Abstract
Purpose
While the strength of a tendon repair is clearly important, the friction of the repair is also a relevant consideration. The purpose of this study was to characterize the frictional coefficient, gliding resistance and breaking strength of suture materials and a suture construct commonly used for flexor tendon repair.
Methods
We measured the friction coefficients of 3-0 braided nylon enclosed in a smooth nylon outer shell (Supramid, S. Jackson, Alexandria, VA), 3-0 braided polyester coated with polybutilate (Ethibond, Ethicon, Somerville, NJ), and a 3-0 braided polyester/monofilament polyethylene composite (FiberWire, Arthrex, Naples, FL) sutures. We also measured the gliding resistance, linear breaking strength and resistance to gapping of zone 2 modified Pennington tendon repairs with the two lowest friction sutures in 20 human cadaveric flexor digitorum profundus (FDP) tendons.
Results
The braided polyester/monofilament polyethylene composite had a significantly lower friction coefficient (0.054) than either the coated polyester (0.076) or nylon (0.130) sutures (p<0.001). The gliding resistances of the repaired tendons with braided/monofilament polyethylene composite suture and coated, braided polyester were similar (p> 0.05). The strength of the two repairs, force to produce a 2mm gap, and resistance to gap formation than coated, braided polyester repairs were also not significantly different.
Conclusion
Braided polyester composite is a low friction suture material. However, when this suture was used for tendon repair with a locking suture technique, it did not show a significant effect on the gliding resistance and repair strength compared with the same repair using coated polyester suture.
Keywords: Gliding Resistance, Suture, Tendon, Tendon Repair
INTRODUCTION
The main challenge facing flexor tendon repair procedures is allowing the sutured tendon to heal while avoiding the formation of adhesions and repair rupture (1, 2). A protocol of early rehabilitation hinders the process of adhesion formation and is associated with better clinical outcomes (3, 4). Since aggressive therapy may result in rupture of the tendon repair before healing has been accomplished (5–8), it is critical to understand how to effectively produce a strong tendon repair which does not rupture or gap (9–13). Tendon rupture or gapping is inversely related to the strength of the repair and directly related to the load experienced by the tendon during the healing process (9, 10, 13).
The tendon loading depends on several components: tension from the muscle, stiffness of the joints, resistance of edematous soft tissue, external load, and the gliding resistance inside the synovial sheath and pulley system (9, 14). Since immobilization is inadvisable if the formation of adhesions is to be prevented (3, 4), it is imperative to achieve the quality of repair that will allow rehabilitation therapy to take place by employing a suture material and technique with the greatest strength while at the same time minimizing gliding resistance within the synovial sheath. Some studies have been aimed at testing the strength of suture materials and the repairs produced with them; many more studies have been aimed at developing strong suture techniques (4, 15–18).
FiberWire (Arthrex, Naples, FL), a suture made from long-chain polyester in a braided polyester jacket, has been reported to have breaking strength superior to nylon and Ethibond sutures of similar caliber, when used in a locking MGH configuration (19). However, previous research has demonstrated that MGH repairs are associated with adhesion formation (20). A high-friction repair can more than offset the advantage gained in strength by increasing gliding resistance and abrasion of the tendon sheath. The gliding resistance of braided polyester/monofilament polyethylene composite sutures is therefore a relevant concern. If braided polyester/monofilament polyethylene composite suture has high friction, its strength advantage may be undermined in the context of flexor tendon repair. In contrast, if this suture has lower friction than comparable materials, it would provide a double advantage: higher strength and lower friction. The purpose of this study was to characterize the frictional coefficient, gliding resistance and strength of tendon repairs made with braided polyester/monofilament polyethylene composite suture and compare those with values for other currently used materials, braided nylon enclosed in a smooth nylon outer shell, and polybutilate coated, braided polyester.
MATERIALS & METHODS
Friction Coefficient of Suture Material
The friction coefficient of three representative suture materials were measured using the method described in Uchiyama et al (21, 22). Briefly, the measurement system consisted of one mechanical actuator with a linear potentiometer, two tensile load transducers, a nylon rod, a mechanical pulley, and a 4.9-Newton dead weight. By measuring the proximal and distal forces (F1 and F2) at various arcs of contact between the suture and nylon rod, the friction coefficient is calculated from the least squares fit of the natural logarithm of F2/F1 versus arc of contact (23).
Five different arcs of contact (20°, 30°, 40°, 50° and 60°) with a nylon rod were studied to determine the coefficient of friction of three 3-0 suture materials: (1) braided nylon enclosed in a smooth nylon outer shell (Supramid, S. Jackson, Alexandria, VA), (2) polybutilate coated, braided polyester (Ethibond, Ethicon, Somerville, NJ), and (3) braided polyester/monofilament polyethylene composite (FiberWire, Arthrex, Naples, FL).
Gliding Resistance of Repaired Tendon
The two lowest friction suture materials were selected for further study. After IRB approval, the right hands of 5 human cadavers were obtained, including 4 females and one male, with an average age of 83.2 years (range 75 – 91). The flexor digitorum profundus (FDP) tendons from the index, middle, ring and little fingers were accessed through a transverse incision in the flexor sheath, just distal to the A2 pulley. The FDP tendons were marked at the distal end of the A2 pulley both at full passive extension and flexion, with a 5N load attached at the proximal FDP tendon to maintain tendon tension. The distance between theses two marks represented the FDP tendon excursion during full finger range of motion. A transverse laceration was made 10 mm distal to the proximal mark in order to ensure movement of the repair site through the pulley system during normal movement.
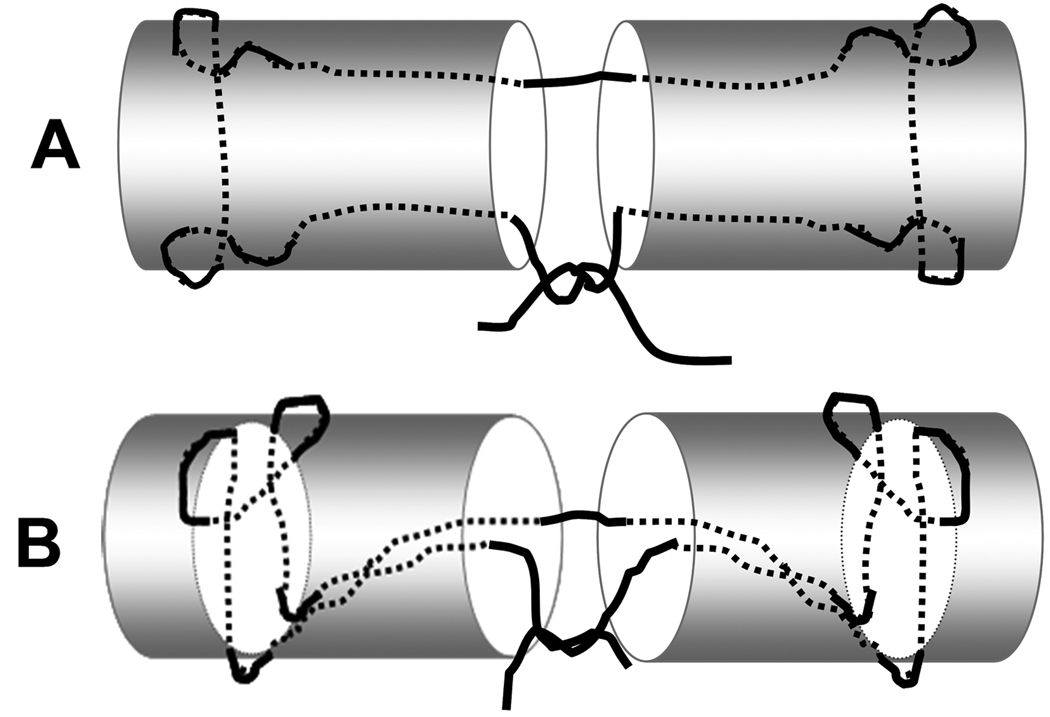
The tendons were randomly allocated into two different groups. One group was repaired using 3-0 coated, braided polyester (Ethibond, Ethicon, Somerville, NJ) and the other was repaired with 3-0 braided polyester/monofilament polyethylene composite (FiberWire, Arthrex, Naples, FL). All repairs were made using a locking modified Pennington core suture technique (16) and a monofilament polypropylene (Prolene, Ethicon, Somerville, NJ) 6-0 running epitendinous suture (Figure 1). The locking loops of the Pennington repair were 1 cm (pre measured with ruler and marked on the tendon surface) from the laceration, and the epitendinous throws were approximately 2 mm from the lacerated tendon ends, with about 1 mm between each stitch. The number of epitendinous throws ranged from 12 to 16, depending upon the tendon size. To minimize the frictional force, the four core suture knots were placed inside the repair site and contralateral to the peripheral suture knot for both repair techniques (24). All repairs were performed by the same investigator to ensure consistency of the results.
Figure 1.
A: Coronal view of the FDP tendon repaired using a modified Pennington technique. B: Lateral view of the Pennington repair with locking configuration.
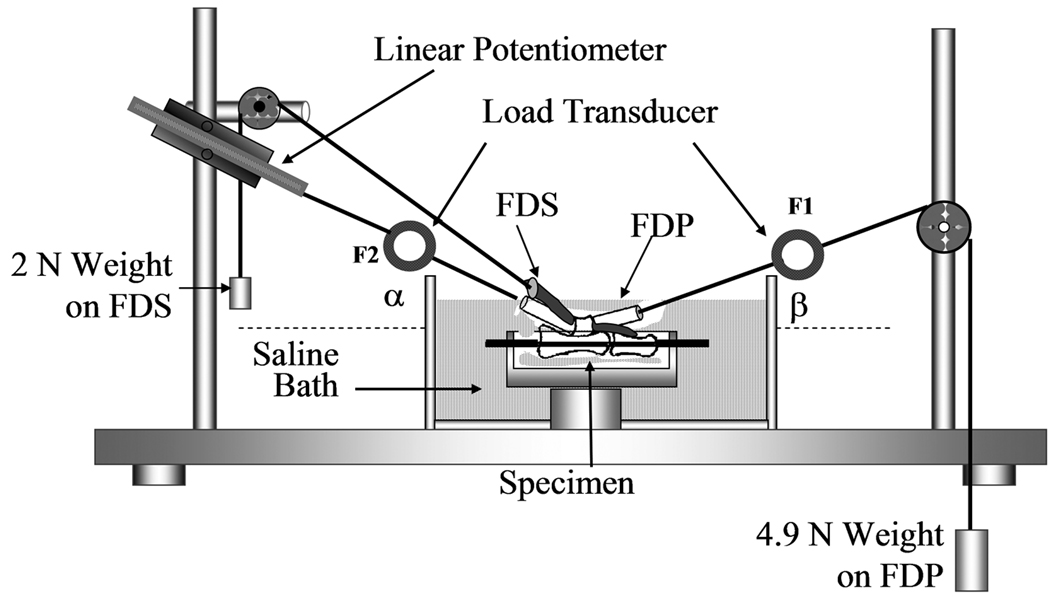
The friction between tendon and pulley was also measured using the method of Uchiyama et al (25). Briefly, the proximal and middle phalanges, proximal pulley, flexor digitorum superficialis (FDS), parietal membrane of proximal pulley, and visceral membrane of flexor digitorum profundus were preserved, while removing the remaining tendon sheath and bone. A Kirschner wire was used to fix the proximal interphalangeal joint in the extended position. Each specimen was mounted on the custom testing device with the palmar side upward. To maintain tension in the FDS tendon, a 2N weight was attached to its proximal end. The magnitude of this weight was determined based on previous in vivo tendon research, on the magnitude of load experienced by normal tendons in situ (26, 27). The measurement system consisted of one mechanical actuator with a linear potentiometer, two tensile load transducers, and a mechanical pulley (Figure 2). The load transducers were attached to the proximal and distal ends of the FDP tendon. The distal transducer was connected to a 4.9 N weight to simulate passive mobilization of the finger, again based on previous in vivo tendon research (26, 27). The proximal load transducer was connected to the mechanical actuator. Based on the experience of previous studies, a set arc of contact, 30° and 20 ° between the horizontal plane and the proximal and distal transducer cables, respectively, was sufficient to provide adequate measurement of the gliding resistance (14, 25). The tendon was pulled proximally by the actuator against the weight at a rate of 2mm/second. Excursion was limited to the distance between the two FDP tendon markers. The forces at the proximal and distal tendon ends and the tendon excursion were recorded. The specimens were kept moist throughout testing by immersion in a saline bath. The force differential between the proximal and distal tendon ends represents the gliding resistance. The mean and peak gliding resistance over the excursion range were calculated and reported.
Figure 2.
Experimental setup and testing apparatus for the gliding resistance of repaired tendon/pulley system. The specimen with repaired FDP tendon was mounted on the testing apparatus which the same mechanism for the suture frictional coefficient measurement.
Tendon Repair Strength
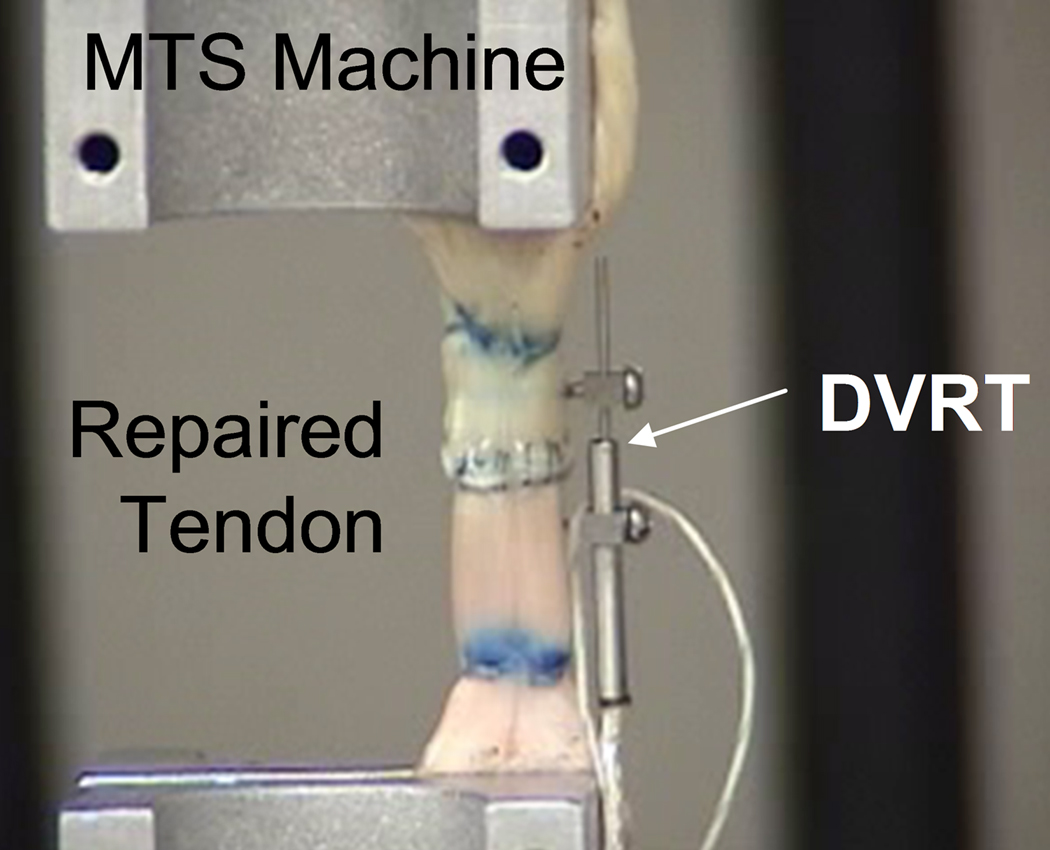
Following gliding resistance testing, the repaired tendon strength was measured using a servo-hydraulic testing machine (MTS, Minneapolis, MN). The repaired tendon was removed from the tendon sheath, secured in the materials testing machine, and linearly distracted to failure at a rate of 20 mm/min. A differential variable reluctance transducer with low friction nitinol couplings (DVRT, Microstrain, Williston, VT) was attached to the tendon with fine wires, inserted proximal and distal to the repair construct, in order to measure gap formation during testing (Fig. 3). Tensile force, grip-to-grip displacement, and gap displacement measured by the DVRT transducer were collected at a rate of 20 Hz. Throughout testing the tendons were kept moist by spraying with physiologic saline. Maximum breaking force and the force to produce a 2 mm gap were recorded. In addition, a regression line was fit to the linear region of the force versus gap formation (as measured by the DVRT) to measure the resistance to gap formation.
Figure 3.
Following frictional testing, the repaired tendons were mounted on the MTS machine for the failure strength testing. A DVRT was attached to the tendon at the repair site to measure the gap formation.
Statistical Analysis
The sample size requirements were determined by a power calculation. Prior study has shown that normal tendon friction is 0.25 (S.D. 0.07) N while the modified Pennington repair has an average friction of 0.80 (S.D. 0.16) N (16). We believe that a difference of approximately 0.25N (100% increase from normal) between repaired tendons with different suture materials is the minimum clinically important difference in gliding resistance, as differences of this magnitude have been shown to affect in vivo results in an animal model(20). For a two-sided comparison, 10 specimens per experimental group are sufficient to detect such a difference with an alpha of 0.05 and a power of 0.8.
The data obtained from the gliding resistance, tensile strength tests, and gap formation were analyzed using one-way analysis of variance (ANOVA). A Tukey-Kramer post-hoc test for individual comparisons was used if there was a significant difference. A p<0.05 significance level was used in all cases.
Results
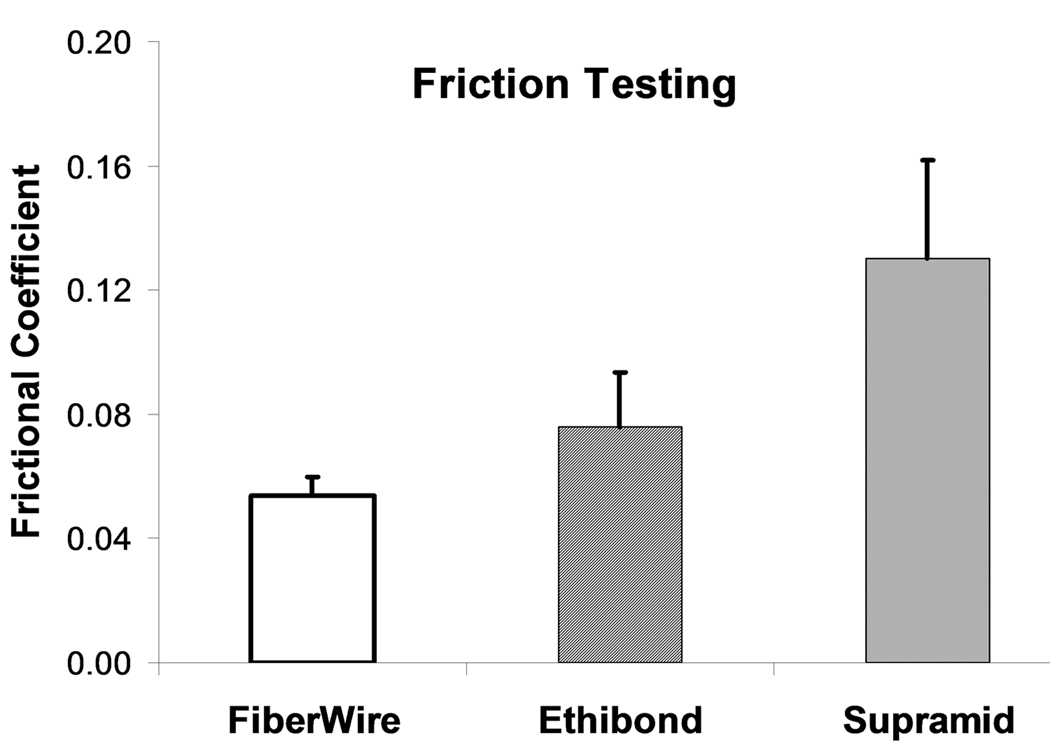
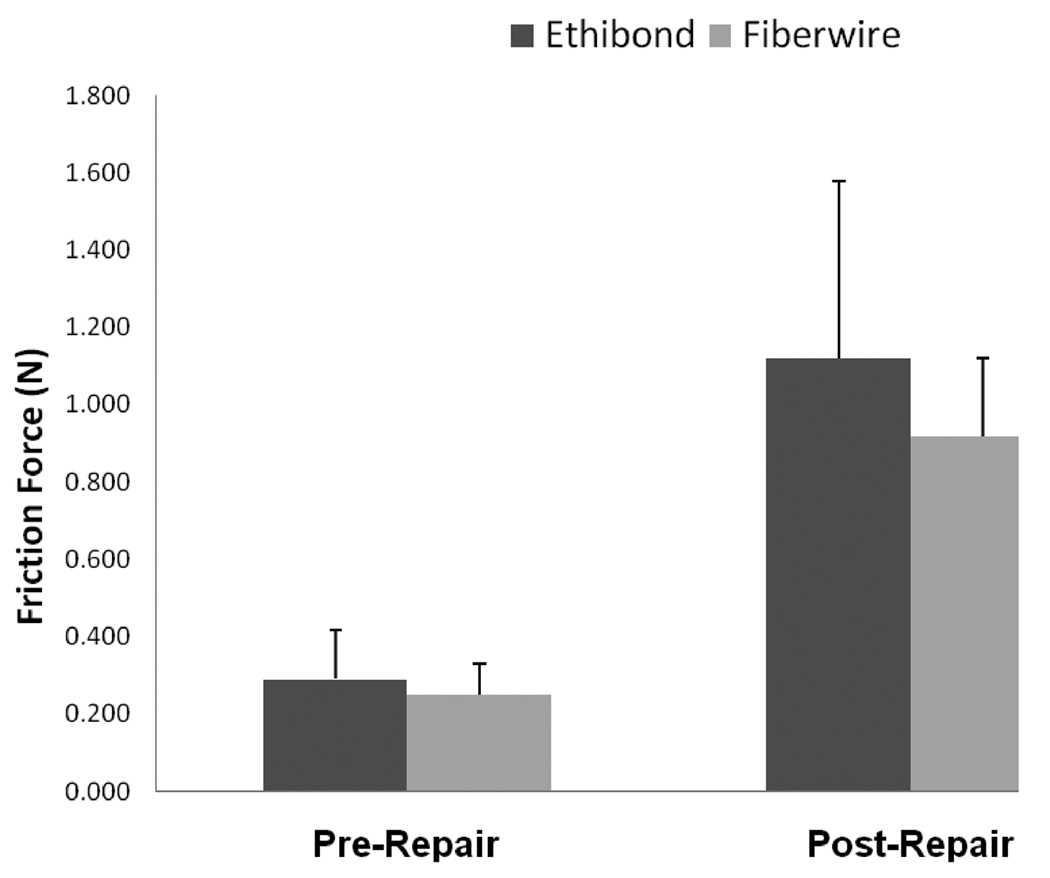
The braided polyester/monofilament polyethylene composite had the lowest frictional coefficient (0.054 ± 0.006) of the 3 sutures tested, followed by polybutilate coated, braided polyester (0.076 ± 0.018) and braided nylon enclosed in a smooth nylon outer shell (0.130 ± 0.032). These differences were statistically significant (p < 0.001) (Figure 4). For the repaired tendon evaluation, the differences were not statistically significant (p > 0.05) (Figure 5).
Figure 4.
Frictional coefficient braided nylon enclosed in a smooth nylon outer shell (Supramid), polybutilate coated, braided polyester (Ethibond), and braided polyester/monofilament polyethylene composite (FiberWire) sutures (p<0.001).
Figure 5.
Mean gliding resistance of repairs made with polybutilate coated, braided polyester (Ethibond) and braided polyester/monofilament polyethylene composite (FiberWire) sutures.
The results of tendon repair testing are shown in table 1. Seven (70%) repaired tendons in each group failed by suture breakage, and 3 tendons (30%) failed due to suture pulling out from the tendon stump. These differences were not statistically significant (p > 0.05).
Table 1.
Gliding Resistance, Ultimate Tensile Strength, Gap Force at 2mm and Stiffness
|
Repair Method and Material |
Gliding Resistance (N) (Mean ± SD) |
Maximal Failure Strength (N) (Mean ±SD) |
Gap Force at 2-mm (N) (Mean ±SD) |
Stiffness (N/mm) (Mean ± SD) |
|---|---|---|---|---|
|
Pennington Repair with FiberWire Suture |
0.916 ± 0.19 | 49.52 ± 6.97 | 45.29 ± 7.23 | 13.64 ± 3.40 |
|
Pennington Repair with Ethibond Suture |
1.119 ± 0.45 | 45.91 ± 6.93 | 42.32 ± 8.47 | 13.17 ± 3.89 |
DISCUSSION
Our results show that braided polyester/monofilament polyethylene composite suture is a low friction material, and might thus lead to a lower friction repair for similar constructs, as compared to braided nylon enclosed in a smooth nylon outer shell and polybutilate coated, braided polyester. In the current study, however, we did not find a significant effect on the repaired tendon gliding resistance when comparing repairs made with those two suture materials. We believe that this may indicate that the variability in friction between individual repairs is more than the variability in friction between suture materials.
The repaired tendon strength to failure and gap was similar to previous reports with the same repair technique and materials (28, 29). Miller et al compared Fiberwire suture with nylon and Ethibond suture in repaired tendon strength (28, 30). They found than FiberWire outperforms both Ethibond and nylon suture when using a locked flexor tendon repair suture (MGH) but not a non locking repair (Strickland repair). However, our results have not demonstrated this superior effect of the FiberWire suture, which might due to different suture techniques being used in these two studies. Specifically, the MGH repair has four strands and the modified Pennington has only two. Also, the MGH repair had a 99% failure by suture breakage in the study reported by Miller et al, but the modified Pennington had 70% suture breakage in our study.
Waitayawinyu et al (28) have shown that FiberWire suture is more likely to unravel if fewer than five knot throws are made. We did not observe this problem in our own specimens, using four throws, but we do believe that it is possible that this tendency to unravel may be related to the lower friction of the FiberWire suture material.
There are several imitations to this study. It is possible that the DVRT device that we used to measure gap formation could affect breaking strength. However, the device was inserted into an intact segment of tendon above and below the suture loops. Thus, while the device may have damaged a few tendon fascicles, it would not have weakened the repair construct in any way. It is also possible that the DVRT may have displaced during testing, affecting the gap recordings. However, we directly observed each tendon during testing and noted no such displacement. In addition, this was purely an in vitro study, not involving tendon healing. The tendon glided against a segment of an annular pulley, not the intact digital sheath, thus the resistance recorded here was merely frictional force, and angles of loading on tendon were unchanged. These are very different from in vivo human situations. Finally, our sample size, designed to detect differences between repair types, may not have been sufficient to detect a differences due to small variations in the surgical technique between individual tendons, as no surgeon is able to repair two tendons so that the repairs are perfectly identical and indistinguishable in all characteristics. Within these limits of human ability, it would appear that the lower friction of FiberWire, while real, may not convey a specific clinical advantage.
In conclusion, the braided polyester/monofilament polyethylene composite suture has shown some theoretical advantage in terms of lower friction. However, when this suture was used for tendon repair with a locking suture technique, it did not t show a significant effect on the repaired tendon gliding resistance and strength compared with the same repair used coated polyester suture.
Acknowledgments
This work was supported by grant R25 GM 55252 from the National Institutes of Health and by the Mayo Foundation. The authors thank Dr. Yuichi Yoshii and Ms. Kelly Predmore for their invaluable experimental assistance and Arthrex (Naples, FL) for their generous donation of suture material.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Boyer MI. Flexor tendon biology. Hand Clin. 2005;21:159–166. doi: 10.1016/j.hcl.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Tang JB. Indications, methods, postoperative motion and outcome evaluation of primary flexor tendon repairs in Zone 2. J Hand Surg [Br] 2007;32(2):118–129. doi: 10.1016/J.JHSB.2006.12.009. 32:118–29. [DOI] [PubMed] [Google Scholar]
- 3.Grewal R, Saw SS, Bastidas JA, Fischer KJ, Sotereanos DG. Passive and active rehabilitation for partial lacerations of the canine flexor digitorum profundus tendon in zone II. J Hand Surg [Am] 1999;24:743–750. doi: 10.1053/jhsu.1999.0743. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C, Amadio PC, Paillard P, Tanaka T, Zobitz ME, Larson DR, An KN. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg [Am] 2004;86-A:320–327. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Hung LK, Pang KW, Yeung PL, Cheung L, Wong JM, Chan P. Active mobilisation after flexor tendon repair: comparison of results following injuries in zone 2 and other zones. J Orthop Surg. 2005;13:158–163. doi: 10.1177/230949900501300209. [DOI] [PubMed] [Google Scholar]
- 6.Braga-Silva J, Kuyven CR. Early active mobilization after flexor tendon repairs in zone two. Chirurgie de la Main. 2005;24:165–168. doi: 10.1016/j.main.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Harris SB, Harris D, Foster AJ, Elliot D. The aetiology of acute rupture of flexor tendon repairs in zones 1 and 2 of the fingers during early mobilization. J Hand Surg [Br] 1999;32(2):118–129. doi: 10.1054/jhsb.1998.0212. 24:275–280. [DOI] [PubMed] [Google Scholar]
- 8.Kitsis CK, Wade PJ, Krikler SJ, Parsons NK, Nicholls LK. Controlled active motion following primary flexor tendon repair: a prospective study over 9 years. J Hand Surg [Br] 1998;32(2):118–129. doi: 10.1016/s0266-7681(98)80055-7. 23:344–349. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia D, Tanner KE, Bonfield W, Citron ND. Factors affecting the strength of flexor tendon repair. J Hand Surg [Br] 1992;17:550–552. doi: 10.1016/s0266-7681(05)80240-2. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence TM, Davis TR. A biomechanical analysis of suture materials and their influence on a four-strand flexor tendon repair. J Hand Surg [Am] 2005;30:836–841. doi: 10.1016/j.jhsa.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Mishra V, Kuiper JH, Kelly CP. Influence of core suture material and peripheral repair technique on the strength of Kessler flexor tendon repair. J Hand Surg [Br] 2003;28:357–362. doi: 10.1016/s0266-7681(03)00080-9. [DOI] [PubMed] [Google Scholar]
- 12.Smith AM, Evans DM. Biomechanical assessment of a new type of flexor tendon repair. J Hand Surg [Br] 2001;26:217–219. doi: 10.1054/jhsb.2000.0542. [DOI] [PubMed] [Google Scholar]
- 13.Zobitz ME, Zhao C, Amadio PC, An KN. Comparison of mechanical properties of various suture repair techniques in a partially lacerated tendon. J Biomech Eng. 2000;122:604–607. doi: 10.1115/1.1324668. [DOI] [PubMed] [Google Scholar]
- 14.Zhao C, Amadio PC, Zobitz ME, An KN. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19:580–586. doi: 10.1016/S0736-0266(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 15.Momose T, Amadio PC, Zhao C, Zobitz ME, Couvreur PJ, An KN. Suture techniques with high breaking strength and low gliding resistance: experiments in the dog flexor digitorum profundus tendon. Acta Orthop Scand. 2001;72:635–641. doi: 10.1080/000164701317269085. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T, Amadio PC, Zhao C, Zobitz ME, Yang C, An KN. Gliding characteristics and gap formation for locking and grasping tendon repairs: a biomechanical study in a human cadaver model. J Hand Surg [Am] 2004;29:6–14. doi: 10.1016/j.jhsa.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Wade PJ, Muir IF, Hutcheon LL. Primary flexor tendon repair: the mechanical limitations of the modified Kessler technique. J Hand Surg [Br] 1986;11:71–76. doi: 10.1016/0266-7681(86)90018-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, Amadio PC, Tanaka T, Kutsumi K, Tsubone T, Zobitz ME, An KN. Effect of Gap Size on Gliding Resistance After Flexor Tendon Repair. J Bone Joint Surg [Am] 2004;86:2482–2488. doi: 10.2106/00004623-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Miller B, Dodds SD, deMars A, Zagoreas N, Waitayawinyu T, Trumble TE. Flexor Tendon Repairs: The Impact of Fiberwire on Grasping and Locking Core Sutures. J Hand Surg [Am] 2007;32:591–596. doi: 10.1016/j.jhsa.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51:917–921. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Uchiyama S, Amadio PC, Ishikawa J, An KN. Boundary lubrication between the tendon and the pulley in the finger. J Bone Joint Surg [Am] 1997;79:213–218. [PubMed] [Google Scholar]
- 22.Coert JH, Uchiyama S, Amadio PC, Berglund LJ, An KN. Flexor tendon-pulley interaction after tendon repair. A biomechanical study. J Hand Surg [Br] 1995;20:573–577. doi: 10.1016/s0266-7681(05)80113-5. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama S, Coert JH, Berglund L, Amadio PC, An K-N. Method for the measurement of friction between tendon and pulley. J Orthop Res. 1995;13:83–89. doi: 10.1002/jor.1100130113. [DOI] [PubMed] [Google Scholar]
- 24.Momose T, Amadio PC, Zhao C, Zobitz ME, An KN. The effect of knot location, suture material, and suture size on the gliding resistance of flexor tendons. J Biome Mater Res. 2000;53:806–811. doi: 10.1002/1097-4636(2000)53:6<806::aid-jbm23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Uchiyama S, Amadio PC, Coert JH, Berglund LJ, An KN. Gliding resistance of extrasynovial and intrasynovial tendons through the A2 pulley. J Bone Joint Surg [Am] 1997;79:219–224. doi: 10.2106/00004623-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Lieber RL, Amiel D, Kaufman KR, Whitney J, Gelberman RH. Relationship between joint motion and flexor tendon force in the canine forelimb. J Hand Surg [Am] 1996;21:957–962. doi: 10.1016/S0363-5023(96)80299-1. [DOI] [PubMed] [Google Scholar]
- 27.Schuind F, Garcia-Elias M, Cooney WP, An KN. Flexor tendon forces: In vivo measurements. J Hand Surg. 1985;8A:182–185. doi: 10.1016/0363-5023(92)90408-h. [DOI] [PubMed] [Google Scholar]
- 28.Waitayawinyu T, Martineau PA, Luria S, Hanel DP, Trumble TE. Comparative Biomechanic Study of Flexor Tendon Repair Using FiberWire. J Hand Surg [Am] 2008;33:701–708. doi: 10.1016/j.jhsa.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T, Amadio PC, Zhao C, Zobitz ME, Yang C, An K-N. Gliding Characteristics and Gap Formation for Locking and Grasping Tendon Repairs: A Biomechanical Study in a Human Cadaver Model. J Hand Surg [Am] 2004;29:6–14. doi: 10.1016/j.jhsa.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Miller B, Dodds SD, deMars A, Zagoreas N, Waitayawinyu T, Trumble TE. Flexor tendon repairs: the impact of fiberwire on grasping and locking core sutures. J Hand Surg [Am] 2007;32:591–596. doi: 10.1016/j.jhsa.2007.03.003. [DOI] [PubMed] [Google Scholar]