Abstract
A variety of 2-oxoamides and related amides based on natural and non-natural amino acids were synthesized. Their activity on two human intracellular phospholipases (GIVA cPLA2 and GVIA iPLA2) and one human secretory phospholipase (GV sPLA2) was evaluated. We show that an amide based on (R)-γ-norleucine is a highly selective inhibitor of GV sPLA2.
Keywords: Amides, Amino acids, Inhibitors, 2-Oxoamides, Phospholipase A2
1. Introduction
The phospholipase A2 (PLA2) superfamily consists of a broad range of structurally distinct enzymes that catalyze the hydrolysis of the sn-2 ester bond of phospholipids. The PLA2 superfamily has been systematically grouped based on the source (i.e. organism) and primary sequence.1-3 In mammals, some of these enzymes are particularly interesting anti-inflammatory drug targets, in part due to their ability to liberate arachidonic acid for subsequent eicosanoid biosynthesis.1-4 Specifically in human macrophages, three of these groups are known to play significant roles in immune response: cytosolic Ca2+-dependent PLA2 (GIVA cPLA2), cytosolic Ca2+-independent PLA2 (GVIA iPLA2) and Ca2+-dependent secretory PLA2 (GV sPLA2).
GIVA cPLA2 is an attractive target for drug development since it is the rate-limiting provider of arachidonic acid and lysophospholipids that are subsequently converted into prostaglandins, leukotrienes and PAF, respectively.5 Various studies on transgenic mice lacking GIVA cPLA2 showed a 90% reduction in the production of prostaglandins and leukotrienes.6,7 Recently, Kalyvas and David demonstrated that GIVA cPLA2 plays an important role in the pathogenesis of experimental autoimmune encephalomyelitis, the animal model of multiple sclerosis,8 while it was reported that cytosolic phospholipase A2-deficient mice were resistant to experimental autoimmune encephalomyelitis.9 While the exact physiological roles remain in question, multiple studies confirm the primacy of GIVA cPLA2 in lipid mediator production. Consequently, much effort has been focused on the design, synthesis and characterization of GIVA cPLA2 inhibitors as potential agents for experimental exploration as well as treatment of various inflammatory conditions.
The role of the GVIA iPLA2 in the inflammatory process is still unclear, and it has not been a target for the development of novel medicines up to now.10 This enzyme appears to be the primary PLA2 for basal metabolic functions within the cell.
It has been shown that in macrophages and other cells, GIVA cPLA2 and secretory phospholipase A2 work together to release arachidonic acid.11-15 Group GV sPLA2 was identified at the DNA-level in humans,16 and subsequently expressed and charactized.17 Several experiments suggested that GV sPLA2 has a role in amplifying the action of GIVA cPLA2 in supplying arachidonic acid for eicosanoid production.18 In addition, GV sPLA2 has cellular functions, independent of its ability to provide arachidonic acid, that include regulation of phagocytosis and foam cell formation.19 Thus, this enzyme was recently proposed to have a unique role in atherosclerosis.19b
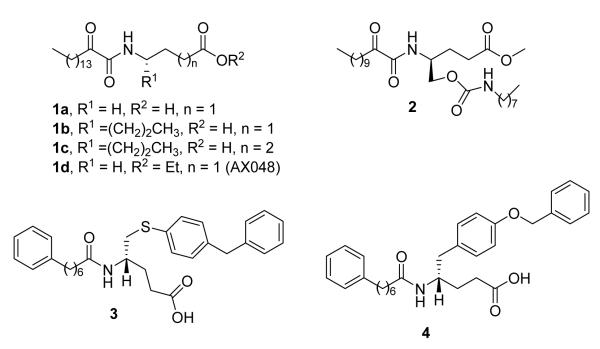
Kokotos and Magrioti recently reviewed many synthetic inhibitors of GIVA cPLA2 and GVIA iPLA2.20 Since then, new N-benzydryl indole inhibitors21-23 as well as 1-indol-1-yl-propan-2-ones24 have been reported. Our recent efforts are focused on the development of a novel class of GIVA cPLA2 inhibitors, initially designed to contain 2-oxoamide functionality and a free carboxyl group.25-32 2-Oxoamides based on γ-aminobutyric acid (1a, Figure 1) and the non-natural amino acids γ-norleucine or δ-norleucine (1b and 1c, Figure 1) are potent inhibitors of GIVA cPLA2 presenting in-vivo anti-inflammatory and analgesic activity.26,30 We have also demonstrated that ethyl 4-[(oxohexadecanoyl)amino]butanoate (1d, Figure 1) inhibits both GIVA cPLA2 and GVIA iPLA2 and is the first systemically bioavailable compound with a significant affinity for GIVA cPLA2, which produces potent hyperalgesia.28 In-vivo, intrathecal and systemic administration of inhibitor 1d blocked carrageenan hyperalgesia.28 Recently, a large number of lipophilic 2-oxoamides have been tested for inhibition of GV sPLA2.30 Only one compound (2, Figure 1) significantly inhibited GV sPLA2 with an XI(50) of 0.035 mole fraction. However, compound 2 inhibited both GIVA cPLA2 and GVIA iPLA2, in addition to GV sPLA2, with no statistical preference.
Figure 1.
Amino acid-based inhibitors of phospholipase A2 enzymes.
Known inhibitors of secreted PLA2 enzyme groups have been reviewed.33 Among them, non-phospholipid amide inhibitors based on non-natural amino acids, such as compounds 334 and 435 (Figure 1), presented interesting inhibition of pancreatic and non-pancreatic GI and GII sPLA2. Inhibitor 4 has been reported to protect rat small intestine from ischaemia and reperfusion injury36 and trinitrobenzene sulfonic acid-induced colitis.37
The selective inhibition of the each PLA2 class is very important to understand enzyme-specific roles in cells and in-vivo. Therefore, we synthesized a variety of 2-oxoamide esters and related amides based on natural and non-natural amino acids and we evaluated their activity on GIVA cPLA2, GV s PLA2 and GVIA iPLA2. While most of these compounds exhibited inhibitory properties for all three PLA2 classes, we demonstrate that an amide based on (R)-γ-norleucine is a highly selective inhibitor of GV sPLA2.
2. Results
2.1. Design and synthesis of inhibitors
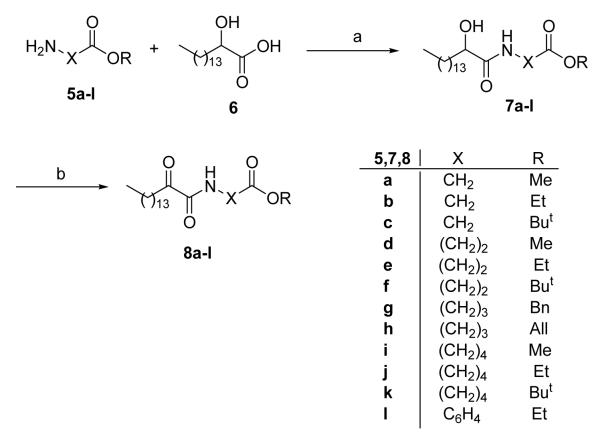
Our previous work has shown that 2-oxoamides containing a free carboxyl group are selective inhibitors of GIVA cPLA2, not affecting GVIA iPLA2 and GV sPLA2.26,29,30 On the contrary, methyl or ethyl esters of 2-oxoamides may inhibit both GIVA cPLA2 and GVIA iPLA2.29 In addition, the 2-oxoamide that contains a methyl ester group (2) inhibits all the three forms: GIVA cPLA2, GVIA iPLA2 and GV sPLA2.30 In this study, we decided to systematically evaluate: (a) the distance between the ester group and the 2-oxoamide functionality, and (b) the nature of the ester group. For structure-activity relationship (SAR) studies, we evaluated a variety of amino acid and ester substitutions such as glycine, β-alanine, γ-aminobutyric acid, δ-aminovaleric acid, and p-aminobenzoic acid as well as methyl, ethyl, and tert-butyl esters. The esters of amino acids 5a-l were coupled with 2-hydroxy-hexadecanoic acid (6) using 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide as a condensing agent in the presence of 1-hydroxybenzotriazole (Scheme 1). The 2-hydroxyamides 7a-l were oxidized to target compounds 8a-l by treatment with Dess-Martin reagent.38
Scheme 1. Reagents and conditions.
(a) CH3(CH2)13CH(OH)COOH, WSCI, HOBt, Et3N, CH2Cl2; (b) Dess-Martin periodinane, CH2Cl2.
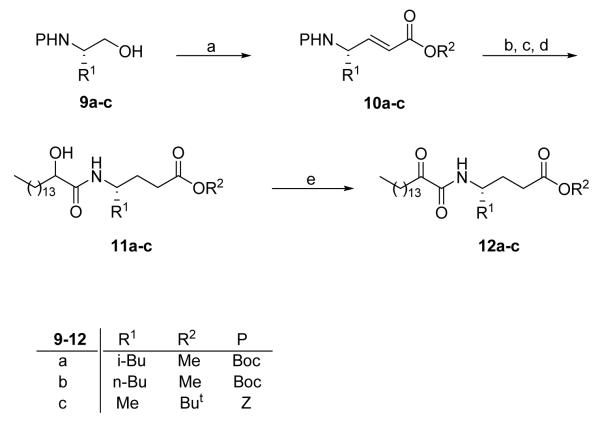
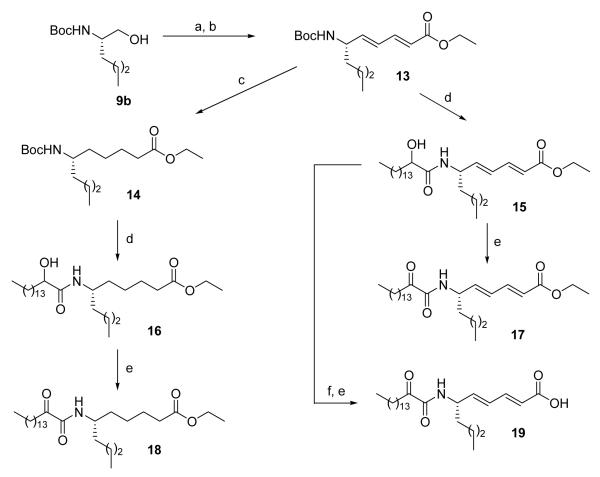
Three 2-oxoamide esters based on γ-leucine, γ-norleucine and γ-alanine were prepared starting from the corresponding amino alcohols 9a-c (Scheme 2) according to procedures we described previously.26,30 Oxidation by the NaOCl/AcNH-TEMPO method39,40 followed by Wittig reaction with methyl or tert-butyl (triphenylphosphoranylidene)acetate led to α,β-unsaturated esters 10a-c. Oxidation of compounds 11a-c by the Dess-Martin method produced the target compounds 12a-c. To prepare 2-oxoamide esters based on ε-norleucine, Boc-L-norleucinol (9b) was oxidized to aldehyde and directly reacted with triethyl phosphonocrotonate41 (Scheme 3). Catalytic hydrogenation of compound 13, removal of the Boc group by HCl/Et2O and coupling with 2-hydroxy-hexadecanoic acid produced compound 16. The unsaturated 2-hydroxyamide 15 was prepared by similar reaction. Both compounds 15 and 16 were oxidized to the corresponding 2-oxoamides 17 and 18 by the Dess-Martin method. 2-Oxoamide 19 containing a free carboxyl group was also prepared.
Scheme 2. Reagents and conditions.
(a) i. NaOCl, TEMPO, NaBr, NaHCO3, EtOAc/PhCH3/H2O 3:3:0.5, −5 °C; ii. Ph3P=CHCOOR2, THF, reflux; (b) H2, 10% Pd/C, EtOH; (c) 4 N HCl/Et2O; (d) CH3(CH2)13CH(OH)COOH, WSCI, HOBt, Et3N, CH2Cl2; (e) Dess-Martin periodinane, CH2Cl2.
Scheme 3. Reagents and conditions.
(a) NaOCl, TEMPO, NaBr, NaHCO3, EtOAc/PhCH3/H2O 3:3:0.5, −5 °C; (b) (EtO)2P(=O)CH2CH=CHCOOEt, LiOH, PhCH3, r.t.; (c) H2, 10% Pd/C, EtOH; (d) i. 4 N HCl/Et2O; ii. CH3(CH2)13CHOHCOOH, WSCI, HOBt, Et3N, CH2Cl2; (e) Dess-Martin periodinane, CH2Cl2; (f) 1 N NaOH, CH3OH.
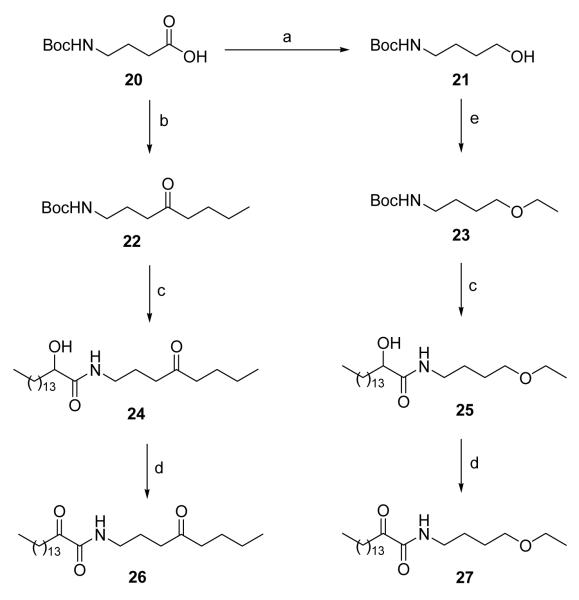
Ketone and ether analogues of the in-vivo active inhibitor 1d were prepared as described in Scheme 4. Treatment of Boc-GABA with n-BuLi led to ketone 22 in moderate yield. Etherification of compound 21 under phase-transfer conditions produced ether 23. Both compounds 22 and 23 were deprotected, coupled and oxidized to obtain the target derivatives 26 and 27.
Scheme 4. Reagents and conditions.
(a) i. NMM, ClCO2Et, THF; ii. NaBH4, MeOH; (b) n-BuLi/hexane, THF; (c) i. 4 N HCl/Et2O; ii. CH3(CH2)13CHOHCOOH, WSCI, HOBt, Et3N, CH2Cl2; (d) Dess-Martin periodinane, CH2Cl2; (e) EtBr, Bu4NHSO4, 50% NaOH, C6H6.
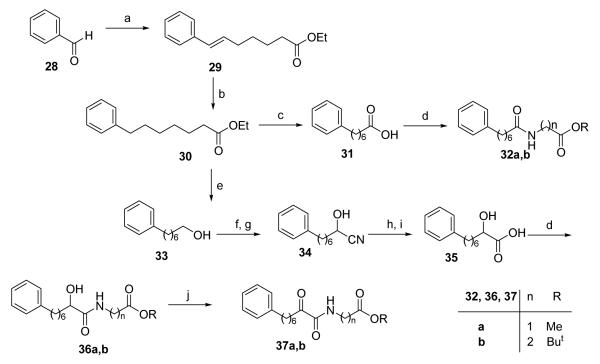
The known inhibitors 3 and 4 (Figure 1) of GI and GII sPLA2 are amides consisting of a 7-phenylheptanoyl acyl residue and a non-natural γ-amino acid bearing a side chain with two aromatic groups. We decided to synthesize 2-oxoamides containing the 7-phenylheptanoyl residue instead of the long aliphatic chain. The synthesis procedure of 2-oxoamides 37a,b and amides 32a,b, based on methyl ester of glycine and tert-butyl ester of β-alanine, is depicted in Scheme 5.
Scheme 5. Reagents and conditions.
(a) Br−Ph3P+(CH2)5COOEt, NaH, THF; (b) H2, 10% Pd/C, EtOH; (c) 1 N NaOH, dioxane; (d) H2N(CH2)nCOOR, WSCI, HOBt, Et3N, CH2Cl2; (e) DIBALH, dry Et2O; (f) NaOCl, TEMPO, NaBr, NaHCO3, EtOAc/PhCH3/H2O 3:3:0.5, −5 °C; (g) NaHSO3, KCN, H2O, CH2Cl2; (h) conc. HCl; (i) KOH, EtOH/H2O; (j) Dess-Martin periodinane, CH2Cl2.
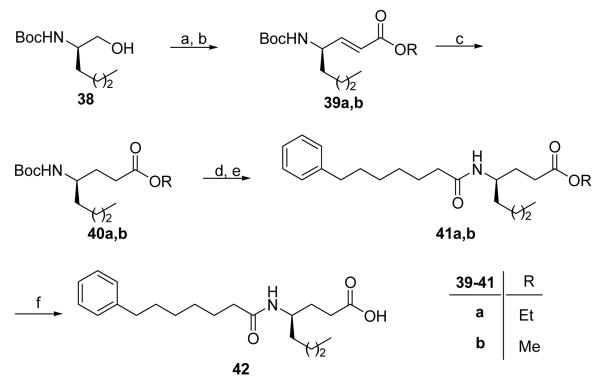
In addition, we synthesized a γ-norleucine-based amide containing a 7-phenylheptanoyl chain. Boc-D-norleucinol (38), prepared according to the literature,42,43 was oxidized to aldehyde and treated with methyl or ethyl (phosphoranylidene)acetate to produce compounds 39a,b (Scheme 6). After hydrogenation and removal of Boc group, coupling with 7-phenylheptanoic acid35 gave amides 41a,b. The target compound 42 was obtained after saponification of either 41a or 41b. Its enantiomer 43 was synthesized following similar reactions.
Scheme 6. Reagents and conditions.
(a) NaOCl, TEMPO, NaBr, NaHCO3, EtOAc/PhCH3/H2O 3:3:0.5, −5 °C; (b) Ph3P=CHCOOMe, THF, reflux; (c) H2, 10% Pd/C, MeOH; (d) 4 N HCl/Et2O, (e) Ph(CH2)6COOH, WSCI, HOBt, Et3N, CH2Cl2; (f) 1 N NaOH, dioxane.
2.2. In vitro inhibition of GIVA cPLA2, GVIA iPLA2 and GV sPLA2
Twenty six new derivatives including 2-oxoamides and amides were tested for inhibition of human GIVA cPLA2, GVIA iPLA2 and GV sPLA2 using previously described mixed micelle-based assays.26,29,30 The relative degrees of inhibition are presented in Tables 1, 2 and 3 as either percent inhibition or XI(50) values. Initially, the percent of inhibition for each PLA2 enzyme at 0.091 mole fraction of each inhibitor was determined, and XI(50) values were estimated for compounds that displayed greater than 90% inhibition. Each XI(50) value is derived from the mole fraction of the inhibitor in the total substrate interface required to inhibit the enzyme by 50%.
Table 1.
Inhibition of PLA2 by 2-oxoamide esters. Average percent inhibition and standard error (n=3) reported for each compound at 0.091 mole fraction. N.D. signifies compounds with less than 25% inhibition (or no detectable inhibition).
| Entry | Structure | GIVA cPLA2 |
GVIA iPLA2 |
GV sPLA2 |
|---|---|---|---|---|
| 8a | 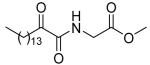 |
62 ± 1 | 45 ± 13 | 52 ± 4 |
| 8b | 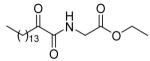 |
74 ± 1 | 62 ± 13 | 62 ± 5 |
| 8c | 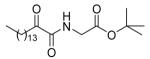 |
80 ± 2 | 53 ± 13 | 82 ± 8 |
| 8d | 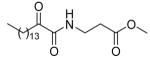 |
N.D | N.D. | 48 ± 9 |
| 8e | 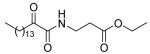 |
44 ± 2 | 51 ± 13 | 59 ± 8 |
| 8f | 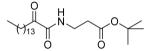 |
79 ± 2 | 54 ± 13 | 72 ± 7 |
| 8g | 53 ± 2 | 63 ± 5 | 38 ± 8 | |
| 8h | 35 ± 1 | N.D. | 58 ± 12 | |
| 8i | N.D. | N.D. | 42 ± 8 | |
| 8j | 31 ± 2 | 35 ± 12 | 47 ± 6 | |
| 8k | 74 ± 2 | 62 ± 13 | 72 ± 10 |
Table 2.
Inhibition of PLA2 by 2-oxoamides based on esters of γ- and ε-amino acids and related compounds. Average percent inhibition and standard error (n=3) reported for each compound at 0.091 mole fraction. N.D. signifies compounds with less than 25% inhibition (or no detectable inhibition).
| Entry | Structure | GIVA cPLA2 |
GVIA iPLA2 |
GV sPLA2 |
|---|---|---|---|---|
| 12a | 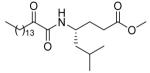 |
83 ± 4 | 65 ± 9 | 65 ± 9 |
| 12b | 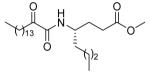 |
87 ± 13 | 78 ± 9 | 86 ± 6 |
| 12c | 85 ± 5 | 72 ± 2 | 87 ± 4 | |
| 17 | 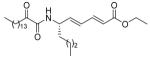 |
64 ± 4 | 74 ± 5 | 52 ± 7 |
| 18 | 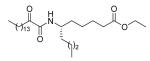 |
62 ± 12 | 66 ± 5 | N.D. |
| 19 | 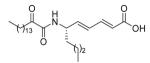 |
85 ± 4 | N.D. | N.D. |
| 8l | 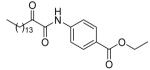 |
87 ± 1 | N.D. | 78 ± 8 |
| 26 | 32 ± 5 | N.D. | N.D. | |
| 27 | 62 ± 14 | 51 ± 2 | 64 ± 12 |
Table 3.
Inhibition of PLA2 by 2-oxoamide and amide containing 7-phenylheptanoyl chain. Average percent inhibition and standard error (n=3) reported for each compound at 0.091 mole fraction. N.D. signifies compounds with less than 25% inhibition (or no detectable inhibition).
| Entry | Structure | GIVA cPLA2 |
GVIA iPLA2 |
GV sPLA2 |
|---|---|---|---|---|
| 37a | 54 ± 4 | 31 ± 10 | 30 ± 2 | |
| 37b | 82 ± 9 | 81 ± 10 | 69 ± 9 | |
| 32a | N.D. | N.D. | N.D. | |
| 32b | 36 ± 3 | 57 ± 12 | 66 ± 9 | |
| 42 | N.D. | N.D. | 95 XI(50) 0.003 ± 0.0004 |
|
| 43 | N.D. | N.D. | N.D. |
None of the methyl, ethyl or tert-butyl esters of 2-oxoamides based on glycine (8a-c), β-alanine (8d-f), and δ-aminovaleric acid (8i-k) (Table 1) display potent inhibition of GIVA cPLA2 or GVIA iPLA2. Tert-butyl esters (8c, 8f, and 8k) are better inhibitors than the corresponding methyl and ethyl esters. Allyl ester (8g) and benzyl ester (8h) analogues of inhibitor 1d were found to be weak inhibitors of GIVA cPLA2 and GVIA iPLA2, indicating that the ethyl ester of GABA leads to better in-vitro results.28
The esters of the 2-oxoamides based on γ-leucine (12a), γ-norleucine (12b) and γ-alanine (12c) are good inhibitors of GIVA cPLA2 (Table 2). They also weakly inhibit GVIA iPLA2. Derivative 8l (Table 2) based on the aromatic amino acid seems to selectively inhibit GIVA cPLA2, without affecting GVIA iPLA2 activity. 2-Oxoamide ethyl esters based on ε-norleucine (saturated 18 or unsaturated 17, Table 2) are weak inhibitors of both enzymes. It should be noticed that compound 19 (Table 2) containing a free carboxyl group is a selective inhibitor of GIVA cPLA2, without affecting the activity of GVIA iPLA2. This observation is in full agreement with our previous report that 2-oxoamides based on γ-amino acids are selective inhibitors of GIVA cPLA2.29,30
Among the 2-oxoamides based on esters of γ- and ε-amino acids, two derivatives (12b and 12c, Table 2) based on esters of γ-norleucine and γ-alanine displayed inhibition of GV sPLA2. However, compounds 12b and 12c also inhibited GIVA cPLA2 and GVIA iPLA2, and therefore demonstrated no statistical preference for the inhibition of the three enzymes.
To characterize the mode of interaction between the ethyl ester derivative 1d and the active site of GIVA cPLA2, we prepared two structurally related compounds, a ketone derivative 26 and an ether derivative 27. In compound 26, the carbonyl group is at the same position as in the ester derivative 1d, although the alkoxy group was replaced by an alkyl group. In compound 27, the ethoxy group of the ester derivative was kept constant, while the carbonyl group is absent. For synthetic reasons, we prepared a ketone derivative containing one additional carbon atom in the right chain of the ketone chain. However, this modification was not expected to play an essential role in inhibition. Neither compounds 26 or 27 presented interesting inhibitory properties, thereby confirming the essential inhibitory role of the ester group of compound 1d.
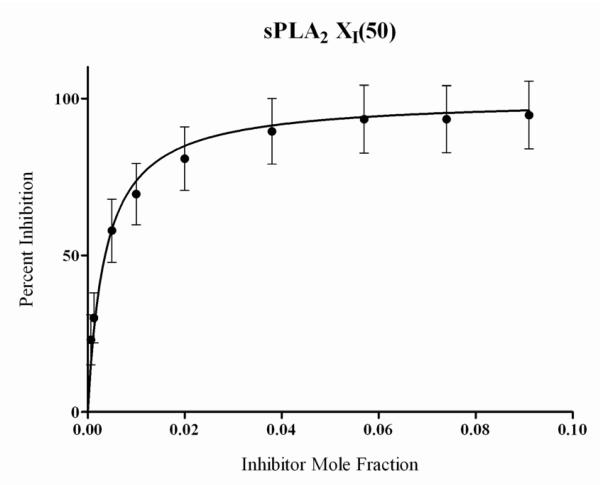
Table 3 summarizes the in-vitro inhibition activities caused by 2-oxoamides and amides containing the 7-phenylheptanoyl chain instead of the long aliphatic chain. Comparing 37a with 8a, it seems that such a replacement of the long chain led to decreased activity for all the enzymes, as observed in the case of glycine methyl ester. However, similar activities were observed for 37b and 8b. The corresponding amides 32a and 32b were either totally inactive or very weak inhibitors. Interestingly, amide 42 based on (R)-γ-norleucine is a potent and selective GV sPLA2 inhibitor, not affecting at all the activities of GIVA cPLA2 and GVIA iPLA2. The dose-response curve for the inhibition of GV sPLA2 by amide 42 is shown in Figure 2, and a XI(50) value 0.003 ± 0.0004 was calculated. The configuration of the substituted amino acid is very important, since the amide 43 based on (S)-γ-norleucine was inactive for all the three PLA2 enzymes.
Figure 2.
Inhibition curve for amide 42 in a mixed-micelle assay with human GV sPLA2. Non-linear regression (hyperbolic) estimated a XI(50) value of 0.003 ± 0.0004. Compound 42 did not inhibit GIVA cPLA2 and GVIA iPLA2 at 0.091 mole fraction.
3. Discussion
We previously demonstrated that 2-oxoamide 1d, which is based on the ethyl ester of γ-aminobutyric acid, inhibits the GIVA cPLA2 and GVIA iPLA2 and exhibits very interesting antihyperalgesic activity.28 The tert-butyl ester variant of compound 1d (AX057) also inhibits both enzymes.28 In addition, 2-oxoamides based on methyl esters of γ-aminobutyric acid or α,β-unsaturated γ-norleucine are able to inhibit the same enzymes.29 From the results of Table 1, we show that 2-oxoamide esters with varying distance between the 2-oxoamide functionality and the ester group (one to four carbon atoms) do not present any significant inhibition of GIVA cPLA2 or GVIA iPLA2. Interestingly, the introduction of a side-chain to the non-natural amino acids (Table 2) increases the potency of methyl or tert-butyl esters of γ-leucine, γ-norleucine, and γ-alanine (12a-c) for GIVA cPLA2; but display rather weak inhibition of GVIA iPLA2. Even so, increasing the spacer length to five carbon atoms,as seen in the ethyl ester of an ε-amino acid variant (17), leads to inhibition of both GIVA cPLA2 and GVIA iPLA2.
The 2-oxoamide compounds are inhibitors of various lipolytic enzymes (pancreatic and gastric lipases,44-48 GIVA cPLA2,25,26,29,30 GVIA iPLA2,29) that utilize a serine residue as the nucleophile in their catalytic mechanism. In the cases of such serine esterases, it has been proposed that the serine hydroxyl group interacts with the activated carbonyl group of the 2-oxoamide functionality. The small, secreted human GV sPLA2, as well as other sPLA2 enzymes, utilizes a histidine residue in the catalytic site to coordinate a water molecule for hydrolysis at the sn-2 ester bond of phospholipids. It is likely that the long chain 2-oxoamides able to inhibit GV sPLA2 resemble the transition state, explaining its high-affinity for the active site of GV sPLA2.
We demonstrate that an amide based on a non-natural amino acid with a short linear aliphatic side chain (42) is a potent and selective inhibitor of GV sPLA2. Comparing the present results with our previous studies, we conclude that a 2-oxoamide based on (S)-γ-norleucine, containing a long aliphatic chain, is a potent and selective inhibitor of GIVA cPLA2,26 while a simple amide based on (R)-γ-norleucine, containing a 7-phenylheptanoyl chain, is a potent and selective inhibitor of GV sPLA2.
4. Conclusions
In conclusion, we synthesized a variety of 2-oxoamide and amides based on various amino acids and studied their activity on two human intracellular phospholipases (GIVA cPLA2 and GVIA iPLA2) and one human secretory phospholipase (GV sPLA2). In summary, compounds with (S) configuration containing the 2-oxoamide functionality and a long fatty acyl chain provide selective inhibition of GIVA cPLA2, while amide functionality in combination with the 7-phenylheptanoyl chain and the (R) configuration lead to selective inhibition of GV sPLA2. Since both of these PLA2s have been shown to contribute to the production of arachidonic acid, selective 2-oxoamide and amide inhibitors will be valuable tools for studies in cells and in-vivo.
5. Experimental Section
5.1. General procedures
Melting points were determined on a Buchi 530 apparatus and are uncorrected. Specific rotations were measured at 25 °C on a Perkin-Elmer 343 polarimeter using a 10 cm cell. NMR spectra were recorded on a Varian Mercury (200 Mz) spectrometer. Fast atom bombardment (FAB) mass spectra were recorded using a VG analytical ZAB-SE instrument. Electron spray ionization (ESI) mass spectra were recorded on a Finnigan, Surveyor MSQ Plus spectrometer. All amino acid derivatives were purchased from Fluka Chemical Co. TLC plates (silica gel 60 F254) and silica gel 60 (70–230 or 230–400 mesh) for column chromatography were purchased from Merck. Visualization of spots was effected with UV light and/or phosphomolybdic acid and/or ninhydrin, both in EtOH stain. THF, toluene, and Et2O were dried by standard procedures and stored over molecular sieves or Na. All other solvents and chemicals were reagent grade and used without further purification.
5.2. Synthesis of 2-Oxoamide Inhibitors
5.2.1. Coupling of 2-hydroxy-acids with amino components
To a stirred solution of 2-hydroxy-acid (1.0 mmol) and the amino component (1.0 mmol) in CH2Cl2 (10 mL), Et3N (3.1 mL, 2.2 mmol) and subsequently 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide (WSCI) (0.21 g, 1.1 mmol) and 1-hydroxybenzotriazole (HOBt) (0.14 g, 1.0 mmol) were added at 0 °C. The reaction mixture was stirred for 1 h at 0 °C and overnight at rt. The solvent was evaporated under reduced pressure and EtOAc (20 mL) was added. The organic layer was washed consecutively with brine, 1N HCl, brine, 5% NaHCO3, and brine, dried over Na2SO4 and evaporated under reduced pressure. The residue was purified by column-chromatography using CHCl3/MeOH as eluent.
5.2.1.1. Methyl 2-(2-hydroxyhexadecanamido)acetate (7a)
Yield 82%; White solid; m.p. 116-118 °C; 1H NMR (200 MHz, CDCl3)δ 7.02 (t, J = 4.8 Hz, 1H,), 4.20-4.14 (m, 1H), 4.10-4.06 (m, 2H), 3.77 (s, 3H), 2.78 (br, 1H), 1.94-1.56 (m, 2H), 1.26 (br s, 24H), 0.89 (t, J = 6.8 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.2, 170.3, 72.2, 52.4, 40.8, 34.8, 31.9, 29.6, 29.5, 29.4, 29.3, 24.9, 22.7, 14.1. Anal. calcd for C19H37NO4: C, 66.43; H, 10.86; N, 4.08. Found: C, 66.65; H, 10.71; N, 4.13.
5.2.1.2. Ethyl 2-(2-hydroxyhexadecanamido)acetate (7b)
Yield 75%; White solid; m.p. 115-117 °C; 1H NMR (200 MHz, CDCl3) δ 6.98 (m, 1H), 4.29-4.07 (m, 5H), 1.93-1.53 (m, 2H), 1.26 (br s, 27H), 0.89 (t, J = 5.8 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.2, 169.9, 72.2, 61.6, 40.9, 34.8, 31.9, 29.6, 29.5, 29.4, 29.3, 24.9, 22.7, 14.1. Anal. calcd for C20H39NO4: C, 67.19; H, 10.99; N, 3.92. Found: C, 67.33; H, 10.83; N, 3.99.
5.2.1.3. tert-Butyl 2-(2-hydroxyhexadecanamido)acetate (7c)
Yield 77%; White solid; m.p. 53-56°C; 1H NMR (200 MHz, CDCl3) δ 7.03 (t, J = 5.2 Hz, 1H), 4.18-4.12 (m, 1H), 3.99-3.93 (m, 2H), 3.15 (br, 1H), 1.89-1.50 (m, 2H), 1.47 (s, 9H), 1.25 (br s, 24H), 0.88 (t, J = 6.8 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.3, 169.1, 82.4, 72.2, 41.5, 34.7, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 28.0, 25.0, 22.7, 14.1. Anal. calcd for C22H43NO4: C, 68.53; H, 11.24; N, 3.63. Found: C, 68.45; H, 11.37; N, 3.71.
5.2.1.4. Methyl 3-(2-hydroxyhexadecanamido)propanoate (7d)
Yield 79%; White solid; m.p. 111-114 °C; 1H NMR (200 MHz, CDCl3) δ 7.00 (br, 1H), 4.12-4.06 (m, 1H), 3.71 (s, 3H), 3.61-3.51 (m, 2H), 2.86 (br, 1H), 2.57 (t, J = 6 Hz, 2H), 1.86-1.55 (m, 2H), 1.25 (br s, 24H), 0.88 (t, J = 6.8 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.0, 172.9, 72.0, 51.9, 34.9, 34.5, 33.8, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 24.9, 22.7, 14.1. Anal. calcd for C20H39NO4: C, 67.19; H, 10.99; N, 3.92. Found: C, 67.03; H, 11.21; N, 3.86.
5.2.1.5. Ethyl 3-(2-hydroxyhexadecanamido)propanoate (7e)
Yield 68%; White solid; m.p. 102-104 °C; 1H NMR (200 MHz, CDCl3) δ 7.07 (t, J = 6 Hz, 1H), 4.15 (q, J = 7.2 Hz, 2H), 4.09-4.04 (m, 1H), 3.59-3.49 (m, 2H), 3.20 (br, 1H), 2.54 (t, J = 6.2 Hz, 2H), 1.86-1.50 (m, 2H), 1.24 (br s, 27H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.1, 172.4, 72.0, 60.8, 34.8, 34.5, 34.0, 31.9, 29.6, 29.5, 29.4, 29.3, 24.9, 22.6, 14.1, 14.0. Anal. calcd for C21H41NO4: C, 67.88; H, 11.12; N, 3.77. Found: C, 67.64; H, 11.34; N, 3.71.
5.2.1.6. tert-Butyl 3-(2-hydroxyhexadecanamido)propanoate (7f)
Yield 63%; White solid; m.p. 95-97 °C; 1H NMR (200 MHz, CDCl3) δ 6.97 (t, J = 5.8 Hz, 1H), 4.11-4.05 (m, 1H), 3.56-3.46 (m, 2H), 2.83 (br, 1H), 2.44 (t, J = 5.8 Hz, 2H), 1.88-1.51 (m, 2H), 1.44 (s, 9H), 1.23 (br s, 24H), 0.86 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 173.8, 171.7, 81.2, 72.0, 35.1, 35.0, 34.7, 31.9, 29.6, 29.5, 29.4, 29.3, 28.1, 24.8, 22.7, 14.1. Anal. calcd for C23H45NO4: C, 69.13; H, 11.35; N, 3.51. Found: C, 69.01; H, 11.48; N, 3.44.
5.2.1.7. Benzyl 4-(2-hydroxyhexadecanamido)butanoate (7g)
Yield 64%; White solid; m.p. 89-91 °C; 1H NMR (200 MHz, CDCl3) δ 7.36 (m, 5H), 6.72 (t, J = 5.8 Hz, 1H), 5.13 (s, 2H), 4.09-4.03 (m, 1H), 3.37-3.28 (m, 2H), 2.85 (br, 1H), 2.42 (t, J = 7 Hz, 2H), 1.95-1.50 (m, 4H), 1.26 (br s, 24H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.1, 173.1, 135.7, 128.6, 128.3, 128.2, 72.1, 66.4, 38.4, 34.9, 31.9, 31.6, 29.7, 29.62, 29.58, 29.5, 29.4, 29.3, 25.0, 24.6, 22.7, 14.1. Anal. calcd for C27H45NO4: C, 72.44; H, 10.13; N, 3.13. Found: C, 72.61; H, 9.95; N, 3.23.
5.2.1.8. Allyl 4-(2-hydroxyhexadecanamido)butanoate (7h)
Yield 71%; White solid; m.p. 91-93 °C; 1H NMR (200 MHz, CDCl3) δ 6.71 (t, J = 5.4 Hz, 1H), 6.02-5.82 (m, 1H), 5.36-5.22 (m, 2H), 4.61-4.57 (m, 2H), 4.12-4.06 (m, 1H), 3.39-3.29 (m, 2H), 2.59 (br, 1H), 2.40 (t, J = 7.2 Hz, 2H), 1.95-1.52 (m, 4H), 1.25 (br s, 24H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.0, 173.0, 132.0, 118.4, 72.1, 65.3, 38.5, 34.9, 31.9, 31.6, 29.7, 29.6, 29.5, 29.4, 29.3, 25.0, 24.6, 22.7, 14.1. Anal. calcd for C23H43NO4: C, 69.48; H, 10.90; N, 3.52. Found: C, 69.62; H, 10.75; N, 3.57.
5.2.1.9. Methyl 5-(2-hydroxyhexadecanamido)pentanoate (7i)
Yield 66%; White solid; m.p. 105-107 °C; 1H NMR (200 MHz, CDCl3) δ 6.73 (t, J = 5.8 Hz, 1H), 4.10-4.04 (m, 1H), 3.66 (s, 3H), 3.32-3.22 (m, 2H), 3.17 (br, 1H), 2.34 (t, J = 7 Hz, 2H), 1.79-1.51 (m, 6H), 1.24 (br s, 24H), 0.87 (t, J = 7 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.2, 174.0, 72.1, 51.6, 38.5, 34.9, 33.4, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 28.9, 25.0, 22.6, 22.0, 14.1. Anal. calcd for C22H43NO4: C, 68.53; H, 11.24; N, 3.63. Found: C, 68.77; H, 11.13; N, 3.58.
5.2.1.10. Ethyl 5-(2-hydroxyhexadecanamido)pentanoate (7j)
Yield 68%; White solid; m.p. 95-97 °C; 1H NMR (200 MHz, CDCl3) δ 6.69 (t, J = 5.8 Hz, 1H), 4.17-4.06 (m, 3H), 3.33-3.23 (m, 2H), 3.15 (br, 1H), 2.33 (t, J = 7 Hz, 2H), 1.79-1.58 (m, 6H), 1.24 (br s, 27H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.0, 173.5, 72.1, 60.4, 38.5, 34.9, 33.7, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 28.9, 25.0, 22.7, 22.0, 14.1. Anal. calcd for C23H45NO4: C, 69.13; H, 11.35; N, 3.51. Found: C, 68.97; H, 11.59; N, 3.43.
5.2.1.11. tert-Butyl 5-(2-hydroxyhexadecanamido)pentanoate (7k)
Yield 61%; White solid; m.p. 60-63 °C; 1H NMR (200 MHz, CDCl3) δ 6.71 (t, J = 5.6 Hz, 1H), 4.11-4.06 (m, 1H), 3.32-3.23 (m, 2H), 2.24 (t, J = 7 Hz, 2H), 1.81-1.46 (m, 6H), 1.43 (s, 9H), 1.23 (br s, 24H), 0.87 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.1, 173.0, 80.4, 72.1, 38.6, 34.9, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 28.9, 28.0, 25.0, 22.7, 22.1, 14.1. Anal. calcd for C25H49NO4: C, 70.21; H, 11.55; N, 3.28. Found: C, 70.02; H, 11.68; N, 3.32.
5.2.1.12. Ethyl 4-(2-hydroxyhexadecanamido)benzoate (7l)
Yield 43%; Oily solid; 1H NMR (200 MHz, CDCl3) δ 8.74 (s, 1H), 8.06 (s, 1H), 7.95-7.33 (m, 3H), 4.37 (q, J = 7 Hz, 2H), 4.28-4.22 (m, 1H), 3.35 (br, 1H), 1.90-1.61 (m, 2H), 1.25 (br s, 27H), 0.88 (t, J = 5.8 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 172.7, 166.3, 137.5, 131.1, 129.1, 125.4, 124.1, 120.6, 72.6, 61.2, 34.7, 31.9, 29.7, 29.5, 29.4, 29.3, 25.0, 22.6, 14.2, 14.1. Anal. calcd for C25H41NO4: C, 71.56; H, 9.85; N, 3.34. Found: C, 71.33; H, 9.97; N, 3.42.
5.2.1.13. (4R)-Methyl 4-(2-hydroxyhexadecanamido)-6-methylheptanoate (11a)
Yield 57%; White solid; 1H NMR (200 MHz, CDCl3) δ 6.40-6.20 (m, 1H), 4.20-3.90 (m, 2H), 3.66 (s, 3H), 3.10-2.90 (m, 1H), 2.35 (t, J = 7.8 Hz, 2H), 1.95- 1.47 (m, 5H), 1.40-1.18 (m, 26H), 0.95-0.81 (m, 9H). Anal. calcd for C25H49NO4: C, 70.21; H, 11.55; N, 3.28. Found: C, 70.34; H, 11.46; N, 3.22.
5.2.1.14. (4S)-Methyl 4-(2-hydroxyhexadecanamido)octanoate (11b)
Yield 62%; White solid; 1H NMR (200 MHz, CDCl3) δ 6.35-6.15 (m, 1H), 4.15-4.00 (m, 1H), 4.00-3.80 (m, 1H), 3.67 (s, 3H), 2.80-2.70 (m, 1H), 2.35 (t, J = 7.8 Hz, 2H), 1.97-1.42 (m, 6H), 1.38-1.15 (m, 28H), 0.98-0.81 (m, 6H). Anal. calcd for C25H49NO4: C, 70.21; H, 11.55; N, 3.28. Found: C, 70.04; H, 11.62; N, 3.36.
5.2.1.15. (4S)-tert-Butyl 4-(2-hydroxyhexadecanamido)pentanoate (11c)
Yield 58%; White solid; 1H NMR (200 MHz, CDCl3) δ 6.60-6.40 (m, 1H), 4.10-3.90 (m, 2H), 3.15-2.95 (m, 1H), 2.27 (t, J = 7.8 Hz, 2H), 1.98-1.52 (m, 4H), 1.44 (s, 9H), 1.40-1.21 (m, 24H), 1.16 (d, J = 6.6 Hz, 3H), 0.88 (t, J = 6.6 Hz, 3H). Anal. calcd for C25H49NO4: C, 70.21; H, 11.55; N, 3.28. Found: C, 70.05; H, 11.74; N, 3.21.
5.2.1.16. (2E,4E,6S)-Ethyl 6-(2-hydroxyhexadecanamido)deca-2,4-dienoate (15)
Yield 58%; Waxy solid; 1H NMR (200 MHz, CDCl3) δ 7.35 (dd, J1 = 15.6 Hz, J2 = 10.8 Hz, 1H), 6.73-6.67 (m, 1H), 6.26 (dd, J1 = 15.2 Hz, J2 = 11 Hz, 1H), 6.12(dd, J1 = 15 Hz, J2 = 5.8 Hz, 1H), 5.95 (d, J = 15.4 Hz, 1H), 4.73-4.57 (m, 1H), 4.35-4.24 (m, 3H), 3.11 (d, J = 4.8 Hz, ⅔H), 3.03 (d, J = 4.8 Hz, ⅓H), 1.92-1.58 (m, 4H), 1.29 (br s, 31H), 0.89 (t, J = 6.6 Hz, 6H); 13C NMR (50 MHz, CDCl3) δ 173.2, 167.0, 143.7, 142.5, 128.0, 121.5, 72.2, 60.4, 50.1, 34.9, 34.5, 31.9, 29.7, 29.6, 29.4, 29.3, 27.8, 24.9, 22.7, 22.4, 14.2, 14.1, 13.9. Anal. calcd for C28H51NO4: C, 72.21; H, 11.04; N, 3.01. Found: C, 72.34; H, 10.97; N, 2.97.
5.2.1.17. (6S)-Ethyl 6-(2-hydroxyhexadecanamido)decanoate (16)
Yield 55%; White solid; 1H NMR (200 MHz, CDCl3) δ 6.28-6.18 (m, 1H), 4.16-4.06 (m, 3H), 3.92-3.89 (m, 1H), 3.11 (d, J = 4.4 Hz, ⅔H), 2.94 (d, J = 5 Hz, ⅓H), 2.28 (t, J = 7.4 Hz, 2H), 1.93-1.21 (m, 41H), 0.88 (t, J = 6.6 Hz, 6H); 13C NMR (50 MHz, CDCl3) δ 173.9, 173.5, 72.2 (72.0), 60.3, 48.6 (48.8), 35.1, 34.9, 34.8, 34.7, 34.1, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 28.0, 25.4, 25.3, 24.9, 24.8, 24.7, 22.7 (22.6), 14.2, 14.1, 14.0. Anal. calcd for C28H55NO4: C, 71.59; H, 11.80; N, 2.98. Found: C, 71.28; H, 11.95; N, 2.94.
5.2.1.18. 2-Hydroxy-N-(4-oxooctyl)hexadecanamide (24)
Yield 70%; White solid; m.p. 122-124 °C; 1H NMR (200 MHz, CDCl3) δ 6.73 (t, J = 5.8 Hz, 1H), 4.22-4.06 (m, 1H), 3.34-3.28 (m, 2H), 2.56-2.40 (m, 4H), 1.97-1.73 (m, 3H), 1.73-1.20 (m, 30H), 0.88 (t, J = 6.6 Hz, 6H); 13C NMR (50 MHz, CDCl3) δ 211.2, 174.0, 72.1, 42.7, 40.0, 38.7, 34.9, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 25.9, 25.0, 23.3, 22.7, 22.3, 14.1, 13.8. Anal. calcd for C24H47NO3: C, 72.49; H, 11.91; N, 3.52. Found: C, 72.38; H, 11.99; N, 3.56.
5.2.1.19. N-(4-Ethoxybutyl)-2-hydroxyhexadecanamide (25)
Yield 67%; White solid; m.p. 92-95 °C; 1H NMR (200 MHz, CDCl3) δ 6.75 (t, J = 5.8 Hz, 1H), 4.10-4.02 (m, 1H), 3.52-3.26 (m, 7H), 1.95-1.50 (m, 6H), 1.25 (br s, 27H), 0.87 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.1, 72.0, 70.1, 66.2, 38.8, 35.0, 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 27.1, 26.4, 25.0, 22.6, 15.1, 14.1. Anal. calcd for C22H45NO3: C, 71.11; H, 12.21; N, 3.77. Found: C, 71.24; H, 12.15; N, 3.72.
5.2.1.20. Methyl 2-(7-phenylheptanamido)acetate (32a)
Yield 45%; White solid; m.p. 48-50 °C; 1H NMR (200 MHz, CDCl3) δ 7.40-7.21 (m, 5H), 6.30 (b, 1H), 4.11 (d, J = 5.6 Hz, 2H), 3.83 (s, 3H), 2.69 (t, J = 7.2 Hz, 2H), 2.32 (t, J = 7.4 Hz, 2H), 1.81-1.62 (m, 4H), 1.55-1.38 (m, 4H); 13C NMR (50 MHz, CDCl3) δ 173.3, 170.4, 142.5, 128.2, 128.1, 125.5, 52.1, 41.0, 36.1, 35.7, 31.1, 28.9, 28.8, 25.3; MS (ESI) m/z(%): 278 (100) [M+H]+; Anal. calcd for C16H23NO3: C, 69.29; H, 8.36; N, 5.05. Found: C, 68.92; H, 8.47; N, 5.11.
5.2.1.21. tert-Butyl 3-(7-phenylheptanamido)propanoate (32b)
Yield 45%; Oil; 1H NMR (200 MHz, CDCl3) δ 7.40-7.23 (m, 5H), 6.18 (br s, 1H), 3.56 (q, 2H), 2.68 (t, J = 7.4 Hz, 2H), 2.53 (t, J = 6.2 Hz, 2H), 2.23 (t, J = 7.0 Hz, 2H), 1.80-1.62 (m, 4H), 1.54 (s, 9H), 1.51-1.38 (m, 4H); 13C NMR (50 MHz, CDCl3) δ 173.0, 172.2, 142.6, 128.3, 128.2, 125.5, 81.0, 36.7, 35.8, 35.0, 34.8, 31.2, 29.0, 28.9, 28.0, 25.6; MS (ESI) m/z(%): 356 (100) [M+Na]+; Anal. calcd for C20H31NO3: C, 72.04; H, 9.37; N, 4.20. Found: C, 71.76; H, 9.59; N, 4.36.
5.2.1.22. Methyl 2-(2-hydroxy-8-phenyloctanamido)acetate (36a)
Yield 41%; White solid; m.p. 65-67 °C; 1H NMR (200 MHz, CDCl3) δ 7.39-7.25 (m, 6H), 4.27-4.08 (m, 3H), 3.83 (s, 3H), 3.32 (br s, 1H), 2.68 (t, J = 7.4 Hz, 2H), 1.98-1.62 (m, 4H), 1.58-1.35 (m, 6H); 13C NMR (50 MHz, CDCl3) δ 174.7, 170.4, 142.7, 128.3, 128.2, 125.5, 72.1, 52.3, 40.7, 35.8, 34.6, 31.3, 29.2, 29.1, 24.8; Anal. calcd for C17H25NO4: C, 66.43; H, 8.20; N, 4.56. Found: C, 66.29; H, 8.11; N, 4.61.
5.2.1.23. tert-Butyl 3-(2-hydroxy-8-phenyloctanamido)propanoate (36b)
Yield 32%; White solid; m.p. 62-64 °C; 1H NMR (200 MHz, CDCl3) δ 7.40-7.23 (m, 5H), 7.13 (t, J = 5.8 Hz, 1H), 4.16 (dd, J1= 3.6 Hz, J2 = 7.2 Hz, 1H), 3.59 (q, J = 6.4 Hz, 2H), 3.10 (br s, 1H), 2.68 (t, J = 7.4 Hz, 2H), 2.54 (t, J = 6.2 Hz, 2H), 1.95-1.78 (m, 1H), 1.76-1.61 (m, 3H), 1.54 (s, 9H), 1.42-1.35 (m, 6H); 13C NMR (50 MHz, CDCl3) δ 174.0, 171.7, 142.7, 128.3, 128.2, 125.5, 81.2, 71.9, 35.9, 35.2, 34.8, 34.7, 31.4, 29.2, 29.1, 28.0, 24.8; Anal. calcd for C21H33NO4: C, 69.39; H, 9.15; N, 3.85. Found: C, 69.28; H, 9.21; N, 3.94.
5.2.1.24. (R)-Ethyl 4-(7-phenylheptanamido)octanoate (41a)
Yield 89%; White solid; m.p. 54-56 °C; [α]D = +8.2 (c 1 CHCl3); 1H NMR (200 MHz, CDCl3) δ 7.39-7.23 (m, 5H), 5.88 (d, J = 9.0 Hz, 1H), 4.19 (q, J = 7.0 Hz, 2H), 4.03 (br s, 1H), 2.69 (t, J = 7.4 Hz, 2H), 2.43 (t, J = 7.6 Hz, 2H), 2.24 (t, J = 6.8 Hz, 2H), 1.99-1.87 (m, 2H), 1.83-1.60 (m, 6H), 1.56-1.36 (m, 8H), 1.32 (t, J = 7.4 Hz, 3H), 0.98 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 173.5, 172.6, 142.4, 128.1, 128.0, 125.4, 60.2, 48.5, 36.6, 35.6, 34.8, 31.1, 30.9, 29.8, 28.9, 28.8, 27.8, 25.6, 22.3, 14.0, 13.9; MS (ESI) m/z(%): 398 (100) [M+Na]+; Anal. calcd for C23H37NO3: C, 73.56; H, 9.93; N, 3.73. Found: C, 73.22; H, 10.18; N, 3.79.
5.2.1.25. (R)-Methyl 4-(7-phenylheptanamido)octanoate (41b)
Yield 70%; White solid; m.p. 62-64 °C; [α]D = +9.3 (c 1 CHCl3); 1H NMR (200 MHz, CDCl3) δ 7.40-7.23 (m, 5H), 5.45 (d, J = 8.2 Hz, 1H), 4.12-3.95 (m, 1H), 3.74 (s, 3H), 2.69 (t, J = 7.0 Hz, 2H), 2.43 (t, J =7.4 Hz, 2H), 2.23 (t, J = 7.4 Hz, 2H), 2.05-1.85 (m, 1H), 1.79-1.60 (m, 5H), 1.55-1.36 (m, 10H), 0.97 (t, J = 6.2 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 174.2, 172.7, 142.6, 128.3, 128.2, 125.5, 51.6, 48.8, 36.8, 35.8, 31.2, 30.8, 30.0, 29.1, 28.9, 28.0, 25.7, 22.5, 13.9; Anal. calcd for C22H35NO3: C, 73.09; H, 9.76; N, 3.87. Found: C, 72.77; H, 9.97; N, 3.95.
5.2.2. Oxidation of 2-hydroxy-amides
To a solution of 2-hydroxy-amide (1 mmol) in dry CH2Cl2 (10 mL) Dess-Martin periodinane was added (0.64 g, 1.5 mmol) and the mixture was stirred for 1 h at rt. The organic solution was washed with 10% aqueous NaHCO3, dried over Na2SO4 and the organic solvent was evaporated under reduced pressure. The residue was purified by column-chromatography using CHCl3 as eluent.
5.2.2.1. Methyl 2-(2-oxohexadecanamido)acetate (8a)
Yield 91%; White solid; m.p. 79-81 °C; 1H NMR (200 MHz, CDCl3) δ 7.42-7.36 (m, 1H), 4.09 (d, J = 5.8 Hz, 2H), 3.79 (s, 3H), 2.91 (t, J = 7.8 Hz, 2H), 1.68-1.52 (m, 2H), 1.25 (br s, 22H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 198.2, 169.3, 160.2, 52.5, 40.9, 36.7, 31.9, 29.62, 29.56, 29.4, 29.33, 29.30, 29.0, 23.1, 22.7, 14.1; MS (ESI): m/z (%): 364 (51) [M + Na]+; Anal. calcd for C19H35NO4: C, 66.83; H, 10.33; N, 4.10. Found: C, 66.92; H, 10.25; N, 4.04.
5.2.2.2. Ethyl 2-(2-oxohexadecanamido)acetate (8b)
Yield 84%; White solid; m.p. 66-68 °C; 1H NMR (200 MHz, CDCl3) δ 7.10-6.91 (m, 1H), 4.22 (q, J = 7 Hz, 2H), 4.07 (d, J = 5.4 Hz, 2H), 2.90 (t, J = 7.2 Hz, 2H), 1.89-1.56 (m, 4H), 1.53-1.08 (br s, 23H), 0.86 (t, J = 7 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 198.2, 168.8, 160.2, 61.7, 41.0, 36.7, 31.9, 29.6, 29.5, 29.4, 29.30, 29.26, 29.0, 23.1, 22.6, 14.1; MS (ESI): m/z (%): 378 (100) [M + Na]+, 356 (5) [M + H]+; Anal. calcd for C20H37NO4: C, 67.57; H, 10.49; N, 3.94. Found: C, 67.71; H, 10.42; N, 3.89.
5.2.2.3. tert-Butyl 2-(2-oxohexadecanamido)acetate (8c)
Yield 81%; White solid; m.p. 54-56 °C; 1H NMR (200 MHz, CDCl3) δ 7.43-7.32 (m, 1H), 3.97 (d, J = 5.6 Hz, 2H), 2.91 (t, J = 7.4 Hz, 2H), 1.65-1.52 (m, 2H), 1.48 (s, 9H), 1.25 (br s, 22H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 198.3, 167.9, 160.1, 82.7, 41.7, 36.7, 31.9, 29.63, 29.57, 29.4, 29.34, 29.30, 29.0, 28.0, 23.1, 22.7, 14.1; MS (ESI): m/z (%): 406 (78) [M + Na]+; Anal. calcd for C22H41NO4: C, 68.89; H, 10.77; N, 3.65. Found: C, 68.95; H, 10.69; N, 3.72.
5.2.2.4. Methyl 3-(2-oxohexadecanamido)propanoate (8d)
Yield 74%; White solid; m.p. 83-86 °C; 1H NMR (200 MHz, CDCl3) δ 7.45-7.33 (m, 1H), 3.71 (s, 3H), 3.62-3.53 (m, 2H), 2.89 (t, J = 6.6 Hz, 2H), 2.58 (t, J = 6.2 Hz, 2H), 1.62-1.52 (m, 2H), 1.25 (br s, 22H), 0.88 (t, J = 7 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 198.9, 172.2, 160.2, 51.9, 36.7, 34.7, 33.5, 31.9, 29.61, 29.56, 29.4, 29.30, 29.32, 29.0, 23.1, 22.7, 14.1; MS (ESI): m/z (%): 378 (100) [M + Na]+, 356 (9) [M + H]+; Anal. calcd for C20H37NO4: C, 67.57; H, 10.49; N, 3.94. Found: C, 67.46; H, 10.53; N, 3.98.
5.2.2.5. Ethyl 3-(2-oxohexadecanamido)propanoate (8e)
Yield 83%; White solid; m.p. 60-62 °C; 1H NMR (200 MHz, CDCl3) δ 7.42 (t, J = 6.2 Hz, 1H), 4.16 (q, J = 7.2 Hz, 2H), 3.61-3.72 (m, 2H), 2.89 (t, J = 7.4 Hz, 2H), 2.56 (t, J = 6.2 Hz, 2H), 1.78-1.18 (m, 27H), 0.87 (t, J = 6.4 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 198.9, 171.8, 160.1, 60.9, 36.7, 34.7, 33.7, 31.9, 29.6, 29.5, 29.4, 29.3, 29.0, 23.1, 22.7, 14.1; MS (FAB): m/z (%): 370 (100) [M + H]+; Anal. calcd for C21H39NO4: C, 68.25; H, 10.64; N, 3.79. Found: C, 68.17; H, 10.61; N, 3.84.
5.2.2.6. tert-Butyl 3-(2-oxohexadecanamido)propanoate (8f)
Yield 75%; White solid; m.p. 44-45 °C; 1H NMR (200 MHz, CDCl3) δ 7.43 (t, J = 6.2 Hz, 1H), 3.57-3.48 (m, 2H), 2.90 (t, J = 7.4 Hz, 2H), 2.47 (t, J = 5.8 Hz, 2H), 1.62-1.55 (m, 2H), 1.45 (s, 9H), 1.24 (br s, 22H), 0.87 (t, J = 7 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 199.0, 171.0, 160.0, 81.4, 36.7, 34.8, 31.9, 29.61, 29.55, 29.4, 29.3, 29.0, 28.0, 23.1, 22.7, 14.1; MS (ESI): m/z (%): 420 (100) [M + Na]+; Anal. calcd for C23H43NO4: C, 69.48; H, 10.90; N, 3.52. Found: C, 69.41; H, 10.84; N, 3.62.
5.2.2.7. Benzyl 4-(2-oxohexadecanamido)butanoate (8g)
Yield 91%; White solid; m.p. 68-70 °C; 1H NMR (200 MHz, CDCl3) δ 7.42-7.29 (m, 5H), 7.11 (t, J = 5.8 Hz, 1H), 5.13 (s, 2H), 3.40-3.30 (m, 2H), 2.90 (t, J = 7.4 Hz, 2H), 2.42 (t, J = 7.2 Hz, 2H), 1.98-1.84 (m, 2H), 1.68-1.53 (m, 2H), 1.26 (br s, 22H), 0.89 (t, J = 6.2 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 199.2, 172.7, 160.3, 135.7, 128.6, 128.31, 128.25, 66.5, 38.6, 36.7, 31.9, 31.5, 29.6, 29.5, 29.4, 29.3, 29.0, 24.4, 23.1, 22.7, 14.1; MS (ESI): m/z (%): 468 (100) [M + Na]+; Anal. calcd for C27H43NO4: C, 72.77; H, 9.73; N, 3.14. Found: C, 72.86; H, 9.57; N, 3.23.
5.2.2.8. Allyl 4-(2-oxohexadecanamido)butanoate (8h)
Yield 83%; White solid; m.p. 60-62 °C; 1H NMR (200 MHz, CDCl3) δ 7.10 (t, J = 6.2 Hz, 1H), 6.02-5.82 (m, 1H), 5.37-5.22 (m, 2H), 4.61-4.58 (m, 2H), 3.41-3.31 (m, 2H), 2.91 (t, J = 7.6 Hz, 2H), 2.40 (t, J = 7.2 Hz, 2H), 2.05-1.84 (m, 2H), 1.64-1.56 (m, 2H), 1.26 (br s, 22H), 0.88 (t, J = 6.8 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 199.2, 172.5, 160.3, 132.0, 118.5, 65.3, 38.6, 36.7, 31.9, 31.5, 29.6, 29.5, 29.4, 29.3, 29.0, 24.4, 23.2, 22.7, 14.1; MS (ESI): m/z (%): 418 (100) [M + Na]+; Anal. calcd for C23H41NO4: C, 69.83; H, 10.45; N, 3.54. Found: C, 69.72; H, 10.52; N, 3.47.
5.2.2.9. Methyl 5-(2-oxohexadecanamido)pentanoate (8i)
Yield 93%; White solid; m.p. 68-70 °C; 1H NMR (200 MHz, CDCl3) δ 7.03 (t, J = 6.2 Hz, 1H), 3.68 (s, 3H), 3.36-3.27 (m, 2H), 2.91 (t, J = 7.2 Hz, 2H), 2.35 (t, J = 6.6 Hz, 2H), 1.70-1.56 (m, 6H), 1.25 (br s, 22H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 199.3, 173.7, 160.2, 51.6, 38.8, 36.7, 33.4, 31.9, 29.6, 29.5, 29.4, 29.3, 29.0, 28.6, 23.1, 22.7, 22.0, 14.1; MS (ESI): m/z (%): 406 (100) [M + Na]+; Anal. calcd for C22H41NO4: C, 68.89; H, 10.77; N, 3.65. Found: C, 68.97; H, 10.71; N, 3.62.
5.2.2.10. Ethyl 5-(2-oxohexadecanamido)pentanoate (8j)
Yield 87%; White solid; m.p. 59-61 °C; 1H NMR (200 MHz, CDCl3) δ 7.03 (t, J = 6.2 Hz, 1H), 4.13 (q, J = 7.2 Hz, 2H), 3.36-3.26 (m, 2H), 2.91 (t, J = 7.4 Hz, 2H), 2.33 (t, J = 7 Hz, 2H), 1.75-1.56 (m, 6H), 1.25 (m, 25H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 199.3, 173.3, 160.2, 60.4, 38.8, 36.7, 33.7, 31.9, 29.62, 29.57, 29.4, 29.31, 29.33, 29.0, 28.6, 23.1, 22.7, 22.0, 14.2, 14.1; MS (FAB): m/z (%): 398 (100) [M + H]+; Anal. calcd for C23H43NO4: C, 69.48; H, 10.90; N, 3.52. Found: C, 69.34; H, 10.97; N, 3.58.
5.2.2.11. tert-Butyl 5-(2-oxohexadecanamido)pentanoate (8k)
Yield 77%; White solid; m.p. 48-50 °C; 1H NMR (200 MHz, CDCl3) δ 7.04 (t, J = 6.2 Hz, 1H), 3.34-3.24 (m, 2H), 2.89 (t, J = 7.4 Hz, 2H), 2.23 (t, J = 6.6 Hz, 2H), 1.65-1.48 (m, 6H), 1.43 (s, 9H), 1.23 (br s, 22H), 0.86 (t, J = 7 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 199.3, 172.6, 160.1, 80.3, 38.9, 36.7, 34.8, 31.9, 29.6, 29.5, 29.4, 39.3, 29.0, 28.6, 28.0, 23.1, 22.6, 22.1, 14.1; MS (ESI): m/z (%): 448 (100) [M + Na]+; Anal. calcd for C25H47NO4: C, 70.54; H, 11.13; N, 3.29. Found: C, 70.65; H, 11.05; N, 3.21.
5.2.2.12. Ethyl 4-(2-oxohexadecanamido)benzoate (8l)
Yield 84%; White solid; m.p. 86-88 °C; 1H NMR (200 MHz, CDCl3) δ 8.87 (s, 1H), 8.19-7.43 (m, 4H), 4.39 (q, J = 7.0 Hz, 2H), 3.02 (t, J = 7.2 Hz, 2H), 1.78-1.12 (m, 27H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 199.2, 165.9, 157.7, 136.5, 131.5, 129.3, 126.2, 123.9, 120.6, 61.2, 36.3, 31.9, 29.63, 29.57, 29.4, 29.34, 29.30, 29.1, 23.3, 22.7, 14.3, 14.1; MS (FAB): m/z (%): 418 (100) [M + H]+; Anal. calcd for C25H39NO4: C, 71.91; H, 9.41; N, 3.35. Found: C, 71.74; H, 9.57; N, 3.29.
5.2.2.13. (R)-Methyl 6-methyl-4-(2-oxohexadecanamido)heptanoate (12a)
Yield 76%; Yellowish solid; m.p. 41-43 °C; [α]D = −9.0 (c 1.0, CHCl3); 1H NMR (200 MHz, CDCl3) δ 6.75-6.60 (m, 1H), 4.10-3.85 (m, 1H), 3.66 (s, 3H), 2.91 (t, J = 7.4 Hz, 2H), 2.32 (t, J = 7.4 Hz, 2H), 1.95- 1.40 (m, 5H), 1.40-1.18 (m, 24H), 0.95-0.81 (m, 9H). Anal. calcd for C25H47NO4: C, 70.54; H, 11.13; N, 3.29. Found: C, 70.39; H, 11.35; N, 3.38.
5.2.2.14. (S)-Methyl 4-(2-oxohexadecanamido)octanoate (12b)
Yield 92%; White solid; m.p. 56-58 °C; [α]D = −4.6 (c 1.0, CHCl3); 1H NMR (200 MHz, CDCl3) δ 6.80-6.60 (m, 1H), 4.20-3.80 (m, 1H), 3.66 (s, 3H), 2.91 (t, J = 7.4 Hz, 2H), 2.35 (t, J = 7.4 Hz, 2H), 1.97-1.42 (m, 6H), 1.38-1.15 (m, 26H), 0.98-0.81 (m, 6H); 13C NMR (50 MHz, CDCl3) δ 199.5, 173.6, 160.0, 51.7, 49.3, 36.8, 34.8, 31.9, 30.7, 29.9, 29.6, 29.5, 29.4, 29.3, 29.1, 27.9, 23.2, 22.7, 22.4, 14.1, 13.9. Anal. calcd for C25H47NO4: C, 70.54; H, 11.13; N, 3.29. Found: C, 70.25; H, 11.32; N, 3.25.
5.2.2.15. (S)-tert-Butyl 4-(2-oxohexadecanamido)pentanoate (12c)
Yield 95%; White solid; m.p. 52-53 °C; [α]D = −1.6 (c 1.0, CHCl3); 1H NMR (200 MHz, CDCl3) δ 6.95-6.80 (m, 1H), 4.05-3.80 (m, 1H), 2.85 (t, J = 7.4 Hz, 2H), 2.25 (t, J = 7.4 Hz, 2H), 1.85-1.65 (m, 2H), 1.65-1.45 (m, 2H), 1.40 (s, 9H), 1.40-1.10 (m, 25H), 0.88 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 199.3, 172.3, 159.6, 80.4, 45.2, 36.6, 32.1, 31.8, 31.3, 29.5, 29.4, 29.3, 29.2, 29.0, 28.0, 23.1, 22.6, 20.6, 14.0. Anal. calcd for C25H47NO4: C, 70.54; H, 11.13; N, 3.29. Found: C, 70.38; H, 11.25; N, 3.21.
5.2.2.16. (S,2E,4E)-Ethyl 6-(2-oxohexadecanamido)deca-2,4-dienoate (17)
Yield 91%; White solid; m.p. 53-55 °C; [α]D = −11.2 (c 0.5 CHCl3); 1H NMR (200 MHz, CDCl3) δ 7.22 (dd, J1 = 15.2 Hz, J2 = 10.8 Hz, 1H), 6.95 (d, J = 8.8 Hz, 1H), 6.25 (dd, J1 = 15.2 Hz, J2 = 10.6 Hz, 1H), 6.00 (dd, J1 = 15.2 Hz, J2 = 6.2 Hz, 1H), 5.86 (d, J = 15.8 Hz, 1H), 4.74 (m, 1H), 4.19 (q, J = 7.2 Hz, 2H), 2.91 (t, J = 7.4 Hz, 2H), 1.73-1.44 (m, 4H), 1.28 (br s, 29H), 0.88 (m, 6H); 13C NMR (50 MHz, CDCl3) δ 199.3, 166.7, 159.4, 143.3, 141.0, 128.7, 122.0, 60.3, 50.9, 36.7, 34.3, 31.9, 29.6, 29.5, 29.4, 29.3, 29.0, 27.7, 23.1, 22.6, 22.3, 14.2, 14.1, 13.9; MS (FAB): m/z (%): 464 (37) [M + H]+; Anal. calcd for C28H49NO4: C, 72.53; H, 10.65; N, 3.02. Found: C, 72.28; H, 10.74; N, 3.09.
5.2.2.17. (S)-Ethyl 6-(2-oxohexadecanamido)decanoate (18)
Yield 93%; White solid; m.p. 47-49 °C; [α]D = −1.6 (c 0.5 CHCl3); 1H NMR (200 MHz, CDCl3) δ 6.66 (d, J = 9.6 Hz, 1H), 4.11 (q, J = 7.2 Hz, 2H), 4.00-3.81 (m, 1H), 2.92 (t, J = 7.4 Hz, 2H), 2.28 (t, J = 7.4 Hz, 2H), 1.80-1.21 (m, 39H), 0.88 (t, J = 6.2 Hz, 6H); 13C NMR (50 MHz, CDCl3) δ 199.7, 173.5, 159.8, 60.2, 49.4, 36.7, 34.7, 34.1, 31.9, 29.6, 29.4, 29.3, 29.1, 27.9, 25.4, 24.7, 23.2, 22.7, 22.5, 14.2, 14.1, 13.9; MS (FAB): m/z (%): 468 (87) [M + H]+; Anal. calcd for C28H53NO4: C, 71.90; H, 11.42; N, 2.99. Found: C, 71.77; H, 11.51; N, 3.08.
5.2.2.18. (S,2E,4E)-6-(2-Oxohexadecanamido)deca-2,4-dienoic acid (19)
Saponification of 15 took place before oxidation. Yield 53%; White solid; m.p. 91-93 °C; [α]D = −35.1 (c 1 CHCl3); 1H NMR (200 MHz, CDCl3) δ 7.20 (dd, J1 = 15.2 Hz, J2 = 10.8 Hz, 1H), 6.85 (d, J = 9 Hz, 1H), 6.18 (dd, J1 = 15.2 Hz, J2 = 11 Hz, 1H), 5.92 (dd, J1 = 15.4 Hz, J2 = 6.2 Hz, 1H), 5.77 (d, J = 15.4 Hz, 1H), 4.57-4.43 (m, 1H), 2.82 (t, J = 7.2 Hz, 2H), 1.76-1.43 (m, 4H), 1.14 (br s, 26H), 0.88 (m, 6H); 13C NMR (50 MHz, CDCl3) δ 199.3, 171.8, 159.5, 145.7, 142.3, 128.4, 121.0, 50.9, 36.7, 34.3, 31.9, 29.6, 29.5, 29.4, 29.3, 29.0, 27.7, 23.1, 22.6, 22.3, 14.1, 13.9; MS (FAB): m/z (%): [M + H]+; Anal. calcd for C26H45NO4: C, 71.68; H, 10.41; N, 3.22. Found: C, 71.51; H, 10.52; N, 3.25.
5.2.2.19. 2-Oxo-N-(4-oxooctyl)hexadecanamide (26)
Yield 79%; White solid; m.p. 73-76 °C; 1H NMR (200 MHz, CDCl3) δ 7.09 (t, J = 6.4 Hz, 1H), 3.35-3.25 (m, 2H), 2.90 (t, J = 7.2 Hz, 2H), 2.50-2.36 (m, 4H), 1.98-1.76 (m, 2H), 1.67-1.45 (m, 4H), 1.25 (br s, 24H), 0.88 (t, J = 6.6 Hz, 6H); 13C NMR (50 MHz, CDCl3) δ 210.3, 199.2, 160.3, 42.6, 39.7, 38.8, 36.7, 31.9, 29.6, 29.5, 29.4, 29.3, 29.0, 25.9, 23.2, 23.1, 22.7, 22.3, 14.1, 13.8; MS (FAB): m/z (%): 396 (100) [M + H]+; Anal. calcd for C24H45NO3: C, 72.86; H, 11.46; N, 3.54. Found: C, 72.74; H, 11.51; N, 3.59.
5.2.2.20. N-(4-Ethoxybutyl)-2-oxohexadecanamide (27)
Yield 88%; White solid; m.p. 55-57 °C; 1H NMR (200 MHz, CDCl3) δ 7.19 (t, J = 6.4 Hz, 1H), 3.52-3.25 (m, 6H), 2.90 (t, J = 7.8 Hz, 2H), 1.78-1.48 (m, 6H), 1.40-1.15 (m, 25H), 0.87 (t, J = 6.6 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 199.4, 160.2, 69.9, 66.2, 39.0, 36.7, 31.9, 29.6, 29.5, 29.4, 29.3, 29.0, 27.1, 26.2, 23.1, 22.6, 15.1, 14.1; MS (FAB): m/z (%): 370 (100) [M + H]+; Anal. calcd for C22H43NO3: C, 71.50; H, 11.73; N, 3.79. Found: C, 71.43; H, 11.65; N, 3.91.
5.2.2.21. Methyl 2-(2-oxo-8-phenyloctanamido)acetate (37a)
Yield 82%; Oily solid; 1H NMR (200 MHz, CDCl3) δ 7.55-7.42 (m, 1H), 7.40-7.23 (m, 5H), 4.16 (d, J = 5.4 Hz, 2H), 3.86 (s, 3H), 3.00 (t, J = 6.8 Hz, 2H), 2.69 (t, J = 7.2 Hz, 2H), 1.80-1.62 (m, 4H), 1.55-1.35 (m, 4H); 13C NMR (50 MHz, CDCl3) δ 198.1, 169.2, 160.2, 142.6, 128.3, 128.2, 125.6, 52.5, 40.8, 36.6, 35.8, 31.2, 28.9, 28.8, 23.0; MS (ESI) m/z(%): 328 (100) [M+Na]+; Anal. calcd for C17H23NO4: C, 66.86; H, 7.59; N, 4.59. Found: C, 66.48; H, 7.78; N, 4.65.
5.2.2.22. tert-Butyl 3-(2-oxo-8-phenyloctanamido)propanoate (37b)
Yield 83%; Oil; 1H NMR (200 MHz, CDCl3) δ 7.58-7.42 (m, 1H), 7.40-7.22 (m, 5H), 3.61 (q, J = 6.2 Hz, 2H), 2.99 (t, J = 7.0 Hz, 2H), 2.68 (t, J = 7.4 Hz, 2H), 2.56 (t, J = 6.2 Hz, 2H), 1.79-1.60 (m, 4H), 1.54 (s, 9H), 1.49-1.40 (m, 4H); 13C NMR (50 MHz, CDCl3) δ 198.9, 171.0, 160.0, 142.6, 128.3, 128.2, 125.5, 81.3, 36.6, 35.8, 34.8, 34.7, 31.2, 28.9, 28.8, 28.0, 23.0; MS (ESI) m/z(%): 384 (100) [M+Na]+; Anal. calcd for C21H31NO4: C, 69.78; H, 8.64; N, 3.87. Found: C, 69.40; H, 8.89; N, 3.94.
5.2.3. (R,E)-Ethyl 4-(tert-butoxycarbonyl)oct-2-enoate (39a)
To a solution of 38 (0.22g, 1.00 mmol) in a mixture of toluene-EtOAc (6 mL), a solution of NaBr (0.11 g, 1.05 mmol) in water (0.5 mL) was added, followed by AcNH-TEMPO (2.2 mg, 0.010 mmol). To the resulting biphasic system, which was cooled at −5 °C, an aqueous solution of 0.35 M NaOCl (3.14 mL, 1.10 mmol) containing NaHCO3 (0.25 g, 3 mmol) was added dropwise while stirring vigorously at −5 °C over a period of 1 h. After the mixture had been stirred for a further 15 min at 0 °C, EtOAc (6 mL) and H2O (2 mL) were added. The aqueous layer was separated and washed with EtOAc (4 mL). The combined organic layers were washed consecutively with 5% aqueous citric acid (6 mL) containing KI (0.04g), 10% aqueous Na2S2O3 (6 mL), and brine and dried over Na2SO4. The solvents were evaporated under reduced pressure, and the residue was used immediately in the next step without any purification. To the solution of the N-protected α-amino aldehyde in dry THF (5 mL), Ph3P=CHCOOEt (1.1 mmol) was added and the reaction mixture was refluxed for 1 h. Saturated aqueous NH4Cl (4 mL) was added and extracted with Et2O (3 × 5 mL). The combined organic phases were washed with brine and dried (Na2SO4). The solvent was evaporated under reduced pressure, and the residue was purified by column chromatography [EtOAc-petroleum ether (bp 40-60 °C) 2:8]. Yield 80% (0.23g); White solid; m.p. 48-50 °C; [α]D = +10.1 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δ 6.84 (dd, J1 = 5.6 Hz, J2 = 15.8 Hz, 1H), 5.90 (dd, J1 = 1.4 Hz, J2 = 15.8 Hz, 1H), 4.52 (m, 1H), 4.38-4.05 (m, 3H), 1.80-1.10 (m, 18H), 0.89 (t, J = 6.2 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 166.4, 155.1, 148.6, 120.5, 79.7, 60.4, 51.4, 34.3, 28.3, 27.7, 22.4, 14.2, 13.9; Anal. calcd for C15H27NO4: C, 63.13; H, 9.54; N, 4.91. Found: C, 63.02; H, 9.69; N, 4.85.
5.2.4. General Method for Catalytic Hydrogenation
To a solution of the substrate (1.00 mmol) in MeOH (10 mL) (through which N2 had been passed for 5 min), 10% Pd/C catalyst (10 mg, 0.01 mmol) was added. The reaction mixture was stirred under H2 atmosphere overnight at room temperature. The catalyst was removed by filtration through a pad of Celite, and the organic solvent was evaporated under reduced pressure.
5.2.4.1. (S)-Ethyl 6-(tert-butoxycarbonylamino)decanoate (14)
Yield 89%; Oil; 1H NMR (200 MHz, CDCl3) δ 4.26 (d, J = 9.2 Hz, 1H), 4.10 (q, J = 7 Hz, 2H), 3.64-3.41 (m, 1H), 2.28 (t, J = 7.4 Hz, 2H), 1.71-1.16 (m, 24H), 0.87 (t, J = 6.2 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 173.7, 155.7, 78.8, 60.1, 50.4, 35.2, 34.2, 28.3, 28.0, 25.4, 24.9, 22.6, 14.2, 14.0. Anal. calcd for C17H33NO4: C, 64.73; H, 10.54; N, 4.44. Found: C, 64.69; H, 10.61; N, 4.46.
5.2.4.2. (R)-Ethyl 4-(tert-butoxycarbonyl)octanoate (40a)
Yield 99%; White solid; m.p. 32-34 °C; [α]D = +3.4 (c 1 CHCl3); 1H NMR (200 MHz, CDCl3) δ 4.29 (d, J = 9.2 Hz, 1H), 4.13 (q, J = 6.4 Hz, 2H), 3.62-3.45 (m, 1H), 2.36 (t, J = 7.6 Hz, 2H) 1.89-1.78 (m, 2H), 1.76-1.58 (m, 2H), 1.43 (s, 9H), 1.40-1.21 (m, 7H), 0.88 (t, J = 6.4 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 173.7, 155.7, 79.0, 60.4, 50.3, 35.5, 31.1, 30.5, 28.4, 28.0, 22.5, 14.2, 14.0; Anal. calcd for C15H29NO4: C, 62.69; H, 10.17; N, 4.87. Found: C, 62.55; H, 10.23; N, 4.95.
5.2.5. tert-Butyl 4-oxooctylcarbamate (22)
To a stirred solution of Boc-γ-aminobutyric acid (0.40 g, 2.0 mmol) in dry THF (2 mL), cooled at −78 °C, a solution of n-BuLi in hexane (3 mL, 2.5M) was added dropwise. After completion of the addition, the reaction mixture was allowed to warm to room temperature and stirring was continued for 4 hours. Then, saturated NH4Cl (5 mL) and EtOAc (10 mL) were added. The aqueous layer was separated and extracted with EtOAc (2×10 mL). The combined organic layers were dried over Na2SO4, the organic solvent was evaporated under reduced pressure and the residue was purified by column-chromatography using CHCl3 as eluent. Yield 46%; White solid; m.p. 36-38 °C; 1H NMR (200 MHz, CDCl3) δ 4.68 (br, 1H), 3.13-3.03 (m, 2H), 2.45-2.33 (m, 4H), 1.87-1.65 (m, 2H), 1.55-1.23 (m, 13H), 0.86 (t, J = 7.2 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 210.7, 156.0, 79.0, 42.5, 40.0, 39.7, 28.3, 25.8, 23.9, 22.2, 13.7. Anal. calcd for C13H25NO3: C, 64.16; H, 10.36; N, 5.76. Found: C, 64.10; H, 10.42; N, 5.71.
5.2.6. tert-Butyl 4-ethoxybutylcarbamate (23)
To a stirred solution of tert-butyl 4-hydroxybutylcarbamate (0.38 g, 2.0 mmol) and ethyl bromide (0.65 g, 6 mmol) in benzene (2 mL) at 10 °C, aq. 50% NaOH (2 mL) and phase transfer catalyst Bu4NHSO4 (0.08 g, 0.3 mmol) were added. The reaction mixture was vigorously stirred for 4 h at 10 °C and overnight at room temperature. The aqueous layer was separated and extracted with EtOAc (2×5 mL). The combined organic layers were washed with brine and dried over Na2SO4. The organic solvent was evaporated under reduced pressure and the residue was purified by column-chromatography using CHCl3 as eluent. Yield 95%; Oil; 1H NMR (200 MHz, CDCl3) δ 4.72 (br, 1H), 3.46-3.34 (m, 4H), 3.19-3.06 (m, 2H), 1.60-1.43 (m, 4H), 1.38 (s, 9H), 1.14 (t, J = 7.4 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 155.9, 78.7, 70.1, 66.0, 40.3, 28.3, 27.0, 26.8, 15.0. Anal. calcd for C11H23NO3: C, 60.80; H, 10.67; N, 6.45. Found: C, 60.55; H, 10.81; N, 6.32.
5.2.7. 2-Hydroxy-8-phenyloctanenitrile (34)
To a stirred solution of 33 (0.45 g, 2.4 mmol) in CH2Cl2 (3 mL), a solution of NaHSO3 (0.37 g, 3.5 mmol) in water (0.6 mL) was added and the mixture was vigorously stirred for 30 min at room temperature. The organic solvent was evaporated under reduced pressure, water was added and the mixture was cooled at 0 °C. Then, a solution of KCN (0.23 g, 3.5 mmol) in water (0.6 mL) was added drop-wise over a period of 3.5 h and then the mixture was left under stirring overnight at room temperature. The aqueous suspension was extracted with CH2Cl2 (2×5 mL). The combined organic layers were washed with brine and dried over Na2SO4. The solvent was evaporated under reduced pressure and the residue was purified by column-chromatography using CHCl3 as eluent. Yield 75%; Oil; 1H NMR (200 MHz, CDCl3) δ 7.40-7.22 (m, 5H), 4.58-4.35 (m, 1H), 3.90-3.78 (m, 1H), 2.69 (t, J = 6.6 Hz, 2H), 1.95-1.80 (m, 2H), 1.80-1.60 (m, 2H), 1.60-1.40 (m, 6H); 13C NMR (50 MHz, CDCl3) δ 142.4, 128.2, 128.1, 125.5, 120.0, 60.9, 35.7, 34.8, 31.1, 28.8, 28.6, 24.3; Anal. calcd for C14H19NO: C, 77.38; H, 8.81; N, 6.45. Found: C, 77.11; H, 8.95; N, 6.37.
5.2.8. 2-Hydroxy-8-phenyloctanoic acid (35)
A solution of 34 (0.36 g, 1.7 mmol) in conc. HCl (4.2 mL) was stirred overnight at room temperature. Then, water was added and the aqueous solution was extracted with CHCl3 (3×5 mL). The combined organic layers were washed with brine and dried over Na2SO4. The organic solvent was evaporated under reduced pressure and the resulting 2-hydroxy-8-phenyloctanamide was precipitated using Et2O.
To a solution of 2-hydroxy-8-phenyloctanamide (0.32 g, 1.3 mmol) in a mixture of EtOH/water (2:1, 10 mL), KOH (0.75 g, 13.4 mmol) was added and the reaction mixture was refluxed for 4 h. After cooling, EtOH was removed under reduced pressure, water was added and the aqueous solution was acidified with conc. H2SO4 until pH 1, followed by extraction with Et2O (3×5 mL). The combined organic layers were washed with brine and dried over Na2SO4. The organic solvent was evaporated under reduced pressure and the residue was purified by recrystallization using CH2Cl2/petroleum ether. Yield 85%; White solid; m.p. 84-86 °C; 1H NMR (200 MHz, CDCl3) δ 7.35-7.24 (m, 5H), 4.40-4.25 (m, 1H), 2.68 (t, J = 6.6 Hz, 2H), 2.00-1.82 (m, 2H), 1.80-1.58 (m, 3H), 1.58-1.15 (m, 6H); 13C NMR (50 MHz, CDCl3) δ 179.5, 142.6, 128.4, 128.2, 125.6, 70.2, 35.9, 34.1, 29.0, 24.6; Anal. calcd for C14H20O3: C, 71.16; H, 8.53. Found: C, 70.92; H, 8.84.
5.2.9. (R)-4-(7-Phenylheptanamido)octanoic acid (42)
To a stirred solution of 41a (or 41b) (2.00 mmol) in a mixture of dioxane/H2O (9:1, 20 mL), 1N NaOH (2.2 mL, 2.2 mmol) was added, and the mixture was stirred for 12 h at room temperature. The organic solvent was evaporated under reduced pressure, and H2O (10 mL) was added. The aqueous layer was washed with EtOAc, acidified with 1N HCl, and extracted with EtOAc (3×12 mL). The combined organic layers were washed with brine, dried over Na2SO4, and evaporated under redused pressure. The residue was purified after recrystalliztion [EtOAc/petroleum ether (bp 40-60 °C)]. Yield 90%; White solid; m.p. 73-75 °C; [α]D = −5.7 (c 1 CHCl3); 1H NMR (200 MHz, CDCl3) δ 11.10 (br s, 1H), 7.39-7.23 (m, 5H), 5.86 (d, J = 9.0 Hz, 1H), 4.10-3.94 (m, 1H), 2.68 (t, J = 7.4 Hz, 2H), 2.46 (t, J =7.4 Hz, 2H), 2.26 (t, J = 7.4 Hz, 2H), 2.04-1.87 (m, 1H), 1.82-1.57 (m, 5H), 1.57-1.09 (m, 10H), 0.97 (t, J = 6.4 Hz, 3H); 13C NMR (50 MHz, CDCl3) δ 177.4, 173.9, 142.5, 128.3, 128.1, 125.5, 49.0, 36.6, 35.8, 34.9, 31.2, 31.1, 30.2, 29.0, 28.8, 28.0, 25.7, 22.4, 13.9; MS (ESI) m/z(%): 370 (100) [M+Na]+; Anal. calcd for C21H33NO3: C, 72.58; H, 9.57; N, 4.03. Found: C, 72.26; H, 9.74; N, 4.11.
Compounds 10a-c,26 13,30 29,49 30,50 31,35 33,51 38,52 39b,26 40b26 have been described previously.
5.3. In-vitro PLA2 Assays
Phospholipase A2 activity was determined using the previously described modified Dole assay25 with buffer and substrate conditions optimized for each enzyme as described previously:25,26,29,30 (i) GIVA cPLA2 substrate mixed-micelles were composed of 400 μM Triton X-100, 97 μM PAPC, 1.8 μM 14C-labeled PAPC, and 3 μM PIP2 in buffer containing 100 mM HEPES pH 7.5, 90 μM CaCl2, 2 mM DTT and 0.1 mg/ml BSA; (ii) GVI iPLA2 substrate mixed-micelles were composed of 400 μM Triton X-100, 99 μM DPPC, and 1.5 μM 14C-labeled DPPC in buffer containing 200 mM HEPES pH 7.0, 1 mM ATP, 2 mM DTT and 0.1 mg/ml BSA; and (iii) GV sPLA2 substrate mixed-micelles were composed of 400 μM Triton X-100, 99 μM DPPC, and 1.5 μM 14C-labeled DPPC in buffer containing 50 mM Tris pH 8.0 and 5 mM CaCl2.
5.4. In-vitro PLA2 Inhibition Studies
Initial screening of compounds at 0.091 mole fraction inhibitor in mixed-micelles was carried out. We considered compounds displaying 25% or less inhibition to have no inhibitory effect (designated N.D.). We report average percent inhibition (and standard error, n=3) for compounds displaying more than 25% and less than 90% enzyme inhibition. If percent inhibition was greater than 90%, we determined its XI(50) by plotting percent inhibition vs. inhibitor molar fraction (7 points; typically 0.005 to 0.091 mole fraction). Inhibition curves were modeled in Graphpad Prism using either a linear (x, y intercept = 0) or non-linear regression (one-site binding model - hyperbola, BMAX = 100) to calculate the reported XI(50) and associated error values.
Acknowledgements
The project is co-funded by the European Social Fund and National Resources (EPEAEK II) (G.K.) and by NIH Grant GM 20,501 (E.A.D).
References and notes
- 1.Schaloske RH, Dennis EA. Biochim. Biophys. Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Balsinde J, Winstead MV, Dennis EA. FEBS Lett. 2002;531:2–6. doi: 10.1016/s0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- 3.Six DA, Dennis EA. Biochim. Biophys. Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 4.Kudo I, Murakami M. Prostag. Oth. Lipid M. 2002;68-69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 5.Leslie CC. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70:373–376. doi: 10.1016/j.plefa.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Bonventre JV, Huang Z, Taheri MR, O’Leary E, Li E, Moskowitz MA, Sapirstein A. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 7.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, Miyazaki J, Shimizu T. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 8.Kalyvas A, David S. Neuron. 2004;41:323–335. doi: 10.1016/s0896-6273(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 9.Marusic S, Leach MW, Pelker JW, Azoitei ML, Uozumi N, Cui JQ, Shen MWH, DeClercq CM, Miyashiro JS, Carito BA, Thakker P, Simmons DL, Leonard JP, Shimizu T, Clark JD. J. Exp. Med. 2005;202:841–851. doi: 10.1084/jem.20050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For reviews see: Winstead MV, Balsinde J, Dennis EA. Biochim. Biophys. Acta. 2000;1488:28–39. doi: 10.1016/s1388-1981(00)00107-4.Balsinde J, Balboa MA. Cellular Signalling. 2005;17:1052–1062. doi: 10.1016/j.cellsig.2005.03.002.
- 11.Satake Y, Diaz BL, Balestrieri B, Lam BK, Kanaoka Y, Grusby MJ, Arm JP. J. Biol. Chem. 2004;279:16488–16494. doi: 10.1074/jbc.M313748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mounier CM, Ghomashchi F, Lindsay MR, James S, Singer AG, Parton RG, Gelb MH. J. Biol. Chem. 2004;279:25024–25038. doi: 10.1074/jbc.M313019200. [DOI] [PubMed] [Google Scholar]
- 13.Kuwata H, Nonaka T, Murakami M, Kudo I. J. Biol. Chem. 2005;280:25830–25839. doi: 10.1074/jbc.M500168200. [DOI] [PubMed] [Google Scholar]
- 14.Shirai Y, Balsinde J, Dennis EA. Biochim. Biophys. Acta. 2005;1735:119–129. doi: 10.1016/j.bbalip.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Ni Z, Okeley NM, Smart BP, Gelb MH. J. Biol. Chem. 2006;281:16245–16255. doi: 10.1074/jbc.M513874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Engle SJ, Seilhamer JJ, Tischfield JA. J. Biol. Chem. 1994;269:2365–2368. [PubMed] [Google Scholar]
- 17.Chen Y, Dennis EA. Biochim. Biophys. Acta. 1998;1394:57–64. doi: 10.1016/s0005-2760(98)00098-8. [DOI] [PubMed] [Google Scholar]
- 18.Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I. J. Biol. Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- 19.For recent reviews see: Balestrieri B, Arm JP. Biochim. Biophys. Acta. 2006;1761:1280–1288. doi: 10.1016/j.bbalip.2006.07.008.Rosengren B, Jonsson-Rylander A-C, Peilot H, Camejo G, Hurt-Camejo E. Biochim. Biophys. Acta. 2006;1761:1301–1308. doi: 10.1016/j.bbalip.2006.06.008.
- 20.Magrioti V, Kokotos G. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2006;5:189–203. [Google Scholar]
- 21.McKew JC, Foley MA, Thakker P, Behnke ML, Lovering FE, Sum F-W, Tam S, Wu K, Shem MWH, Zhang W, Gonzalez M, Liu S, Mahadevan A, Sard H. J. Med. Chem. 2006;49:135–158. doi: 10.1021/jm0507882. [DOI] [PubMed] [Google Scholar]
- 22.Lee KL, Foley MA, Chen LR, Behnke ML, Lovering FE, Kirincich SJ, Wang WH, Shim J, Tam S, Shen MWH, Khor SP, Xu X, Goodwin DG, Ramarao MK, Nickerson-Nutter C, Donahue F, Ku MS, Clark JD, Mckew JC. J. Med. Chem. 2007;50:1380–1400. doi: 10.1021/jm061131z. [DOI] [PubMed] [Google Scholar]
- 23.Lee KL, Behnke ML, Foley MA, Chen L, Wang W, Vargas R, Nunez J, Tam S, Mollova N, Xu X, Shen MWH, Ramarao MK, Goodwin DG, Nickerson-Nutter CL, Abraham WM, Williams C, Clark JD, McKew JC. Bioorg. Med. Chem. 2008;16:1345–1358. doi: 10.1016/j.bmc.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig J, Bovens S, Brauch C, Elfringhoff AS, Lehr M. J. Med. Chem. 2006;49:2611–2620. doi: 10.1021/jm051243a. [DOI] [PubMed] [Google Scholar]
- 25.Kokotos G, Kotsovolou S, Six DA, Constantinou-Kokotou V, Beltzner CC, Dennis EA. J. Med. Chem. 2002;45:2891–2893. doi: 10.1021/jm025538p. [DOI] [PubMed] [Google Scholar]
- 26.Kokotos G, Six DA, Loukas V, Smith T, Constantinou-Kokotou V, Hadjipavlou-Litina D, Kotsovolou S, Chiou A, Beltzner CC, Dennis EA. J. Med. Chem. 2004;47:3615–3628. doi: 10.1021/jm030485c. [DOI] [PubMed] [Google Scholar]
- 27.Constantinou-Kokotou V, Peristeraki A, Kokotos CG, Six DA, Dennis EA. J. Pept. Sci. 2005;11:431–435. doi: 10.1002/psc.628. [DOI] [PubMed] [Google Scholar]
- 28.Yaksh TL, Kokotos G, Svensson CI, Stephens D, Kokotos CG, Fitzsimmons B, Hadjipavlou-Litina D, Hua X-Y, Dennis EA. J. Pharmacol. Exp. Ther. 2006;316:466–475. doi: 10.1124/jpet.105.091686. [DOI] [PubMed] [Google Scholar]
- 29.Stephens D, Barbayianni E, Constantinou-Kokotou V, Peristeraki A, Six DA, Cooper J, Harkewicz R, Deems RA, Dennis EA, Kokotos G. J. Med. Chem. 2006;49:2821–2828. doi: 10.1021/jm050993h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Six DA, Barbayianni E, Loukas V, Constantinou-Kokotou V, Hadjipavlou-Litina D, Stephens D, Wong AC, Magrioti V, Moutevelis-Minakakis P, Baker S, Dennis EA, Kokotos G. J. Med. Chem. 2007;50:4222–4235. doi: 10.1021/jm0613673. [DOI] [PubMed] [Google Scholar]
- 31.Moutevelis-Minakakis P, Neokosmidi A, Filippakou M, Stephens D, Dennis EA, Kokotos G. J. Pept. Sci. 2007;13:634–641. doi: 10.1002/psc.889. [DOI] [PubMed] [Google Scholar]
- 32.Antonopoulou G, Magrioti V, Stephens D, Constantinou-Kokotou V, Dennis EA, Kokotos G. J. Pept. Sci. 2008 doi: 10.1002/psc.1048. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid RC. Curr. Med. Chem. 2005;12:3011–3026. doi: 10.2174/092986705774462860. [DOI] [PubMed] [Google Scholar]
- 34.Beaton HG, Bennion C, Connolly S, Cook AR, Gensmantel NP, Hallam C, Hardy K, Hitchin B, Jackson CG, Robinson DH. J. Med. Chem. 1994;37:557–559. doi: 10.1021/jm00031a001. [DOI] [PubMed] [Google Scholar]
- 35.Hansford KA, Reid RC, Clark CI, Tyndall JDA, Whitehouse MW, Guthrie T, McGeary RP, Schafer K, Martin JL, Fairlie DP. ChemBioChem. 2003;4:181–185. doi: 10.1002/cbic.200390029. [DOI] [PubMed] [Google Scholar]
- 36.Arumugam TV, Arnold N, Proctor LM, Newman M, Reid RC, Hansford KA, Fairlie DP, Shiels IA, Taylor SM. Br. J. Pharmacol. 2003;140:71–80. doi: 10.1038/sj.bjp.0705402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodruff TM, Arumugam TV, Shiels IA, Newman ML, Ross PA, Reid RC, Fairlie DP, Taylor SM. Intern. Immunopharm. 2005;5:883–892. doi: 10.1016/j.intimp.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Dess DB, Martin JC. J. Am. Chem. Soc. 1991;113:7277–7287. [Google Scholar]
- 39.Ma Z, Bobbitt JM. J. Org. Chem. 1991;56:6110–6114. [Google Scholar]
- 40.Leanna MR, Sowin TJ, Morton HE. Tetrahedron Lett. 1992;33:5029–5032. [Google Scholar]
- 41.Takacs JM, Jaber MR, Clement F, Walters C. J. Org. Chem. 1998;63:6757–6760. [Google Scholar]
- 42.Kokotos G. Synthesis. 1990:299–301. [Google Scholar]
- 43.Kokotos G, Noula C. J. Org. Chem. 1996;61:6994–6996. doi: 10.1021/jo9520699. [DOI] [PubMed] [Google Scholar]
- 44.Chiou A, Markidis T, Constantinou-Kokotou V, Verger R, Kokotos G. Org. Lett. 2000;2:347–350. doi: 10.1021/ol991295s. [DOI] [PubMed] [Google Scholar]
- 45.Kokotos G, Verger R, Chiou A. Chem. Eur. J. 2000;6:4211–4217. doi: 10.1002/1521-3765(20001117)6:22<4211::aid-chem4211>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Chiou A, Verger R, Kokotos G. Lipids. 2001;36:535–542. doi: 10.1007/s11745-001-0754-0. [DOI] [PubMed] [Google Scholar]
- 47.Kotsovolou S, Chiou A, Verger R, Kokotos G. J. Org. Chem. 2001;66:962–967. doi: 10.1021/jo005705y. [DOI] [PubMed] [Google Scholar]
- 48.Kokotos G. J. Mol. Catal. B-Enzym. 2003;22:255–269. [Google Scholar]
- 49.Fawzi MM, Gutsche CD. J. Org. Chem. 1966;31:1390–1393. [Google Scholar]
- 50.Hadei N, Assen BE, Kantchen B, O’ Brien CJ, Organ MG. J. Org. Chem. 2005;70:8503–8507. doi: 10.1021/jo051304c. [DOI] [PubMed] [Google Scholar]
- 51.Spencer TA, Onofrey TJ, Cann RO, Russel JS, Lee LE, Blanchard DE, Castro A, Gu P, Jiang G, Shechter J. J. Org. Chem. 1999;64:807–818. doi: 10.1021/jo981617q. [DOI] [PubMed] [Google Scholar]
- 52.Kokotos G, Constantinou-Kokotou V, Noula C, Nicolaou A, Gibbons WA. Int. J. Pept. Protein Res. 1996;48:160–166. doi: 10.1111/j.1399-3011.1996.tb00827.x. [DOI] [PubMed] [Google Scholar]