Abstract
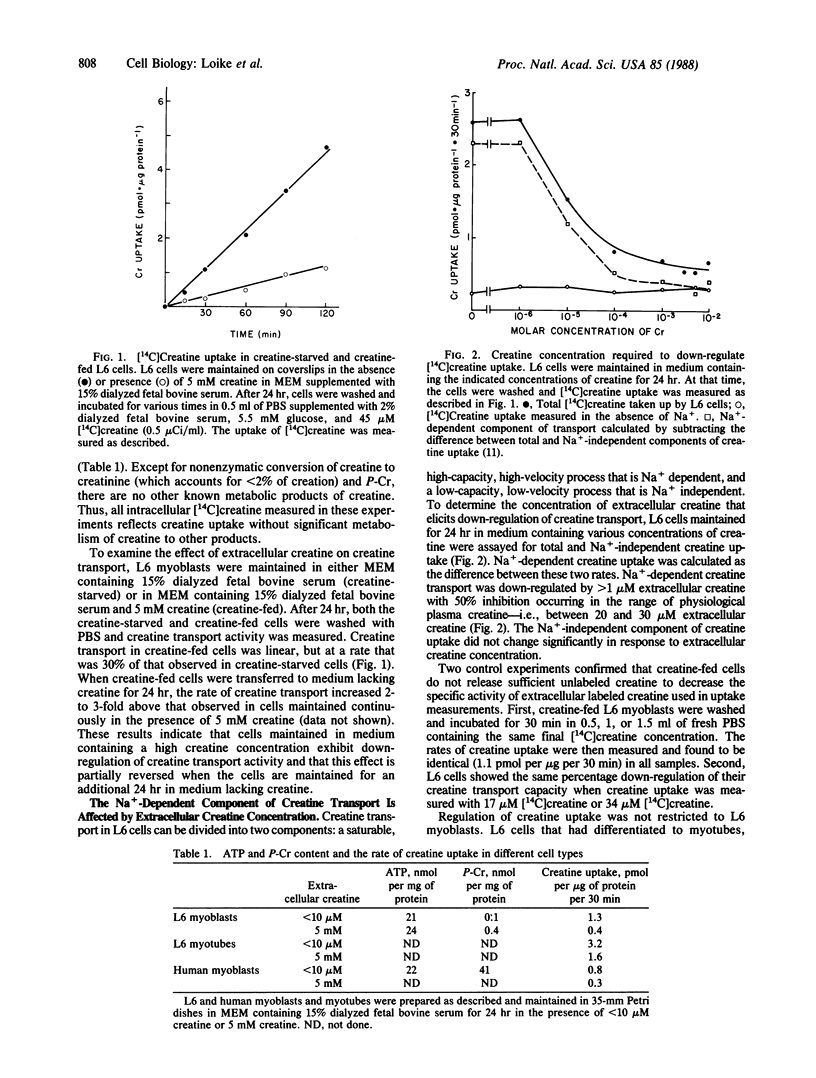
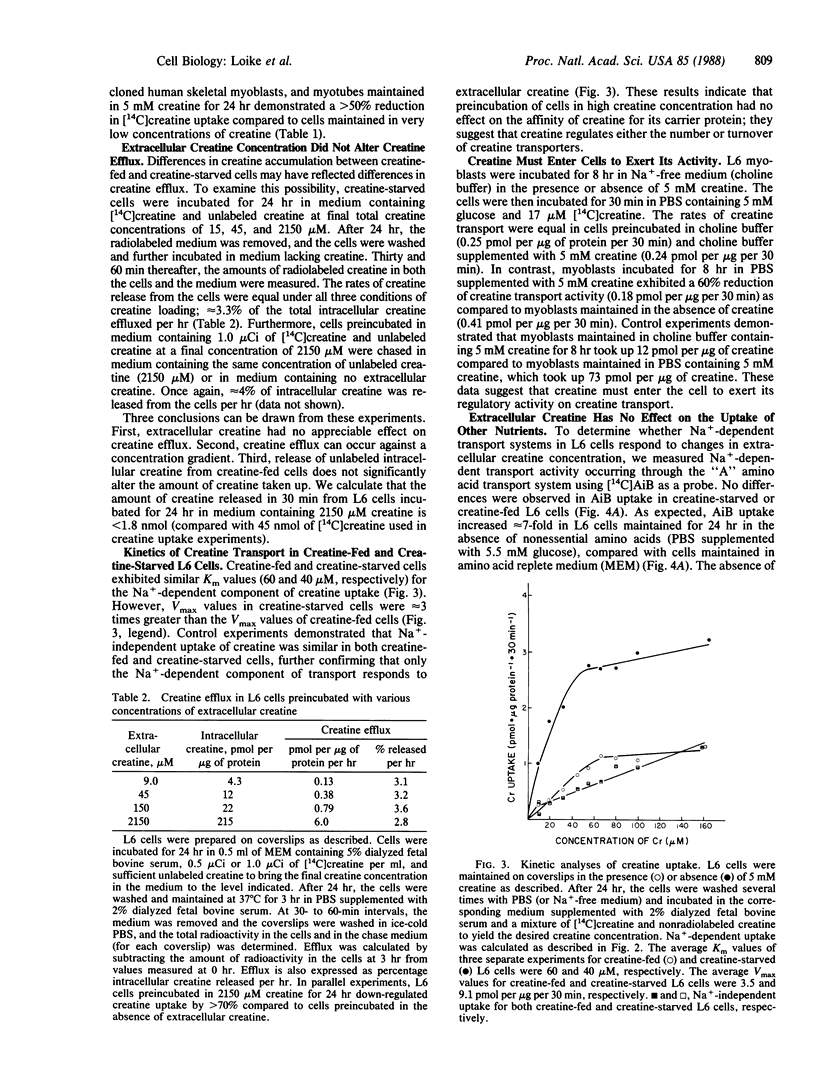
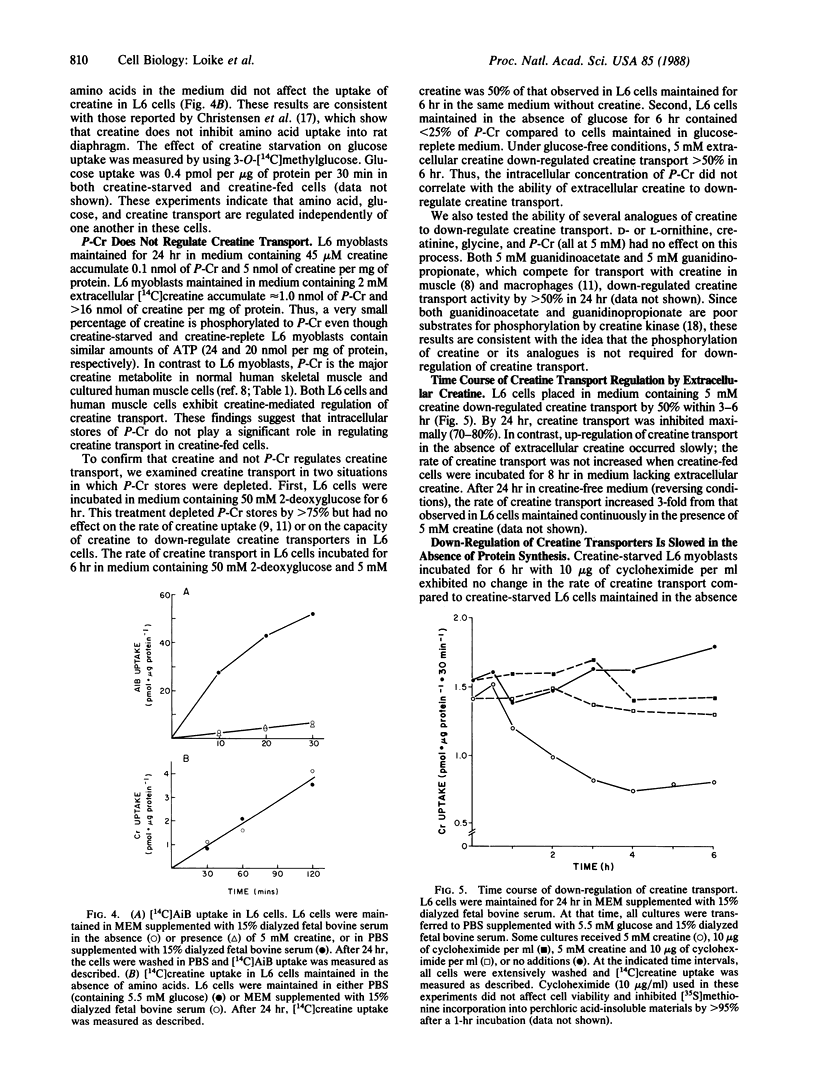
Muscle cells do not synthesize creatine; they take up exogenous creatine by specific Na+-dependent plasma membrane transporters. We found that extracellular creatine regulates the level of expression of these creatine transporters in L6 rat muscle cells. L6 myoblasts maintained for 24 hr in medium containing 1 mM creatine exhibited 1/3rd of the creatine transport activity of cells maintained for 24 hr in medium without creatine. Down-regulation of creatine transport was partially reversed when creatine-fed L6 cells were incubated for 24 hr in medium lacking creatine. Down-regulation of creatine transport occurred independently of amino acid and glucose transport. Furthermore, the down-regulation of creatine transporters by extracellular creatine was slowed by inhibitors of protein synthesis. These results suggest that creatine induces the expression of a protein that functionally inactivates the creatine transporters. Regulation of creatine transport by extracellular creatine also was observed in L6 myotubes and in cultures of human myoblasts and myotubes. Hence, the activity of creatine transport represents another site for the regulation of creatine homeostasis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYSE E. A., OLD L. J., CHOUROULINKOV I. CYTOTOXIC TEST FOR DEMONSTRATION OF MOUSE ANTIBODY. Methods Med Res. 1964;10:39–47. [PubMed] [Google Scholar]
- Boerner P., Saier M. H., Jr Hormonal regulation of the System A amino acid transport adaptive response mechanism in a kidney epithelial cell line (MDCK). J Cell Physiol. 1985 Feb;122(2):316–322. doi: 10.1002/jcp.1041220222. [DOI] [PubMed] [Google Scholar]
- Daly M. M., Seifter S. Uptake of creatine by cultured cells. Arch Biochem Biophys. 1980 Aug;203(1):317–324. doi: 10.1016/0003-9861(80)90182-4. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- Gazzola G. C., Dall'Asta V., Guidotti G. G. Adaptive regulation of amino acid transport in cultured human fibroblasts. Sites and mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3191–3198. [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Handlogten M. E., Kilberg M. S. Induction and decay of amino acid transport in the liver. Turnover of transport activity in isolated hepatocytes after stimulation by diabetes or glucagon. J Biol Chem. 1984 Mar 25;259(6):3519–3525. [PubMed] [Google Scholar]
- Kenyon G. L., Reed G. H. Creatine kinase: structure-activity relationships. Adv Enzymol Relat Areas Mol Biol. 1983;54:367–426. doi: 10.1002/9780470122990.ch6. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Barber E. F., Handlogten M. E. Characteristics and hormonal regulation of amino acid transport system A in isolated rat hepatocytes. Curr Top Cell Regul. 1985;25:133–163. doi: 10.1016/b978-0-12-152825-6.50009-6. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Han H. P., Barber E. F., Chiles T. C. Adaptive regulation of neutral amino acid transport System A in rat H4 hepatoma cells. J Cell Physiol. 1985 Feb;122(2):290–298. doi: 10.1002/jcp.1041220219. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Logan W. J., Klip A., Gagalang E. Regulation of amino acid transport in L6 muscle cells: I. Stimulation of transport system A by amino acid deprivation. J Cell Physiol. 1982 Aug;112(2):229–236. doi: 10.1002/jcp.1041120211. [DOI] [PubMed] [Google Scholar]
- Loike J. D., Kozler V. F., Silverstein S. C. Creatine kinase expression and creatine phosphate accumulation are developmentally regulated during differentiation of mouse and human monocytes. J Exp Med. 1984 Mar 1;159(3):746–757. doi: 10.1084/jem.159.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loike J. D., Kozler V. F., Silverstein S. C. Increased ATP and creatine phosphate turnover in phagocytosing mouse peritoneal macrophages. J Biol Chem. 1979 Oct 10;254(19):9558–9564. [PubMed] [Google Scholar]
- Loike J. D., Somes M., Silverstein S. C. Creatine uptake, metabolism, and efflux in human monocytes and macrophages. Am J Physiol. 1986 Jul;251(1 Pt 1):C128–C135. doi: 10.1152/ajpcell.1986.251.1.C128. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Babiss L. E., Fisher P. B. Measurement of the effect of interferons on cellular differentiation of human skeletal muscle cells. Methods Enzymol. 1986;119:619–628. doi: 10.1016/0076-6879(86)19083-5. [DOI] [PubMed] [Google Scholar]
- Walker J. B. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol. 1979;50:177–242. doi: 10.1002/9780470122952.ch4. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]


