach (12) that takes into account an individual’s age, gender, weight and height (see Supporting Information for more details).
(2) 100% of absorbed OP pesticide dose is expressed in urine as OP metabolites, unless otherwise noted (See Table S5).
(3) OP metabolite concentrations are equivalent to internal doses on a molar basis. Because each OP pesticide molecule devolves into exactly one of its possible OP metabolites, the molar sum of metabolite equals the molar concentration of OP pesticide.
Further, we estimate cumulative 24-hour OP pesticide dose equivalents based on metabolite concentrations by adapting the U.S. EPA’s guidelines for cumulative risk assessment of OP pesticides (11). These guidelines define “cumulative risk” as the risk of a common toxic effect associated with concurrent exposure by all relevant pathways and routes of exposure to a group of chemicals that share a common mechanism of toxicity (11). However in our assessment we assume that food is the dominant pathway and that oral ingestion is the main route of exposure. These assumptions are supported by the EPA cumulative assessment for OPs: food pathway and oral route overwhelmed risks estimated for drinking water and residential pathways (11). Also, because many residential uses of OP pesticides are being phased out in the United States, current exposure to OP pesticides for the general population occurs primarily through ingestion of pesticide residues on foods (1, 13). We assume exposure to a mixture of OP pesticides that share a common mechanism of toxicity (brain cholinesterase inhibition) and similar dose-response curves (11). We rely upon U.S. EPA relative potency factors (RPF) to aggregate toxicity across pesticides in our assessment group:
| Eq(2) |
where chemical n = a member of the cumulative assessment group; index chemical = the chemical selected as the basis for standardization of toxicity of components in a mixture; measure of potency = BMD10 (defined below).
To calculate cumulative dose we convert each relevant pesticide’s dose estimate into its index chemical toxicity equivalent using RPFs and sum these values across all pesticides. We use U.S. EPA’s oral benchmark dose (BMD10) values for cholinesterase inhibition in the female rat brain as the measure of potency in our RPF calculations (See Table S4). BMD10 is the benchmark dose for 10% brain cholinesterase inhibition compared with the background response and is used by EPA for its OP cumulative risk assessment (11). We select chlorpyrifos as the index chemical for cumulative dose estimates because: (1) it is a compound in our cumulative assessment group with extensive hazard assessment and dose-response information available; (2) it metabolizes into urinary DAPs; (3) it is commonly used in agriculture; and (4) its measure of relative toxicity, as expressed by the BMD10, falls in the mid-range of BMD10 values for OP pesticides (chlorpyrifos BMD10=1.48 mg/kg/day) (11). Risk estimates based on cumulative dose equivalents are insensitive to the choice of index chemical (i.e. calculating MOEs using another other index chemical to estimate equivalent dose would result in the same MOE).
Non-Specific Metabolite Cumulative Dose Estimates
In our cumulative assessment based on the non-specific metabolites, we assume that the OP exposure mixture is proportional to OP use patterns. The pesticides in our cumulative assessment group are presented in Table 1.
| Eq(3) |
where DCum = cumulative dose equivalent (µg/kg/day); µMolDiEthyl = total micromoles of diethyl DAP metabolites (DEP, DETP, DEDTP) excreted over a 24-hour period (based on Equation 8 in SI); µMolDiMethyl = total micromoles of dimethyl DAP metabolites (DMP, DMTP, DMDTP) excreted over a 24-hour period (based on Equation 9 in SI; Pi = molar proportion of pesticide i in the mixture from pesticide use data (see Table 1) calculated using Equations 10 and 11 in SI; MWi = molecular weight of ith pesticide (µg/µmol); RPFi = relative potency factor of the ith pesticide in the cumulative assessment group; BW = body weight (kg).
Specific Metabolite Cumulative Dose Estimates
In our cumulative assessment group for the specific metabolites we include diazinon (IMPY) and chlorpyrifos (TCPy). We did not include malathion because the metabolite MDA was not measured in the 2001–2002 NHANES. We did not include PNP because it has several possible precursor compounds (e.g. nitrobenzene) in addition to the OP pesticides ethyl and methyl parathion.
| Eq(4) |
where DCum = cumulative dose equivalents (µg/kg/day); µMolMet = total micromoles of metabolite excreted over a 24-hour period (see Equation 1); MWi = molecular weight of ith pesticide (µg/µmol); RPFi = relative potency factor of the ith pesticide in the cumulative assessment group; Exi = percent dose excreted as urinary metabolite of ith pesticide; BW = body weight (kg).
We adjusted each pesticide specific metabolite concentration for the expected percent of absorbed dose excreted as a urinary metabolite (Exi ) (See Table S5) (14, 15, 16, 17, 18, 19). TCPy concentrations were multiplied by 20%, an estimate of the proportion attributable to chlorpyrifos exposures (20, 21).
Cumulative Risk Estimation
To assess cumulative risk we calculate a margin of exposure (MOE), which is the ratio of the BMD10 to the estimated cumulative dose. The MOE is specifically identified by the U.S. EPA Office of Pesticide Programs as an appropriate metric for characterizing risk from exposure to mixtures of OP pesticides (11). Cumulative MOEs are calculated separately for our 3 use profiles (National, California and Monterey) based on the DAPs and for cumulative exposure to chlorpyrifos and diazinon based on their specific metabolites. We calculate MOEs for different demographic groups (by age, race/ethnicity, gender, education, and nativity).
We evaluate the level of limited risk as an MOE of 1,000. The BMD10 represents 10% brain cholinesterase inhibition in adult female rats (11). To estimate an MOE that represents de minimis risk, we consider several adjustment factors. We use a factor of 10 for differences between observed animal responses and typical human responses, and an additional factor of 10 to adjust for variability in the adult population. We also apply a factor of 10 to adjust for potential insensitivities in the experimental design, in particular that the studies used for calculating the BMD10 do not account for potentially more sensitive early life exposure. Experimental data show that 1 mg/kg/day of chlorpyrifos given to rats prenatally and postnatally resulted in noncholinergic effects on brain development, and that the neurologic deficits from prenatal exposure occurred in adolescence and continued into adulthood (22, 23, 24, 25, 26).
Statistical Analysis
National population estimates for DAPs are derived from NHANES 1999–2002. The Supporting Information provides a detailed description of the NHANES datasets and sampling weights used in our analyses.
The measured pesticide metabolites are frequently below their limits of detection (LOD). We used a Monte Carlo substitution method to impute non-detect concentrations: For each age group, a log-normal distribution was fitted to all the data and used to estimate the distribution of the non-detect concentrations. Values for the non-detects were randomly selected from their estimated distributions. The Supporting Information (p S15) provides more details on how we treated values below the LOD.
To compare geometric means between different demographic groups, we used a one-way analysis of variance of the natural logarithms of the survey data.
To account for the complex survey design, p-values were estimated using SUDAAN® (RTI International, Research Triangle Park, NC) statistical survey software’s Taylor linearization and Wald chi-square statistics with Satterthwaite correction for degrees of freedom (REGRESS procedure).
RESULTS
The estimated geometric mean cumulative dose for the overall U.S. population is highest for the Monterey use profile at 1.2 µg/kg/day (Table 2). The estimated cumulative doses based on the California and National profiles are similar to each other. There are statistically significant differences (p-value <0.001) in geometric mean cumulative doses by age across all 3 pesticide use profiles and the corresponding estimated MOEs. Children age 6–11 years have the highest geometric mean cumulative dose estimates at 1.1 µg/kg/day for the California profile, 2.3 µg/kg/day for the Monterey profile, and 1.0µg/kg/day for the National profile, and experience the lowest corresponding MOEs at 1400 for the California profile, 650 for the Monterey profile, and 1500 for the National profile.
Table 2.
Geometric means of estimated OP Pesticide Cumulative Dose and Margins of Exposure by Age Based on non-specific DAP metabolites NHANES 1999–2002
| N | Cumulative Dose µg/kg/day |
Margins of Exposure | |||||
|---|---|---|---|---|---|---|---|
| California Use Profile |
Monterey County Use Profile |
National Use Profile |
California Use Profile |
Monterey County Use Profile |
National Use Profile |
||
| Total age 6 and older |
4,287 | 0.55 | 1.2 | 0.59 | 2700 | 1300 | 2500 |
| Age group | |||||||
| 6 –11 | 1,018 | 1.1** | 2.3** | 1** | 1400** | 650** | 1500** |
| 12 – 19 | 1,408 | 0.55** | 1.2** | 0.55** | 2700** | 1300** | 2700** |
| 20 + | 1,861 | 0.5** | 1** | 0.55** | 3000** | 1400** | 2700** |
statistically significant differences by age group at 5% level based on Satterthwaite-adjusted chi-square test
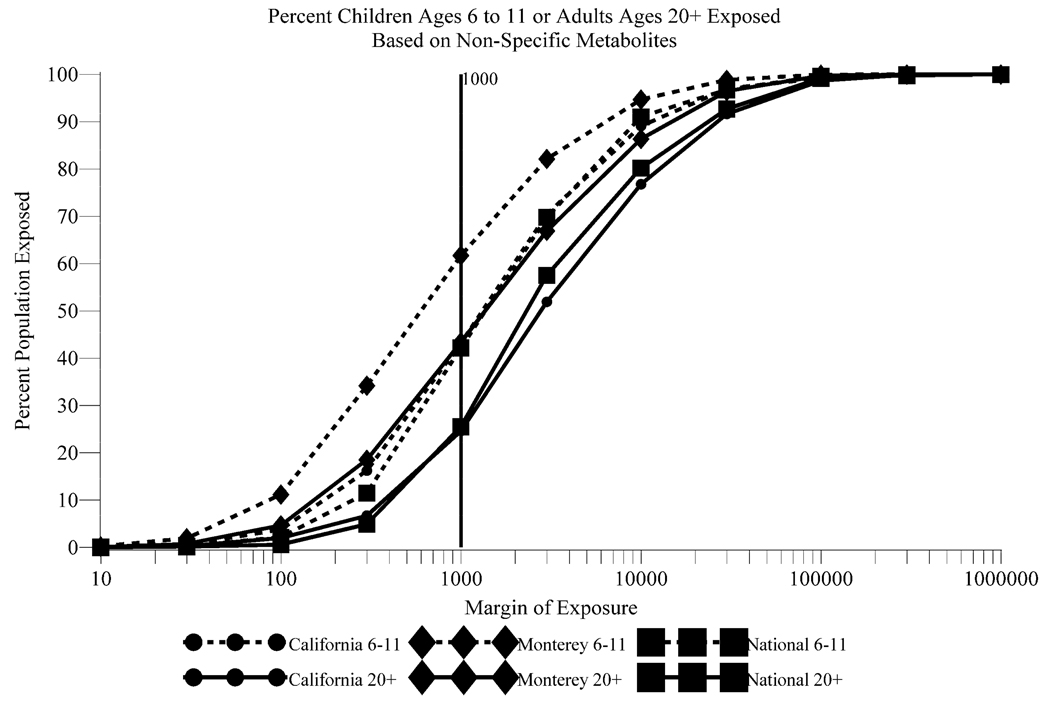
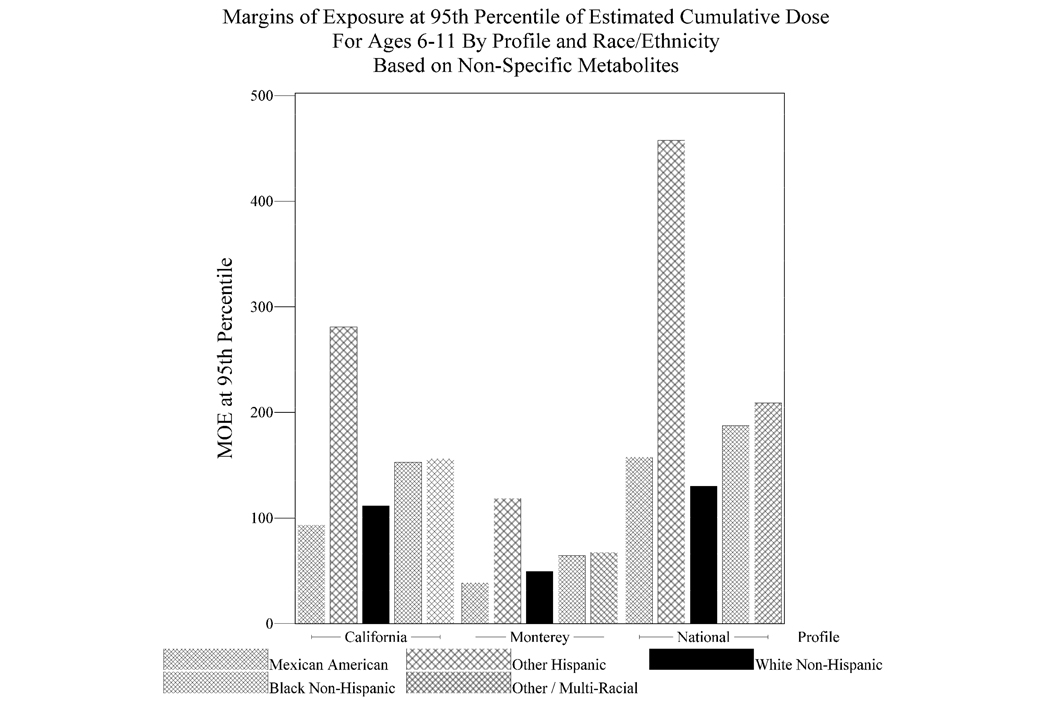
A higher percentage (62%) of children have MOEs of 1,000 or less for the Monterey pesticide use profile compared to California (43%) and National (42%) use profiles (Figure 1). At the 95th percentile cumulative dose equivalents, the MOE for children is less than 100 for the Monterey profile (Figure S2). The differences between MOEs among children and adults at the higher dose equivalent levels are greater for both the National and California use profiles compared to the Monterey profile. These MOEs indicate that children have about 2 times higher risk than adults at the high end of estimated cumulative exposure to OP pesticides. Among children, Mexican Americans and white non-Hispanics have the lowest MOEs across all 3 OP use profiles (Figure 2).
Figure 1.
Margins of Exposure by Use Profile for Children Ages 6 to 11 and Adults Ages 20+ Based on Estimates of Cumulative Exposure to OP Pesticides
Figure 2.
Margins of Exposure at 95th Percentile of Estimated Cumulative Dose for Ages 6–11 by Profile and Race/Ethnicity Based on Non-Specific Metabolites
The geometric mean cumulative dose estimate based on 2 pesticide specific metabolites for chlorpyrifos and diazinon for the U.S. population age 6 years and older is 0.021 µg/kg/day with a population MOE of 70,000. There are statistically significant differences by age (p-value <0.001), gender (p-value 0.011), education (p-value <0.001), and place of birth (p-value 0.004). Consistent with our analysis of the nonspecific metabolites, children age 6–11 years have the highest estimated geometric mean cumulative dose at 0.039 µg/kg/day compared with 0.019 for adults, age 20 + years. At the 95th percentile of estimated cumulative dose equivalents, children have lower MOE (at 6,900) as compared to adults (at 15,000) (Tables S10 and S11).
DISCUSSION
Biomonitoring is a valuable tool for identifying exposures to chemicals that pose potential harm to human health, understanding trends in exposures and identifying populations with higher exposures, fostering public health interventions, and evaluating environmental health policies (2). Biomonitoring can provide unequivocal evidence that exposures and uptake have occurred, though alone it cannot tell you the sources of exposure. However, a health-based or risk-based interpretation of biomonitoring data depends on whether additional information is available on sources and pathways of exposure and pharmacokinetics to aid in reconstructing intake or internal doses of parent chemical compounds. In addition, the non-specificity of the DAP metabolites of the OP pesticides adds another layer of complexity to dose reconstruction compared with other NHANES urinary metabolites. We developed agricultural pesticide use profiles to approximate the potential mixtures of OP pesticides to which NHANES participants are exposed. We combine this information with toxicological benchmarks and OP pesticide biomonitoring results from NHANES 1999–2002 to estimate cumulative dose equivalents and risk. Our deterministic, steady-state model results show that children’s cumulative exposure and risk estimates are most often higher than those of adults. This is consistent with studies reporting age differences in levels of the individual pesticide urinary metabolites (27, 28).
Notably few studies have estimated cumulative dose based on DAPs and OP-specific metabolites for children ages 6–11 as we have in our study. As part of our analysis, we also estimate single chemical doses for each of the 22 OPs that form the 3 pesticide use profiles (Tables S8 and S9). Our dose estimates for chlorpyrifos, oxydemeton-methyl, phosmet and malathion fall within the range of, or are lower than, previously reported estimated pesticide doses for children based on nonspecific DAP and pesticide-specific metabolite data. For example, Barr et al. used NHANES 1999–2000 data for TCPy to estimate a chlorpyrifos median dose for children at 0.024 µg/kg/day and 95th percentile at 0.06 µg/kg/day (20). Based on NHANES 1999–2002 data, our geometric mean chlorpyrifos dose for children ages 6–11 (Table S9) is 0.032 µg/kg/day, which is comparable to Barr et al.
A 1995 study of children living in an agricultural community estimated median phosmet intake of 0.5 µg/kg/day for 26 reference children(10) similar to our geometric mean phosmet estimate of 0.31 µg/kg/day for children 6–11 years. A 2001 study of preschool children in Washington State estimated mean doses of 2.2 µg/kg/day for oxydemeton-methyl, 2.8 µg/kg/day for phosmet, and 2.3 µg/kg/day for malathion for children with conventional diets(9) - substantially higher than our national estimates for school age children. This might be due to different approaches for dose estimation, as the Washington study used volume adjustment instead of creatinine adjustment, and differences in exposure levels, as DAP levels appear higher in this population than in the general U.S. population (9).
Studies focusing on pesticide exposures of farm children and farmworker children offer some comparisons as well. Geometric mean chlorpyrifos dose, based on TCPy from spot urine samples collected in 2001, was estimated at 0.67 µg/kg/day for 66 farm children and 0.58 µg/kg/day for 52 nonfarm children (29). Chlorpyrifos doses were likely overestimated as a result of direct exposure to TCPy (29). For 121 children less than 6 years of age in a heavily farmed U.S./Mexico border community, dose estimates ranged from 2.43–12 µg/kg/day for chlorpyrifos; from 2.11–11.3 µg/kg/day for diazinon; and from 7.98–121 µg/kg/day for malathion (30). A study of farmworker children during spray season in Washington state estimated median dose for phosmet of 1.5 – 2.0 µg/kg/day (10). Our estimates are based on a nationally representative sample of children age 6–11 years old, whereas these 3 studies focused on children who are likely to be more highly exposed via multiple pathways of exposure (children of farmworkers and/or pesticide applicators).
Our cumulative dose estimates based on specific metabolites only consider diazinon and chlorpyrifos. We can estimate a comparable cumulative dose based on the non-specific metabolites if we restrict the calculations to just DEP and DETP for diazinon and chlorpyrifos. Chlorpyrifos and diazinon together represent 53%, 42%, and 17% of the OP pesticides used in California, Monterey County, and nationally. Our geometric mean cumulative dose estimates of 0.034 µg/kg/day for children 6–11 and 0.02 µg/kg/day for the general U.S. population based on the National use profile are similar to the geometric mean cumulative dose results based on the NHANES OP specific metabolites TCPy and IMPY (0.039 and 0.021 µg/kg/day, respectively) suggesting that our method for interpreting nationally representative pesticide biomonitoring results is reasonable.
The unique aspect of our study is the cumulative dose and risk analysis, which represents estimates of exposure to multiple OP pesticides combined. Higher percentages of children have MOEs less than 1,000 compared with adults. We compare the MOEs to the value of 1,000, which implies a 1,000-fold uncertainty factor applied to the BMD10. It is important to ensure that children have adequate MOEs as recent studies have linked prenatal OP pesticide exposure with adverse neurodevelopment effects in children (31) as well as reported human evidence suggesting that newborns may be more susceptible to the adverse effects of specific OP pesticide exposure than adults (32, 33).
Our estimates of cumulative exposure and risk are based on biomonitoring data from 1999–2002. Exposures and risks may have been reduced through local and national mitigation efforts (e.g. use cancellations) since then (11). Our results suggest that these mitigation efforts to reduce exposure to OPs are warranted.
Limitations
Our assumptions for dose calculation and risk estimation introduce uncertainty related to likely precursor pesticide compounds and sources of exposure, intra-individual and temporal variability, and the use of creatinine to estimate 24-hour urinary metabolite excretion. To back-calculate dose based on non-specific urinary metabolite data, we require estimates of the mixture of precursor compounds. For our cumulative dose analyses, we use national level and California agricultural pesticide use reports, reflecting the same time period when the NHANES data were collected, to develop three OP pesticide exposure profiles. We assume that these pesticide profiles are applicable to NHANES participants and that the main route of exposure to OP pesticides is through food.
National- and state-level purchase records for pesticides are a rough surrogate for the proportion of exposure to different OPs as application patterns vary regionally, by crop, between urban and rural setting, and seasonally. Further, the proportion of pesticide applied in the field that will remain as residue on the food as consumed will also vary by crop, by pesticide and by other factors. The proportions from the use profiles may not be representative of the OP exposure mixture for any particular individual and may vary by age of the individual, but serve as an approximation of the proportions of exposure for the US population. A more complex approach, with its own uncertainties, could be to identify an OP mixture based on combined data from national food consumption surveys such as USDA's Continuing Survey of Food Intakes by Individuals and pesticide residue data from USDA's Pesticide Data Program. However, these datasets do not cover all food commodities of interest and the commodities sampled for pesticide residues vary year to year (11). The similarity of our estimates of cumulative exposure for diazinon and chlorpyrifos based on non-specific metabolites to those based on specific metabolites suggests our approach for interpreting national level pesticide biomonitoring data is reasonable.
There could be sources of OP pesticide exposure other than food among the NHANES participants that are not reflected in our assumptions. For example, chlorpyrifos and diazinon residues have been detected in house dust long after indoor residential use was banned (34). Contact with residues in house dust likely contributes to exposure. However, a small study of preschool children (during 2000 and 2001 around the time of the ban on residential use of chlorpyrifos) measured TCPy in both urinary samples and in environmental media (food, dust, air), and concluded that the primary route of exposure to chlorpyrifos was dietary intake (35). Given the available data, our approach is reasonable for evaluating national-scale biomonitoring data on OP metabolites.
In addition to reflecting exposure to precursor pesticides, the level of the metabolite in urine may reflect direct exposure to the metabolite itself, if the metabolite is present in environmental media. We adjusted our pesticide-specific metabolite analysis to reflect findings that 20% of the urinary TCPy is attributable to chlorpyrifos exposure (21). Similar data are not currently available for other urinary metabolites in our study, and therefore those daily dose calculations could be overestimates.
In estimating daily dose we assume that metabolite measurements from a spot urine sample describe chronic exposure. We assume that the input (exposure) and the output (excreted metabolites) are occurring at a constant rate and are equal on a molar basis over a 24-hour period. This is a simplification of both the temporal variability of exposure and the metabolism of these compounds. Our default assumption that 100% of absorbed OP pesticide dose is excreted in urine as nonspecific DAP metabolites may underestimate dose because some metabolites will be excreted in other biologic media (e.g., feces). Although the kinetics of elimination vary among the DAPs, toxicological evidence suggests that the metabolites of many OP compounds are excreted primarily, but not exclusively, in the urine (36, 37).
Adjusting metabolite concentration data for estimated total daily urine volume was necessary because NHANES provides only spot urine samples. Several limitations of using creatinine adjustment in biological monitoring have been reported. For young children there are no standardized creatinine reference values (24, 21, 29, 38, 39, 40). An alternative to creatinine adjustment would be to generate cumulative dose estimates by adjusting for total daily urine volume based on reference values for children. Given results from other studies, we expect that dose estimates derived from volume-adjusted data would be highly correlated with, and slightly higher than, the creatinine adjusted dose estimates presented here (8, 10).
We only focus on the OPs that have a common mechanism of action. But recent studies show that pesticides with different mechanisms of action can have an additive neurological effect (41, 42), and as such we may underestimate risk from interaction of OPs with other neurotoxins.
Children encounter pesticides daily through normal activities such as eating, playing outdoors and indoors where pesticides may have been applied, and frequent hand-to-mouth contact. Children are more susceptible to the health effects associated with OP pesticide exposure than adults because children’s nervous systems are rapidly developing. Further, pound for pound, children generally eat more than adults, and they may be exposed more heavily to certain pesticides because they consume a diet different from that of adults (43). Understanding how and at what levels children are exposed becomes critical for implementing health protective policies and interventions. Biomonitoring initiatives, such as NHANES, provide important information on exposure to contaminants such as OP pesticides. Our results suggest that during 1999–2002 approximately 40% of children in the United States may have had insufficient MOEs (MOE less than 1,000) for cumulative exposures to OP pesticides .Our methods and results may be informative for comparison with models of cumulative exposure based on concentrations of OP pesticides in environmental media and as an approach to use biomonitoring data to assess whether policies to mitigate pesticide exposures are successful.
Supplementary Material
ACKNOWLEDGMENT
We thank EPA’s Office of Children’s Health Protection for its support to conduct the NHANES biomonitoring data analysis . We appreciate the assistance of EPA’s Cynthia Doucure, Arthur Grube and David Widawsky in providing pesticide use data. We appreciate comments from Anna Lowit, David Miller and David Hrdy from EPA’s Office of Pesticide Programs.
R. Castorina was supported in part by U.S. EPA grant RD 83171001 and NIEHS grant PO1 ES009605.
The views expressed in this document are those of the authors and do not represent official U.S. Environmental Protection Agency policy.
Footnotes
Supporting Information Available
Background information on OP pesticides, NHANES, details of assumptions and methods (e.g. creatinine excretion equations) for estimating single and cumulative doses as well as summary of dose estimate results. This information is available free of charge via the Internet at http://pubs.acs.org.
LITERATURE CITED
- 1.National Center for Environmental Health, Division of Laboratory Sciences. Atlanta, Georgia: Centers for Disease Control and Prevention; National Report on Human Exposure to Environmental Chemicals. 2005
- 2.National Research Council. Washington, DC: The National Academies Press; Human Biomonitoring for Environmental Chemicals. 2006
- 3.Landrigan PJ, Schechter CB, Lipton JM, Fahs MC, Schwartz J. Environmental pollutants and disease in American children: estimates of morbidity, mortality, and costs for lead poisoning, asthma, cancer, and developmental disabilities. Environ. Health. Perspect. 2002;110(7):721–728. doi: 10.1289/ehp.02110721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD. The decline in blood lead levels in the United States: The National Health and Nutrition Examination Surveys (NHANES) JAMA. 1994;272(4):284–291. [PubMed] [Google Scholar]
- 5.U.S. Environmental Protection Agency. Washington, DC: Office of Prevention, Pesticides, and Toxic Substances; Pesticide Industry Sales and Usage—2000 and 2001 Market Estimates. 2004
- 6.National Research Council. Washington, DC: National Academy of Sciences Press; Pesticides in the Diets of Infants and Children. 1993
- 7.California Department of Pesticide Regulation Pesticide Use Reports. Available at http://www.cdpr.ca.gov/docs/pur/purmain.htm.
- 8.Castorina R, Bradman A, McKone T, Barr DB, Harnly ME, Eskenazi B. Cumulative organophosphate pesticide exposure and risk assessment among pregnant women living in an agricultural community: a case study from the CHAMACOS cohort. Environ. Health. Perspect. 2003;111(13):1640–1648. doi: 10.1289/ehp.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curl CL, Fenske RA, Elgethun K. Organophosphorus pesticide exposure of urban and suburban preschool children with organic and conventional diets. Environ. Health. Perspect. 2003;111(3):377–382. doi: 10.1289/ehp.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenske RA, Kissel JC, Lu C, Kalman D, Simcox NJ, Allen E. Biologically based pesticide dose estimates for children in an agricultural community. Environ. Health. Perspect. 2000;108(6):515–520. doi: 10.1289/ehp.00108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Environmental Protection Agency. Washington, DC: Office of Pesticide Programs; Organophosphorus Cumulative Risk Assessment 2006 Update. 2006 Available at http://www.epa.gov/pesticides/cumulative/2006-op/index.htm.
- 12.Mage DT, Allen RH, Kodali A. Creatinine corrections for estimating children’s and adult’s pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J. Expo. Sci. Environ. Epidemiol. 2008;18:360–368. doi: 10.1038/sj.jes.7500614. [DOI] [PubMed] [Google Scholar]
- 13.Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R. Organic diets significantly lower children's dietary exposure to organophosphorus pesticides. Environ. Health. Perspect. 2006;114(2):260–263. doi: 10.1289/ehp.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garfitt S, Jones K, Mason H, Cocker J. Exposure to the organophosphate diazinon: data from a human volunteer study with oral and dermal doses. Toxicol. Lett. 2002;134(1–3):105–113. doi: 10.1016/s0378-4274(02)00178-9. [DOI] [PubMed] [Google Scholar]
- 15.Koch HM, Hardt J, Angerer J. Biological monitoring of exposure of the general population to the organophosphorus pesticides chlorpyrifos and chlorpyrifos-methyl by determination of their specific metabolite 3,5,6-trichloro-2-pyridinol. Int. J. Hyg. Environ. Health. 2001;204(2–3):175–180. doi: 10.1078/1438-4639-00082. [DOI] [PubMed] [Google Scholar]
- 16.Krieger RI, Bernard CE, Dinoff TM, Ross JH, Williams RL. Biomonitoring of persons exposed to insecticides used in residences. Ann. Occup. Hyg. 2001;45 Suppl 1:S143–S153. doi: 10.1016/s0003-4878(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 17.Nolan RJ, Rick DL, Freshour NL, Saunders JH. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol. Appl. Pharmacol. 1984;73(1):8–15. doi: 10.1016/0041-008x(84)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Dong MH, Ross JH, Thongsinthusak T, Krieger RI. Use of spot urine sample results in physiologically based pharmacokinetic modeling of absorbed malathion doses in humans. In: Blancato JN, editor. Biomarkers for Agrochemicals and Toxic Substances: Applications and Risk Assessment. Washington, DC: American Chemical Society; 1996. [Google Scholar]
- 19.Ross JH, Thongsinthusak T, Krieger RI, Frederickson S, Fong HR, Taylor S, Begum S, Dong MH. Sacramento, CA: Worker Health and Safety Branch, California Department of Pesticide Regulation; Human Clearance of Malathion. 1991 Technical Report HS-1617.
- 20.Barr DB, Allen R, Olsson AO, Bravo R, Caltabiano LM, Montesano A, Nguyen J, Udunka S, Walden D, Walker RD, Weerasekera G, Whitehead RD, Schober SE, Needham LL. Concentrations of selective metabolites of organophosphorus pesticides in the United States population. Environ. Research. 2005;99:314–326. doi: 10.1016/j.envres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Wilson NK, Chuang JC, Lyu C, Menton R, Morgan MK. Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home. J. Expo. Anal. Environ. Epidemiol. 2003;13(3):187–202. doi: 10.1038/sj.jea.7500270. [DOI] [PubMed] [Google Scholar]
- 22.Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol. Appl. Pharmacol. 1997;145(1):158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- 23.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004;198(2):132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Meyer A, Seidler FJ, Aldridge JE, Tate CA, Cousins MM, Slotkin TA. Critical periods for chlorpyrifos-induced developmental neurotoxicity: alterations in adenylyl cyclase signaling in adult rat brain regions after gestational or neonatal exposure. Environ. Health. Perspect. 2004;112(3):295–301. doi: 10.1289/ehp.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lassiter TL, Padilla S, Mortensen SR, Chanda SM, Moser VC, Barone S., Jr Gestational exposure to chlorpyrifos: apparent protection of the fetus? Toxicol. Appl .Pharmacol. 1998;152(1):56–65. doi: 10.1006/taap.1998.8514. [DOI] [PubMed] [Google Scholar]
- 26.Hunter DL, Lassiter TL, Padilla S. Gestational exposure to chlorpyrifos: comparative distribution of trichloropyridinol in the fetus and dam. Toxicol. Appl. Pharmacol. 1999;158(1):16–23. doi: 10.1006/taap.1999.8689. [DOI] [PubMed] [Google Scholar]
- 27.Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Jr, Olsson AO, Caudill SP, Schober SE, Pirkle JL, Sampson EJ, Jackson RJ, Needham LL. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ. Health. Perspect. 2004;112(2):186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adgate JL, Barr DB, Clayton CA. Measurement of children’s exposure to pesticides: analysis of urinary metabolite levels in a probability-based sample. Environ. Health. Perspect. 2001;109(6):583–590. doi: 10.1289/ehp.01109583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, Reynolds SJ, Alavanja MC. Pesticide dose estimates for children of Iowa farmers and non-farmers. Environ. Research. 2007;105(3):307–315. doi: 10.1016/j.envres.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 30.O’Rourke MK, Lizardi PS, Rogan SP, Freeman NC, Aguirre A, Saint CG. Pesticide exposure and creatinine variation among young children. J. Expo. Anal. Environ. Epidemiol. 2000;10:672–681. doi: 10.1038/sj.jea.7500119. [DOI] [PubMed] [Google Scholar]
- 31.Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, Johnson C, Fenster L, Barr DB. Pesticide toxicity and the developing brain. Basic. Clin. Pharmacol. Toxicol. 2008;102(2):228–236. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 32.Furlong CE, Holland N, Richter RJ, Bradman A, Ho A, Eskenazi B. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenet. Genomics. 2006;16(3):183–190. doi: 10.1097/01.fpc.0000189796.21770.d3. [DOI] [PubMed] [Google Scholar]
- 33.Holland N, Furlong C, Bastaki M, Richter R, Bradman A, Huen K, Beckman K, Eskenazi B. Paraoxonase polymorphisms, haplotypes, and enzyme activity in Latino mothers and newborns. Environ. Health. Perspect. 2006;114(7):985–991. doi: 10.1289/ehp.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stout DM, Bradham KD, Egeghy P, Jones PA, Croghan CW, Ashley P, Pinzer E, Friedman W, Brinkman MC, Nishioka MG, David CC. American Healthy Homes Survey: a national study of residential pesticides measured from floor wipes. Environ. Sci. Technol. 2009;43(12):4294–4300. doi: 10.1021/es8030243. [DOI] [PubMed] [Google Scholar]
- 35.Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, Wilson NK, Lyu CW. Exposures of pre-school children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J. Expo. Anal. Environ. Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- 36.Griffin P, Mason H, Heywood K, Cocker J. Oral and dermal absorption of chlopyrifos: a human volunteer study. Occup. Environ. Med. 1999;56(1):10–13. doi: 10.1136/oem.56.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieger RI, Dinoff TM. Malathion deposition, metabolite clearance, and cholinesterase status of date dusters and harvesters in California. Arch. Environ. Contam. Toxicol. 2000;38(4):546–553. doi: 10.1007/s002449910071. [DOI] [PubMed] [Google Scholar]
- 38.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am. Ind. Hyg. Assoc. J. 1993;54(10):615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 39.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health. Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mage DT, Allen R, Gondy G, Smith W, Barr DB, Needham LL. Estimating pesticide dose from pesticide exposure data by creatinine correction in the third National Health and Nutrition Examination Survey (NHANES-III) J. Expo. Anal. Environ. Epidemiol. 2004;14(6):457–465. doi: 10.1038/sj.jea.7500343. [DOI] [PubMed] [Google Scholar]
- 41.Slotkin TA, Bodwell BE, Ryde IT, Levin ED, Seidler FJ. Exposure of neonatal rats to parathion elicits sex-selective impairment of acetylcholine systems in brain regions during adolescence and adulthood. Environ. Health. Perspect. 2008;116(10):1308–1314. doi: 10.1289/ehp.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cory-Slechta DA, Thiruchelvam M, Barlow BK, Richfield EK. Developmental pesticide models of the parkinson disease phenotype. Environ. Health. Perspect. 2005;113(9):1262–1270. doi: 10.1289/ehp.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Environmental Protection Agency. Washington, DC: National Center for Environmental Economics; America’s Children and the Environment. 2003
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.