Abstract
Nociceptive, parasympathetic and sympathetic nerves play critical roles in regulating glandular, vascular and other processes in airway mucosa. These functions are vital for cleaning and humidifying ambient air before it is inhaled into the lungs. Recent identification of subsets of nociceptive nerves has tipped the donkey cart of dogma and led to the discovery of new receptor and ion channel families that respond to culinary odorants (“aromatherapy”), inhaled irritants, temperature and other “humors”; a new interpretation of airway nociceptive nerve axon responses; and an understanding of the neural plasticity induced by inflammation and different neurotrophic factors.
Nasal Mechanics
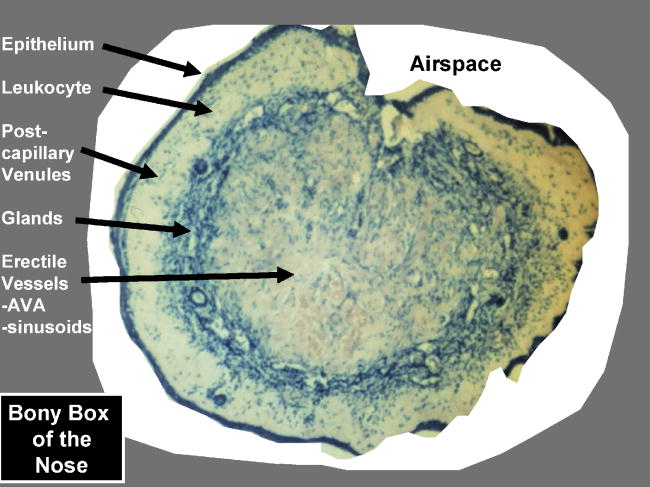
The nose plays vital functions in cleaning and humidifying inhaled air. These processes are under strict physiological control that depends on local mucosal feedback systems, sensory and autonomic reflexes. The functional structures of the nasal cavity are the walls of the anterior nasal vestibule, inferior and other turbinates, and the regulatory engine of the nasal mucosa (Figure 1).
Figure 1.
Cross-section of inferior nasal turbinate within the bony confines of the nose. The concentric structural layers of nasal tissues are shown. The cross-sectional area of the airspace determines nasal airflow.
The mucosa is covered by a 10- to 15-μm deep layer of mucus [1]. One to two liters are secreted per day. It consists of 2.5% to 3% glycoproteins, 1% to 2% salts, and 95% water. The fluid forms a low viscosity sol phase that envelops the cilia, and its superficial covering of the polysaccharide-rich mucin gel phase. The sol layer has a pH of 5.5 to 6.5 and contains many antimicrobial proteins secreted from submucosal glands, and plasma proteins that are extravasated from the post-capillary venules of the superficial lamina propria. Humidification occurs by evaporation of water from the sol phase. This action combined with autoregulation of superficial blood flow maintains the nasal air temperature at about 30°C regardless of the inhaled temperature or absolute humidity. The sol phase also adsorbs water soluble volatile organic compounds (VOCs) such as formaldehyde.
The cilia beat at about 1,000 times per minute. This transports that surface materials at a rate of 3 to 25 mm per minute [2,3]. The cilia are embedded in “islands” of cross-linked, highly viscous and adhesive mucins. Many other proteins are also incorporated into these plaques. Inhaled particles are removed when inhaled air passes through the anterior nasal valve (cross sectional area of 30 to 40 mm2) and around the inferior turbinate [4]]. The anterior nasal valve contributes about half of the total resistance for airflow to the lungs. High speed laminar airflow through the valve at 12 to 18 m/sec is converted to turbulent, low speed (2 to 3 m/sec) flow in the wider diameter region between the septum and inferior turbinate. These conditions increase the contact between suspended particulate materials and the sticky mucus lining. The deposited particulate is transported via the mucociliary escalator anteriorly from the first centimeter of the nasal cavity, and posteriorly to the nasopharynx so it can be swallowed. This process removes nearly 100% of particles > 4 μm in diameter before the air enters the posterior pharynx.
Post-capillary venules
Beneath the epithelial basement membrane are fenestrate capillaries and post-capillary venules [5-7]. The latter are important sites for regulation of vascular extravasation and leukocyte adhesion. Many inflammatory mediators have been shown to have receptors on the endothelium of these vessels including bradykinin and neurokinins. Edema occurs as a pathological event, but the changes in mucosal thickness are small and require microstereometry to be measured [8]. Plasma flux provides the water required for air humidification. Superficial blood flow is insufficient to continuously heat inspired cold air. Instead, exhaled air saturated with water vapor at body temperature passes of the relatively cooler nasal surface leading to condensation of water and mucosal warming. This countercurrent recycling mechanism may recover as much at a third of the water and energy expended in conditioning inhaled ambient air [9].
Submucosal glands
Tubuloalveolar glands form the 3rd concentric ring of the turbinate (Figure 2). Invaginating ducts lead to centrally placed mucous cells that secretion mucin 5B, and peripheral mucous serous “demilunes” that form the distal ends of the secretory apparatus. The serous cells express polymeric immunoglobulin receptors (receptors for J-chains, secretory component) on their interstitial surfaces [10]. These bind locally produced mucosal IgA dimers (connected by J-chains) and transport them through the serous cell by pinocytosis. Lysozyme and secretion IgA (sIgA) account for about 14% each of the total protein in nasal lavage fluid [11]. Lactoferrin, secretory leukocyte protease inhibitor (SPLI), members of the lipocalin and subfamily of palate, lung, upper airway, nasal clone (PLUNC) proteins [12], other antimicrobial proteins, “neutral” mucin 8, and uric acid [13] are other serous cell products.
Figure 2.
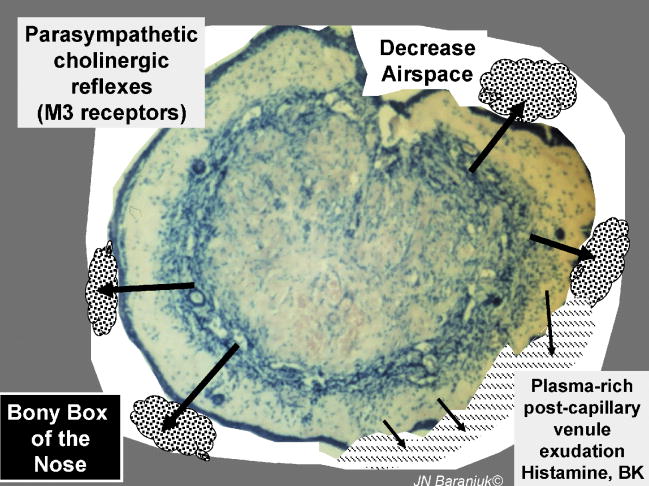
Secretory processes. The submucosal glands contribute about two – thirds of the total protein in nasal lavage fluid. Hypersecretion may lead to filling of narrow spaces with mucoclots or heavy rhinorrhea resulting in decreased airflow. Copious watery rhinorrhea are common in allergic rhinitis (histamine) and the early phase of the common cold. Bradykinin and possibly arachidonic acid metabolites can also stimulate vascular permeability and plasma exudation.
Parasympathetic reflexes and acetylcholine have the greatest effects on gland cell exocytosis in both normal and pathological conditions [11]. The effects are mediated predominantly by muscarinic M3 receptors on gland cells [14]. The gland acini are surrounded by myoepithelial cells that are contracted by factors such as endothelin in order to force the tooth paste – like exocytosed material into the duct lumen.
Water and ion flux is carefully controlled and leads to hydration of the mucins and generation of the less viscous sol phase [15]. CFTR and aquaporins may play key roles in regulating these processes. Dysfunction in cystic fibrosis leads to an hypertonic sol phase, low water flux, the inability to hydrate mucins, and generation of highly tenacious, obstructive mucoclots. Pathological glandular hypertrophy has been reported as a subtype of rhinosinusitis [16], and may be the active process leading to “turbinate hypertrophy” and chronic nasal obstruction.
The nasal erectile apparatus
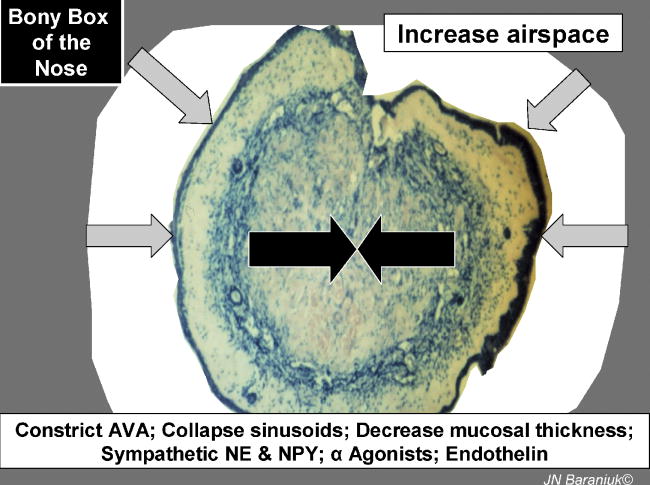
Deep to these structures are the arteriovenous anastomoses, expandable venous sinusoids, and throttle veins that regulate the efflux of blood out of sinusoids [5-7]. Constriction of the anastomoses combined with coordinated relaxation of the throttle veins and elastic recoil of mucosal tissue leads to expulsion of blood from the nasal mucosa (Figure 3). This decreases turbinate diameter (periosteal to epithelial mucosal thickness) and increases the cross – sectional area for airflow through the nasal cavity. The state of patency is normally induced by sympathetic nervous system release of norepinephrine and neuropeptide tyrosine (NPY). All subtypes of α1 and α2 adrenergic receptors are expressed on these vessels, although α2C receptors may be the most important for vasoconstriction [17]. NPY receptors are localized to the anastomoses [18]. α-Agonists such as oxymetazoline, zylometazoline, pseudoephedrine, and cocaine have been important for their medical and surgical vasoconstrictor properties. The role of endogenous vasoconstrictors such as endothelin are not understood.
Figure 3.
Vasoconstriction of deep venous sinusoids leads to nasal decongestion.
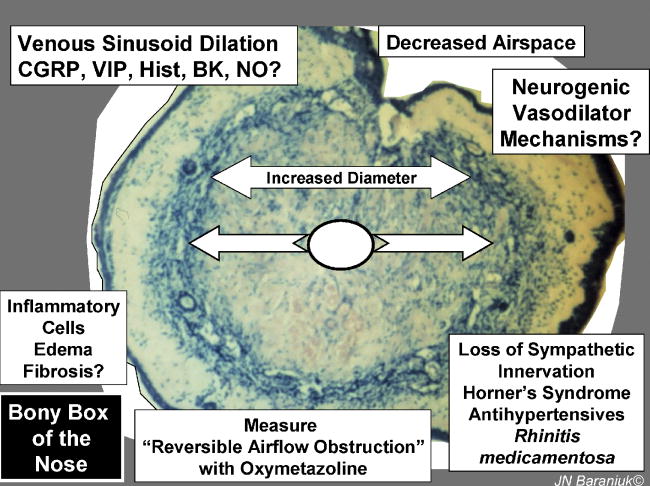
Disruption of sympathetic pathways leads to obstinate vasodilation of the deep sinusoidal structures, mucosal thickening, and the loss of nasal patency (Figure 4). The disruption may be due to stroke and nerve damage as in Horner's syndrome, antihypertensives that block α-receptors and prevent vasoconstriction, and chronic overuse of nasal sympathomimetics that may lead to severe rebound vasodilation and nasal congestion. Many inflammatory mediators such as bradykinin, prostaglandins and leukotrienes have the potential to dilate these deep vessels. Calcitonin gene – related peptide (CGRP) receptors are localized to arteriovenous anastomoses [19]. CGRP is responsible for the cutaneous flare response and is potently obstructs the nose in the pig model [20]. However, neural or other pathways leading to CGRP release in the anastomoses have not been traced to date.
Figure 4.
Dilation of the deep vascular and nasal obstruction.
Nasal cycle
The caliber of the left and right nasal cavities alternate on a 50 min to 4 hr cycle in at least 80% of the population [21]. Total nasal airway cross section and resistance is maintained as one nostril vasodilates and the other vasoconstricts [22]. This endogenous circadian rhythm is poorly understood. It is likely under autonomic control, and can be altered by medications and its effects magnified by allergic, infectious and nonallergic conditions that lead to increased thickness of the lamina propria and mucous secretions.
Nociceptive nerves
Trigeminal neural responses to inhaled irritants have been examined using carbon dioxide, mannitol, adenosine, hypertonic saline (HTS) and other nociceptive agents. Responses to HTS will be described here. Other stimuli are likely to act via parallel or redundant mechanisms.
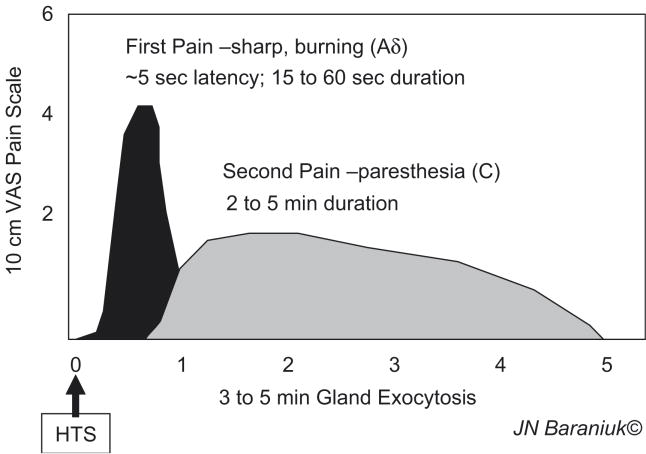
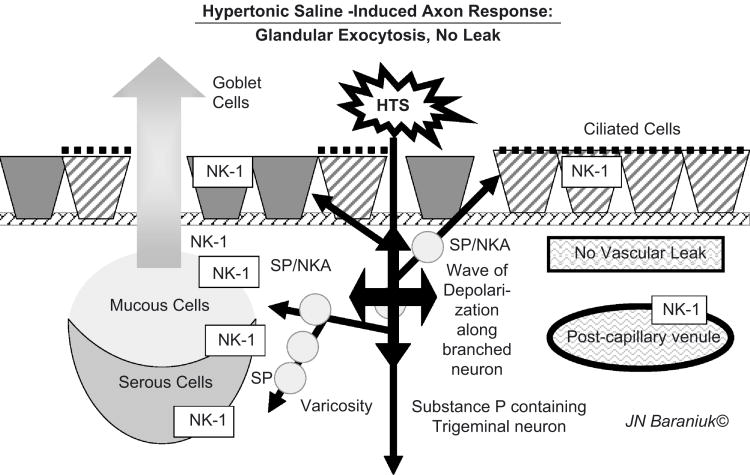
Application of HTS to the inferior turbinate nasal mucosa leads to pain with 2 components. “First pain” has a rapid onset, and sharp burning sensation (Figure 5) [23]. The speed of onset and rapid recovery indicate activation of fast conducting, thinly myelinated Aδ nerve fibres. This was followed by a Type C neuron mediated, slow onset, prolonged paresthetic, tingling sensation. Both the intensity of the 1st pain and duration of the 2nd pain were dependant on the HTS dose [24]. Analysis of nasal lavage fluids found substance P release but little secretion in the 1st 3 minutes. There was robust, dose – dependent glandular exocytosis from 3 to 5 minutes. Ipratropium had not effect indicating that central parasympathetic reflexes were not recruited. Vascular permeability was not stimulated by HTS in normal, acute allergic rhinitis, or acute / chronic rhinosinusitis subjects [25]. Changes in acoustic rhinometry could be related to the volume of increased glandular secretion, suggesting that vascular changes were minimal [26]. Substance P – preferring NK1 receptors were localized to gland acini suggesting that local axon response – mediated substance P release near glands may have preceded and been responsible for the glandular exocytosis [24]. The absence of albumin and IgG secretion was consistent with the relative paucity of tachykinin innervation of the superficial vascular plexus and post-capillary venules [27]. This suggested that HTS – induced axon responses did not involve significant neuropeptide release near these vessels or the stimulation of endothelial cells with plasma exudation. Instead, the human axon response to HTS was copious glandular exocytosis (Figure 6). This rapid onset mucosal defense mechanism would flood the nasal epithelial lining fluid with gel and sol components to trap inhaled irritants and particulate material.
Figure 5.
Time course of 1st and 2nd pain following hypertonic saline administration.
Figure 6.
Hypertonic Saline – Induced Nociceptive Nerve Axon Responses.
Neurone diversity
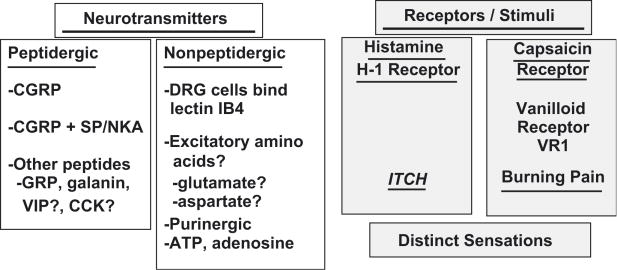
This response to HTS is unlikely to be the only local neural response in the airway mucosa. It is now clear that Type C and Aδ neurons respond to irritants. The Type C fibres fall into several distinct categories [28]. First, peptidergic neurons contain predominantly CGRP. A population of CGRP plus substance P neurons are present. Localization of other neurotransmitters such as gastrin – releasing peptide (GRP) and galanin are less well studied in trigeminal neurons. A second major distinction is the type of sensation conveyed by different sets of nociceptive nerves. A population of very narrow diameter neurons express histamine H1 receptors, respond to histamine, and convey the sensation of itch [29]. They are localized to distinct spinal cord dorsal horn regions, ascending pathways, thalamus, and thalamocortical radiations. Neurons that respond strongly to capsaicin, the hot spicy essences of chili peptides, appear to be distinct. These express the capsaicin vanilloid receptor and ion channel: transient receptor potential vanilloid 1 (TRPV1). These neurons mediated burning pain. Additional sets of neurons with distinct sets of receptors are anticipated as studies begin to focus on different mediators of cellular injury such as ATP, adenosine, H+, K+, and Ca+2 [30]. These distinctions may breakdown under inflammatory conditions. There is significant neuroplasticity of receptor and neurotransmitter expression under the influences of leukotriene B4, nerve growth factor (TrkA receptor), brain derived neurotrophic factor (BDNF) & neurotrophin-4 (NT-4; TrkB receptor), and NT-3 (TrkC receptor) [31].
Voltage gated ion channel protein family
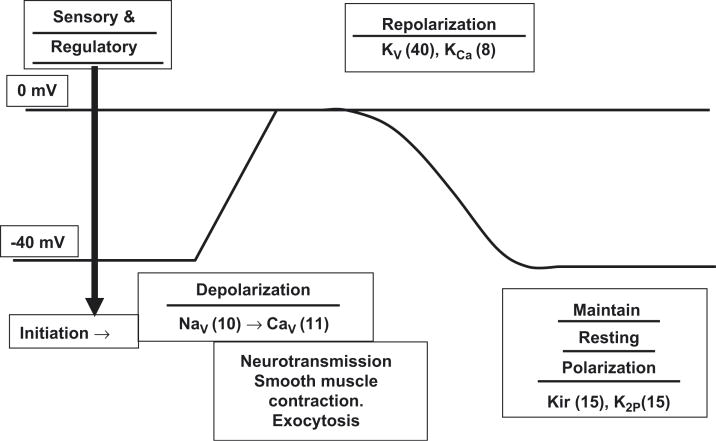
The TRPV1 capsaicin receptor was the first of a family of 143 human ion channels to be recognized as transmitting a specific pain sensation [32]. The superfamily includes sensory receptors that initiate neuron depolarization and the ion channels that mediate sustained depolarization, smooth muscle contraction, glandular exocytosis, and other functions, neuron repolarization, and maintenance of resting membrane potential [33] (Figure 8). The basic motif of these transmembrane proteins is the presence of an extracellular loop that dips into the membrane (Figure 9). Polymers of these proteins form pores that permit the regulated flow of specific ions in or out of the cell.
Figure 8.
Cell membrane voltage (y-axis) is plotted along with the ion channel families that regulate depolarization, repolarization, and other cellular membrane electrical functions.
Figure 9.
TRPV1 ion channel.
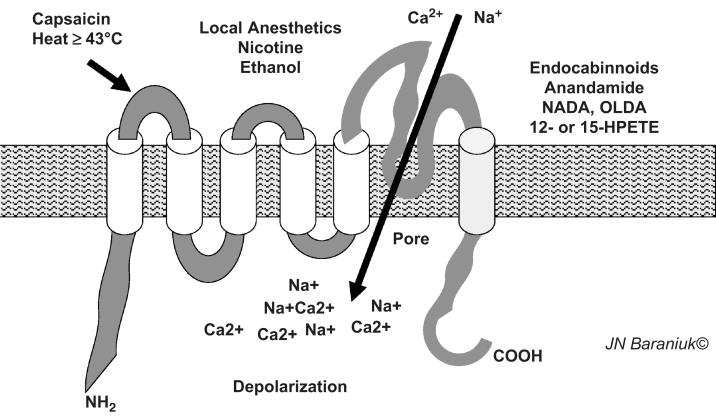
Analysis of the factors that could active TRPV1 showed that this was a highly polymodal chemoreceptor. Capsaicin, temperatures above 43°C, local anesthetics, nicotine and ethanol each interact with distinct portions of the protein to open the ion channel and allow a rapid influx of cations. The endocabinnoids are thought to be engodenous cannibanoid receptor agonists that also active the terminal transmembrane region of TRPV1. Mediators that activate 12- and 15-lipoxygenase can also activate TRPV1 [34]. Activation of intracellular phosphokinases phosphorylates specific protein residues and reduces the threshold for opening the pore and so the threshold for cellular depolarization. In addition to neurons, TRPV1 is also present on airway endothelium and keratinocytes. This dispersion of receptor localization to mucosal surfaces expands the sensory receptor function of this ion channel.
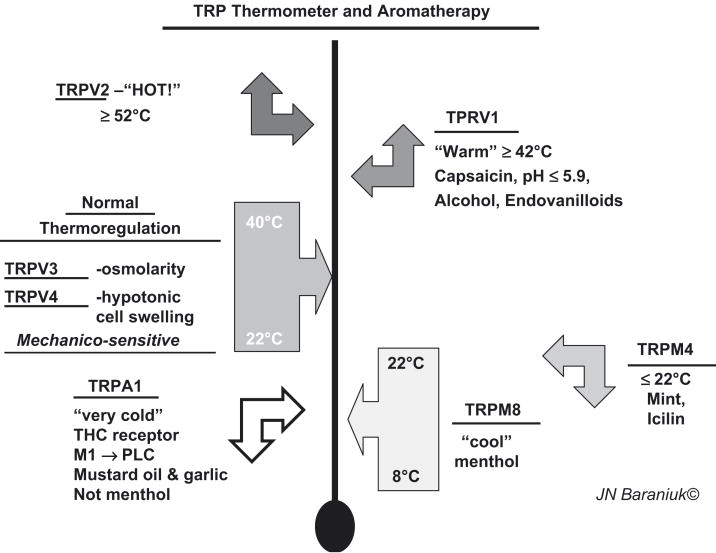
The TRP thermometer
TRPV1 is the only channel to be sensitive to capsaicin. However, nerve activation by other culinary spices has played a major role in the discovery of other TRP protein families [35]. Many of these are multimodal, and include temperature sensitivity. A “TRP thermometer” can be constructed to illustrate the versatility of these receptors (Figure 10). Ambient temperatures are sensed by TRPV3 and TRPV4. They are also mechanicosensitive and are activated by changes in osmotic cell swelling. Temperatures ≥ 42°C activate TRPV1, while TRPV2 responds to dangerously high, tissue damaging temperatures ≥ 52°C. Lower temperatures are sensed by TRP melanostatin 4 (TRPM4). This receptor also responds to mint and the odorless, topical coolant icilin found in many cutaneous ointments. TRPM8 are the menthol receptors. They respond to the range of about 8°C to 22°C. They may alter their frequency of depolarization to convey different temperatures. “Very cold” temperatures, mustard oil, some garlic components, and tetrahydrocannibinol activate the TRPA1 (ankryn) receptor. Activation of muscarinic M1 receptors leads to phospholipase C activation that sensitizes this receptor to its agonists.
Figure 10.
TRP thermometer and aromatherapy: the trigeminal chemosensory nervous system.
Conclusions
The many functions of the nasal mucosa have been appreciated for a considerable time. However, more recent studies have demonstrated specific roles for different classes of neurons and other mediators in regulating epithelial cell, superficial lamina propria vascular, submucosal gland, and deep venous sinusoid activities. The hypertonic saline – induced nociceptive axon response leads to glandular exocytosis with no vascular leak in human airway mucosa. This is in stark contrast to effects in rat trachea and other rodents. The dissection of neurons into peptidergic and nonpeptidergic, H1 receptor-bearing itch and capsaicin receptor-bearing burning pain neurons, and further delineation of the combinations of sensory and regulatory voltage gated and other ion channels including the transient receptor potential (TRP) channels has ushered in a new era with a more highly sophisticated understanding of nociceptive nerve subpopulations and their regulation.
Figure 7.
Subsets of Type C and Aδ Neurons in Human Nasal Mucosa.
Acknowledgments
Supported by Public Health Service Awards 1 RO1 ES015382-01 and P50 DC000214.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Widdicombe J. The physiology of the nose. Clin Chest Med. 1986;7:159–170. [PubMed] [Google Scholar]
- 2.Cole P. Pathophysiology and treatment of airway mucociliary clearance. A moving tale. Minerva Anestesiol. 2001;67:206–209. [PubMed] [Google Scholar]
- 3.Stannard W, O'Callaghan C. Ciliary function and the role of cilia in clearance. J Aerosol Med. 2006;19:110–115. doi: 10.1089/jam.2006.19.110. [DOI] [PubMed] [Google Scholar]
- 4.Swift DL, Proctor DF. Access of air to the respiratory tract. In: Brain JD, Proctor DF, Reid LM, editors. Respiratory Defense Mechanisms. New York, NY: Marcel Dekker; 1977. [Google Scholar]
- 5.Cauna N, Hinderer KH. Fine structure of blood vessels of the human nasal respiratory mucosa. Ann Otol Rhinol Laryngol. 1969;78:865–879. doi: 10.1177/000348946907800418. [DOI] [PubMed] [Google Scholar]
- 6.Cauna N. Electron microscopy of the nasal vascular bed and its nerve supply. Ann Otol Rhinol Laryngol. 1970;79:443–450. doi: 10.1177/000348947007900303. [DOI] [PubMed] [Google Scholar]
- 7.Cauna N. Blood and nerve supply to the nasal lining. In: Proctor DF, Anderson IB, editors. The nose: upper airway physiology and the atmospheric environment. Amsterdam: Elsevier Biomedical Press BV; 1982. pp. 45–69. [Google Scholar]
- 8.Hallen H, Graf P. Evaluation of rhinostereometry compared with acoustic rhinometry. Acta Otolaryngol. 1999;119:921–924. doi: 10.1080/00016489950180298. [DOI] [PubMed] [Google Scholar]
- 9.Scherer PW, Hahn II, Mozell MM. The biophysics of nasal airflow. Otolaryngol Clin North Am. 1989;22:265. [PubMed] [Google Scholar]
- 10.Brandtzaeg P. Immune functions of human nasal mucosa and tonsils in health and disease. In: Bienenstock J, editor. Immunology of the lung and upper respiratory tract. New York: McGrew-Hill; 1984. pp. 28–95. [Google Scholar]
- 11.Baraniuk JN. Mechanisms of rhinitis. In: Lasley MV, Altman LC, editors. Rhinitis Immunology and Allergy Clinics of North America. Vol. 20. W.B. Saunders; Philadelphia: May, 2000. pp. 245–264. [Google Scholar]
- 12.Casado B, Viglio S, Baraniuk JN. Proteomics of sinusitis nasal lavage fluid. In: Thongboonkerd V, editor. Proteomics of Human Body Fluids: principles, methods, and applications. Humana Press; New York: 2006. [Google Scholar]
- 13.Peden DB, Hohman R, Brown ME, Mason RT, Berkebile C, Fales HM, Kaliner MA. Uric acid is a major antioxidant in human nasal airway secretions. Proc Natl Acad Sci U S A. 1990;87:7638–7642. doi: 10.1073/pnas.87.19.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baraniuk JN, Kaliner MA, Barnes PJ. Localization of m3 muscarinic receptor mRNA in human nasal mucosa. Am J Rhinol. 1992;6:145–148. [Google Scholar]
- 15.Assanasen P, Baroody FM, Abbott DJ, Naureckas E, Solway J, Naclerio RM. Natural and induced allergic responses increase the ability of the nose to warm and humidify air. J Allergy Clin Immunol. 2000;106:1045–10520. doi: 10.1067/mai.2000.110472. [DOI] [PubMed] [Google Scholar]
- 16.Malekzadeh S, Hamburger MD, Whelan PJ, Biedlingmaier JF, Baraniuk JN. Density of middle turbinate subepithelial mucous glands in patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2002;127:190–195. doi: 10.1067/mhn.2002.126800. [DOI] [PubMed] [Google Scholar]
- 17.Stafford-Smith M, Baraniuk JN, Wilson K, Schwinn DA. α2-Adrenergic receptor subtypes in human nasal turbinate: expression of mRNA encoding specific α2-adrenergic receptor subtypes in nasal epithelium, duct, gland, and vascular cells. Assoc of Univ Anesthesiol. 1997 [Google Scholar]
- 18.Baraniuk JN, Castellino S, Goff J, Lundgren JD, Mullol J, Merida M, Shelhamer JH, Kaliner MA. Neuropeptide Y (NPY) in human nasal mucosa. Am J Respir Cell Mol Biol. 1990;3:165–173. doi: 10.1165/ajrcmb/3.2.165. [DOI] [PubMed] [Google Scholar]
- 19.Baraniuk JN, Lundgren JD, Goff J, Mullol J, Castellino S, Merida M, Shelhamer JH, Kaliner M. Calcitonin gene related peptide (CGRP) in human nasal mucosa. Am J Physiol. 1990;258:L81–L88. doi: 10.1152/ajplung.1990.258.2.L81. [DOI] [PubMed] [Google Scholar]
- 20.Rinder J. Sensory neuropeptides and nitric oxide in nasal vascular regulation. Acta Physiol Scand. 1996 632:1–45. [PubMed] [Google Scholar]
- 21.Hasegawa M, Kern EB. The human nasal cycle. Mayo Clin Proc. 1977;52:28. [PubMed] [Google Scholar]
- 22.Kennedy DW, Zinreich SJ, Kumar AJ, et al. Physiologic mucosal changes within the nose and the ethmoid sinus: Imaging of the nasal cycle by MRI. Laryngoscope. 1988;98:928. doi: 10.1288/00005537-198809000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Dray A, Urban L, Dickenson A. Pharmacology of chronic pain. TiPS. 1994;15:190–197. doi: 10.1016/0165-6147(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 24.Baraniuk JN, Ali M, Yuta A, Fang SY, Naranch K. Hypertonic saline nasal provocation stimulates nociceptive nerves, substance P release, and glandular mucous exocytosis in normal humans. Am J Respir Crit Care Med. 1999;160:655–662. doi: 10.1164/ajrccm.160.2.9805081. [DOI] [PubMed] [Google Scholar]
- 25.Baraniuk JN, Petrie KN, Le U, Tai CF, Park YJ, Yuta A, Ali M, VandenBussche CJ, Nelson B. Neuropathology in rhinosinusitis. Am J Respir Crit Care Med. 2005;171:5–11. doi: 10.1164/rccm.200403-357OC. [DOI] [PubMed] [Google Scholar]
- 26.Baraniuk JN, Ali M, Naranch K. Hypertonic saline nasal provocation and acoustic rhinometry. Clin Exp Allergy. 2002;32:543–550. doi: 10.1046/j.0954-7894.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- 27.Baraniuk JN, Lundgren JD, Okayama M, Goff J, Mullol J, Merida M, Shelhamer JH, Kaliner MA. Substance P and neurokinin A (NKA) in human nasal mucosa. Am J Respir Cell Mol Biol. 1991;4:228–236. doi: 10.1165/ajrcmb/4.3.228. [DOI] [PubMed] [Google Scholar]
- 28.Tai CF, Baraniuk JN. Upper airway neurogenic mechanisms. Curr Opin Allergy Clin Immunol. 2002;2:11–19. doi: 10.1097/00130832-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Andrew D, Craig AD. Spinothalamic lamina 1 neurons selectively sensitive to histamine: a central neural pathway for itch. Nature Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 30.Vaughan RP, Szewczyk MT, Jr, Lanosa MJ, Desesa CR, Gianutsos G, Morris JB. Adeonsine sensory transduction pathways contribute to activation of the sensory irritation response to inspirited irritant vapors. Toxicol Sci. 2006 doi: 10.1093/toxsci/kfl061. Epub before print. [DOI] [PubMed] [Google Scholar]
- 31.Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med. 2000;161:1985–90. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]
- 32.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 33.Yu FH, Catterall WA. The VGL-Chanome: A protein superfamily specialized for electrical signaling and ionic homeostatis. doi: 10.1126/stke.2532004re15. Science STKE 2004 re15 www.stke.org/cgi/content/full/sigtrans;2004/253/re15. [DOI] [PubMed]
- 34.van der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 35.Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Current Opinions Neurobiol. 2004;14:362–369. doi: 10.1016/j.conb.2004.05.003. [DOI] [PubMed] [Google Scholar]