Bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor, was recently approved for treatment of glioblastoma. Initial data indicate increased response rates and progression-free survival compared to historical controls. Despite these promising data, we have identified several cases of severe optic neuropathy in patients with glioblastoma treated with bevacizumab.
Methods.
We performed a retrospective record review from 2005 to 2008 to identify adult patients with glioblastoma receiving bevacizumab who developed severe optic neuropathy. Five institutions participated, including the University of Virginia, UCLA, Columbia University, Rush University, and the University of Nebraska. The UCLA patient has already been reported in a larger case series discussing patients with glioblastoma receiving bevacizumab.1 Age at diagnosis, gender, radiation therapy data, chemotherapeutic regimens including the bevacizumab dosing schedule, ophthalmologic records, CSF results, and MRI were assessed.
Standard protocol approvals, registrations, and patient consents.
Each institution provided institutional review board approval. Since data were collected retrospectively without identifiers, institutional review boards did not require patient or surrogate consent.
Results.
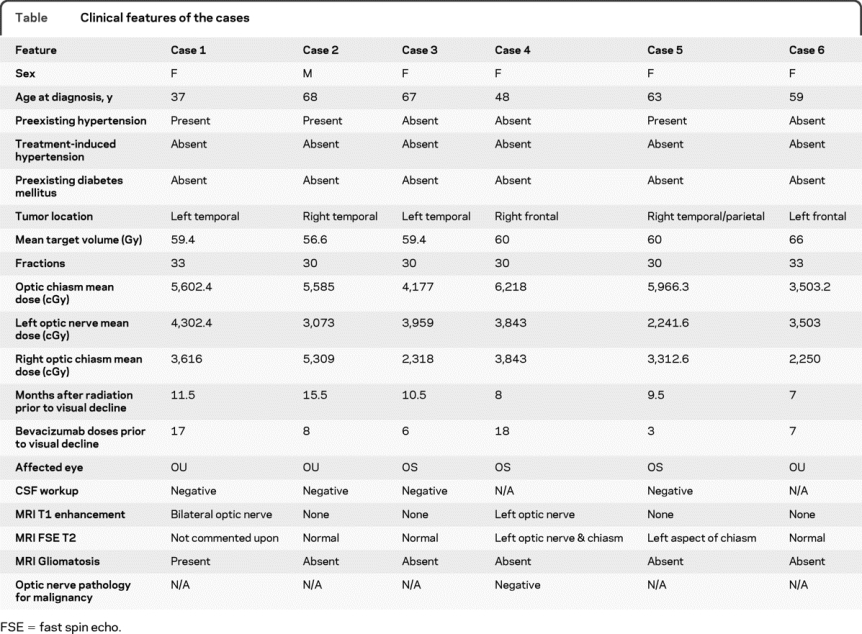
Six patients (5 women) were identified. Median age at diagnosis was 61 years (range 37 to 68). Following surgery, all patients received fractionated radiation therapy with concomitant temozolomide. One patient received bevacizumab at initial diagnosis; 5 received it at progression. Tumors received 60 Gy delivered in a mean of 30 fractions. Mean radiation dose to the optic chiasm, left optic nerve, and right optic nerve was 5,602.4 cGy, 3,673 cGy, and 3,464.3 cGy (table e-1 on the Neurology® Web site at www.neurology.org). Median time from the end of radiotherapy to the onset of visual symptoms was 11.5 months (range 7 to 15.5).
Patients received a median of 7.5 doses (range 3–18) of bevacizumab prior to onset of visual symptoms. Bevacizumab was discontinued a median of 1 month after onset of visual symptoms (range 1 week to 10 months). Visual loss subsequently developed unilaterally in 3 patients and bilaterally in 3 patients. The MRI findings for the optic apparatus are presented in the table. Postmortem pathologic specimen of the patient with left optic nerve enhancement displayed no evidence of tumor infiltration with focal vascular hyalinization and mild gliosis consistent with prior radiotherapy. CSF analysis in 4 patients was negative for myelin basic protein, oligoclonal bands, malignant cells, and pleocytosis. Median time from visual symptom onset to no light perception in at least 1 eye was 1.0 month (range 0.5–4.5 months). Over this epoch, 503 patients with glioblastoma received bevacizumab at the 5 institutions for an incidence of 1.2%. In comparison, 1 of 567 glioblastoma patients treated at these institutions without bevacizumab developed severe optic neuropathy (0.2% incidence, p = 0.056).
Table Clinical features of the cases
Discussion.
Bevacizumab has become a treatment option for recurrent glioblastoma.1–3 A phase II clinical trial (AVF3708g) assessed 167 patients receiving bevacizumab with and without irinotecan at tumor progression. Two of the patients in the current report were included in this clinical trial. Recognized bevacizumab side effects include arterial thrombosis (twofold increase), hypertension, proteinuria, impaired wound healing, and gastrointestinal perforation; visual loss has not previously been reported.1,2,4–6
We report 6 recent patients who developed severe optic neuropathy after bevacizumab treatment. While etiology and mechanism remain uncertain, an association between this rare event and bevacizumab is possible. While not seen in patients treated for non–brain tumor indications, this association appears to require dose independent radiation to the optic apparatus suggesting a priming effect for optic nerve injury. The patients in the current report received standard chemoradiation, with radiation to the optic apparatus generally considered within tolerance levels. Ophthalmologic assessment in all patients confirmed optic neuropathy of unknown etiology. The MRI of the optic apparatus for each case is unique with 3 patients displaying a normal examination. CSF findings did not support the diagnoses of either neoplastic meningitis or autoimmune demyelination. Gliomatosis cerebri was excluded as only 1 patient displayed this finding on MRI and a separate patient with optic nerve enhancement displayed negative pathology. Radiation-induced optic neuropathy was considered less likely secondary to both the severity and timing of the visual decline relative to the radiation and bevacizumab treatment. Proposed mechanisms may involve arterial thrombosis or upregulation of VEGF and subsequent neovascularization after radiotherapy with delayed ischemia following bevacizumab. An animal study analyzing whether bevacizumab decreases optic nerve tolerance to radiation is currently being devised. Until we understand the mechanistic basis for our findings, patients receiving bevacizumab should be followed closely in order to clarify whether this complication represents drug-related optic neuropathy, coincidental radiation optic neuropathy, or an unusual bevacizumab-related pattern of tumor failure with infiltration of the optic pathways from gliomatosis.
Supplementary Material
Supplemental data at www.neurology.org
Disclosure: Dr. Sherman and Dr. Aregawi report no disclosures. Dr. Lai has served on scientific advisory boards for Genentech, Inc. and Schering-Plough Corp.; serves on the editorial board of the Journal of Neuro-Oncology; and receives research support from Genentech, Inc., Schering-Plough Corp., the NIH/NCI [1KO8CA124479-01A1 (PI)], and the American Brain Tumor Association. Dr. Fathallah-Shaykh has served on a scientific advisory board for Genentech, Inc. and serves on the editorial board of the Archives of Neurology. Dr. Bierman reports no disclosures. Dr. Linsky receives research support from the Doris Duke Charitable Foundation (Clinical Research Fellowship for Medical Students). Dr. Larner has served on review panels for the US Department of Defense and receives research support from the NIH [5R01 ES011975-05 (PI), [5 R01 CA120413-02 (PI)]. Dr. Newman serves on editorial boards of the Journal of Neuro-Ophthalmology, Skull Base Surgery, EYENET, Ophthalmology, and Evidence-Based Eye Care; and has received honoraria for lectures and/or educational activities not funded by industry. Dr. Schiff has served on a scientific advisory board for Genentech, Inc.
Received April 3, 2009. Accepted in final form August 13, 2009.
Address correspondence and reprint requests to Dr. David Schiff, Box 800432, Health Sciences Center, Charlottesville, VA 22908; ds4jd@virginia.edu
&NA;
- 1.Lai A, Filka E, McGibbon B, et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys 2008;71:1372–1380. [DOI] [PubMed] [Google Scholar]
- 2.Friedman H, Prados M, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733–4740. [DOI] [PubMed] [Google Scholar]
- 3.Wong ET, Brem S. Taming glioblastoma: targeting angiogenesis. J Clin Oncol 2007;25:4705–4706. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 5.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 2008;70:779–787. [DOI] [PubMed] [Google Scholar]
- 6.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


