Abstract
The 6-deoxyerythronolide B synthase (DEBS) and pikromycin (Pik) modular polyketide synthases are unique multifunctional enzyme systems that are responsible for the biosynthesis of the erythromycin and pikromycin 14-membered ring aglycones, respectively. Together, these natural product biosynthetic systems provide excellent platforms to examine the fundamental structural and catalytic elements that govern polyketide assembly, processing and macrocyclization. In these studies, the native pentaketide intermediate for DEBS was synthesized and employed for in vitro chemoenzymatic synthesis of macrolactone products in engineered monomodules Ery5, Ery5-TE and Ery6. A comparative analysis was performed with the corresponding Pik module 5 (PikAIII) and module 6 (PikAIV), dissecting key similarities and differences between these highly related PKSs. The data revealed that individual modules in the DEBS and Pik PKSs possess distinctive molecular selectivity profiles, and suggest that substrate recognition has evolved unique characteristics in each system.
Introduction
Biosynthetic assembly of polyketide natural products occurs on multi-enzyme megasynthases termed polyketide synthases (PKSs).1 In these systems, natural product scaffolds are built from CoA esters of simple organic acid monomers by an assembly line of enzymatic domains that catalyze their condensation into more complex polyketide chains. These domains are uniquely arranged in bacterial type I PKSs, where they are organized into covalently bound collinear modules. Each module is responsible for a single elongation and processing step in the PKS enzyme pathway.
The unique architecture of type I modular PKS systems affords the opportunity for rational bioengineering of PKS enzymes for the generation of novel chemotypes. Predictable chemical permutations in a polyketide scaffold can be achieved by a variety of engineering strategies such as the modification of individual PKS catalytic domains (i.e. inactivation, substitution, addition, deletion)2,3 or exchange of full modules to form hybrid PKS systems.4-6 To date, hundreds of novel polyketides have been produced utilizing these methods. However, in many cases, engineered PKS proteins have disrupted or significantly attenuated polyketide production, precluding the efficient generation of large libraries of new compounds.5-7 These studies highlight the need for detailed knowledge regarding the fundamental mechanistic underpinnings of PKS catalytic processes.
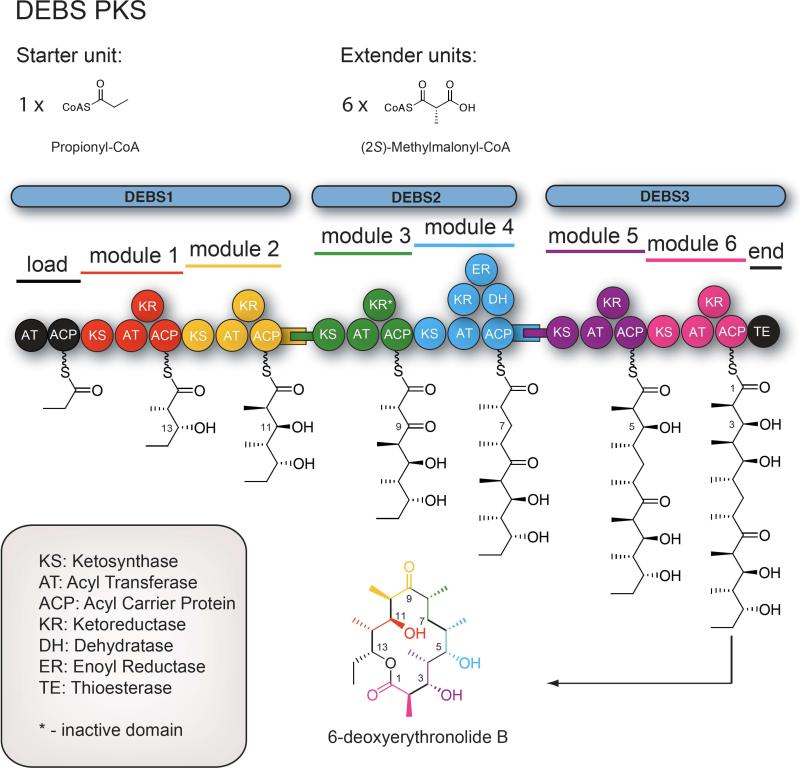
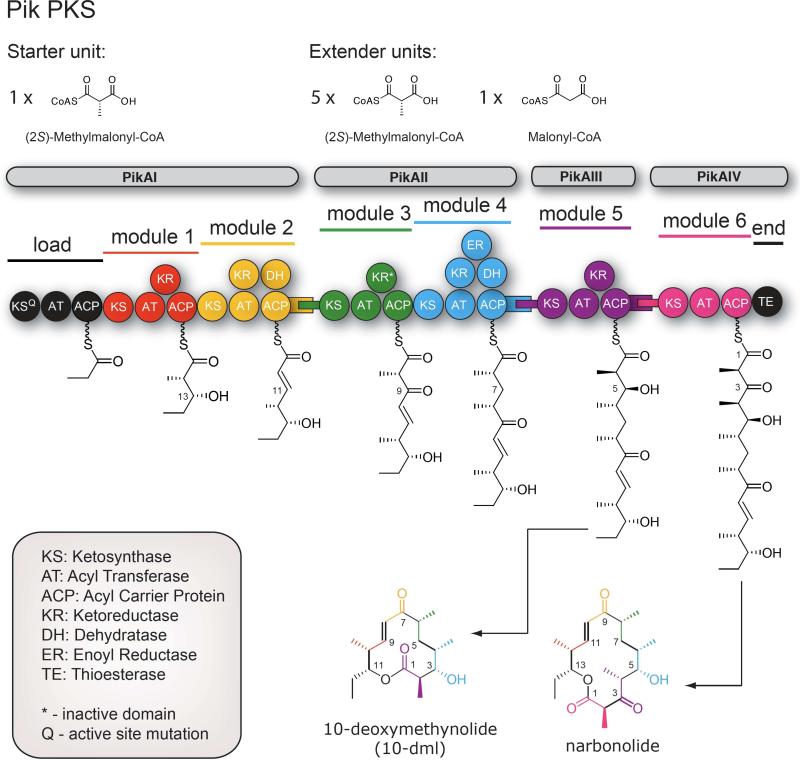
Currently, the most well studied modular type I PKSs are the 6-deoxyerythronolide B synthase (DEBS, Figure 1)8,9 and the pikromycin PKS10 (Pik, Figure 2). These two systems share many similar features, yet contain substantive differences in modular organization, substrate specificity and product distribution. Specific key differences lie in the final two modules (5 and 6) of these PKSs, which are contained on a single bimodular polypeptide in DEBS to produce one 14-membered ring macrolactone 6-deoxyerythronolide B (6-DEB), but are on individual monomodular polypeptides in Pik and produce both the 12-membered ring macrolactone 10-deoxymethynolide (10-DML) and the 14-membered ring macrolactone narbonolide (Nbl). As a result, the “late” modules of these systems have been the subjects of significant detailed analysis.
Figure 1.
Illustration of the DEBS PKS from the erythromycin biosynthetic pathway. DEBS is responsible for generation of the aglycone 6-deoxyerythronolide B (6-DEB).
Figure 2.
Illustration of the Pik PKS from the methymycin/pikromycin biosynthetic pathway. Pik produces the aglycones 10-deoxymethynolide (10-DML) and narbonolide (Nbl).
In the DEBS system, many pioneering experiments have carved out an understanding of modular PKS catalytic efficiency and substrate specificity. Probing of individual DEBS modules, including 5 and 6, with short chain diketide model substrates revealed that they each have an inherent molecular selectivity profile and a high degree of catalytic competence.11-13 However, the optimal substrates in these in vitro experiments correlate poorly to the native substrates that are normally accepted and processed by these modules in vivo. This is especially true of the downstream modules that must accept and process increasingly complex polyketide intermediates in the growing chain. Comparative study of late modules of the pikromycin PKS14,15 showed that extension and processing of model diketides by Pik monomodules were nearly three orders of magnitude less efficient than DEBS. More in depth experiments,16 though, showed that these diketide model compounds do not effectively probe the molecular recognition features of these PKS modules. Rather, utilizing full-length native chain elongation substrates in biochemical studies of PKS modules yields a more valuable assessment of kinetic parameters and modular specificity, as evidenced by the fact that native penta- and hexaketide substrates for Pik modules 5 (PikAIII) and 6 (PikAIV) were elongated, processed and cyclized 2-3 orders of magnitude more efficiently than the corresponding diketide model substrates.
To further our assessment of the molecular recognition features in late-stage DEBS modules, we were motivated to synthesize the native pentaketide substrate and employ it for in vitro biochemical analysis of engineered monomodules from the DEBS3 polypeptide. To establish an effective experimental system, the bimodular DEBS3 was separated into Ery5 (module 5), Ery5-TE (module 5 + engineered TE fusion), Ery6 (module 6 + native TE fusion) and DEBS TE (excised TE domain). The catalytic competence and substrate specificity profiles of these modules were evaluated and compared to corresponding late monomodules (module 5 and module 6) from the Pik PKS.
Results
Total synthesis of DEBS SNAC pentaketide substrate
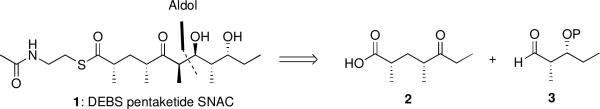
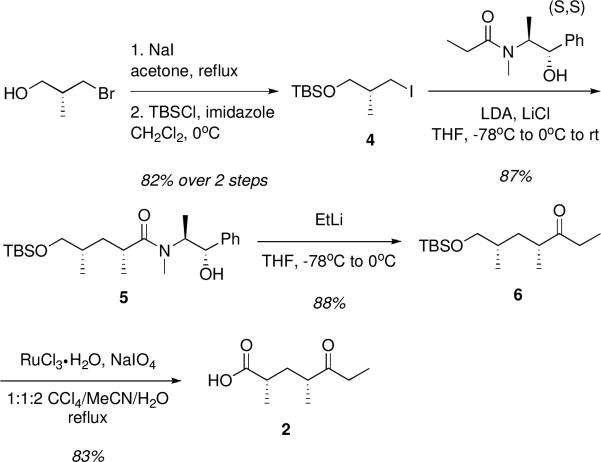
Retrosynthetic analysis of the native DEBS pentaketide (1) revealed an aldol disconnection between C6-C7, to give keto acid 2 and aldehyde 3 as the required fragments (Scheme 1). Selectivity for the desired anti-Felkin syn diastereomer could be achieved in the aldol coupling via a chelation-control element.17,18 Synthesis of the left fragment started with commercially available (R)-3-bromo-2-methyl-1-propanol, which was readily converted to iodide 4 by halide displacement and TBS protection of the primary alcohol (Scheme 2). Diastereoselective Myers alkylation19 of 4 with the enolate of (S,S)-pseudoephedrine propionamide gave amide 5 with high selectivity for the desired 2,4-syn product.20 The amide was then readily ethylated with EtLi to furnish ketone 6. Originally, ketone 6 was employed as the intended aldol-coupling partner, but complications arose in subsequent steps, making the strategy untenable. This was circumvented by instead using the C1-oxidized keto acid 2 as the direct coupling partner. Attempts to oxidize C1 via 2-step deprotection/oxidation procedures were problematic due to varying degrees of epimerization; however, one pot Sharpless oxidation of the TBS ether using RuO421 proved to be a clean and facile route to 2.
Scheme 1.
Retrosynthetic Analysis
Scheme 2.
Synthesis of Left Fragment.
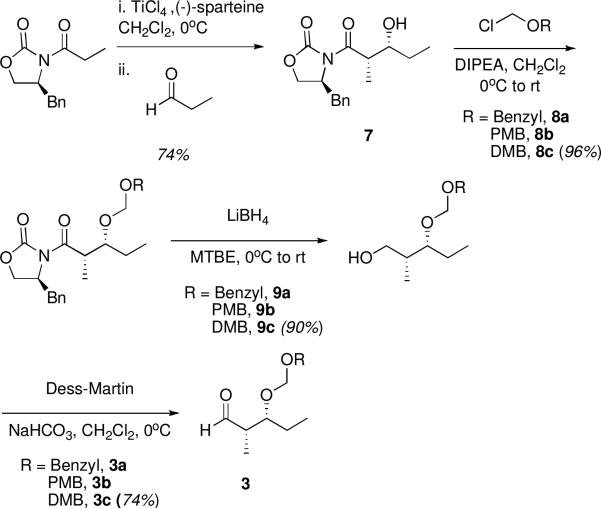
Synthesis of the right fragment 3 began with known Evans aldol product 7,22,23 which was protected with a benzyl formacetal group (Scheme 3). The protecting group strategy applied at the C9 hydroxyl proved crucial for selectivity in the aldol coupling and for final deprotection of the pentaketide substrate. Initially, the BOM ether protecting group was utilized since its extended linker had been thought to allow chelation at the C9 hydroxyl, thus enabling good anti-Felkin selectivity in analogous aldol couplings.17 However, attempts to remove this group at the end of the synthesis either failed or resulted in degradation of the desired pentaketide; therefore, other synthetic strategies were explored, including new protecting groups for the C9 hydroxyl. Of the protecting groups surveyed, only related benzyl formacetals did not abolish aldol selectivity. The p-methoxybenzyl (PMBOM) formacetal derivative also proved difficult to remove at the end of the synthesis, but the 3,4-dimethoxybenzyl (DMBOM) derivative was readily deprotected. Therefore, Evans-syn aldol product 7 was protected using DMBOMCl to give 8c, followed by reductive removal of the Evans auxiliary with LiBH4 to give alcohol 9c. Oxidation to aldehyde 3c was then best accomplished using Dess-Martin periodinane.
Scheme 3.
Synthesis of Right Fragment.
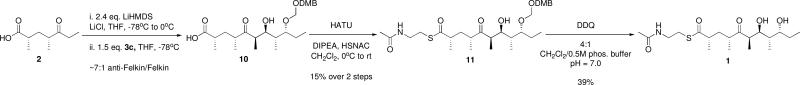
Coupling of the two fragments proceeded first by enolization of keto acid 2 with LiHMDS, followed by an aldol reaction with the DMBOM-protected aldehyde 3c to give the full carbon chain of the DEBS pentaketide with good (approximately 7:1) anti-Felkin/Felkin selectivity24 and modest yield (Scheme 4). This aldol step was followed by subsequent thioesterification with N-acetyl cysteamine (SNAC). Due to the exceptional acid-sensitivity of 1, buffered conditions were imperative in the final oxidative deprotection of the DMBOM group with DDQ, and deprotection yields suffered somewhat due to competing oxidation to form the DMB orthoester. Nonetheless, this convergent synthetic strategy provided the target pentaketide SNAC substrate in a sequence of only eight linear steps, avoiding excessive protecting group and functional group manipulations.
Scheme 4.
Aldol Coupling and Deprotection
Assay of DEBS monomodule activity
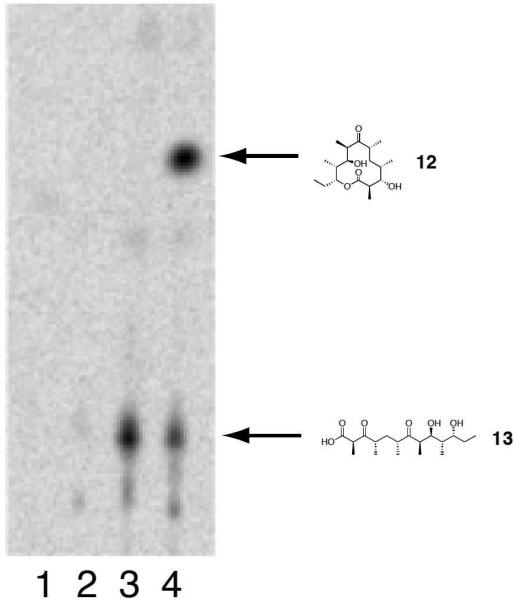
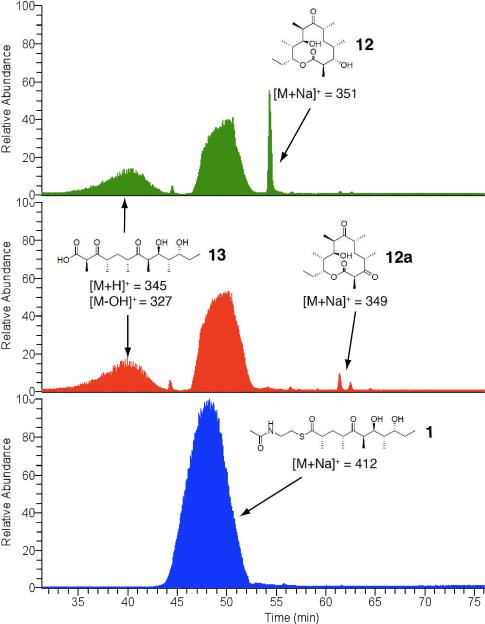
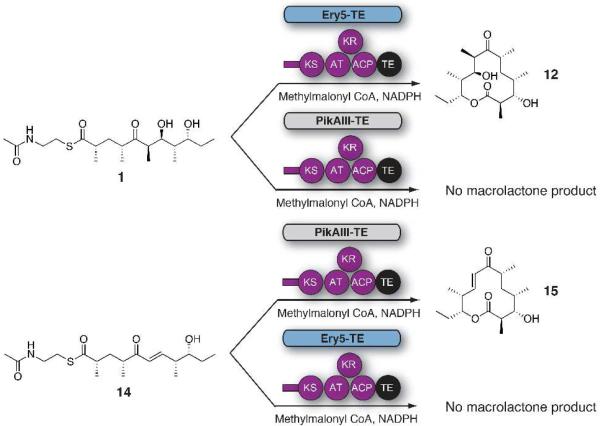
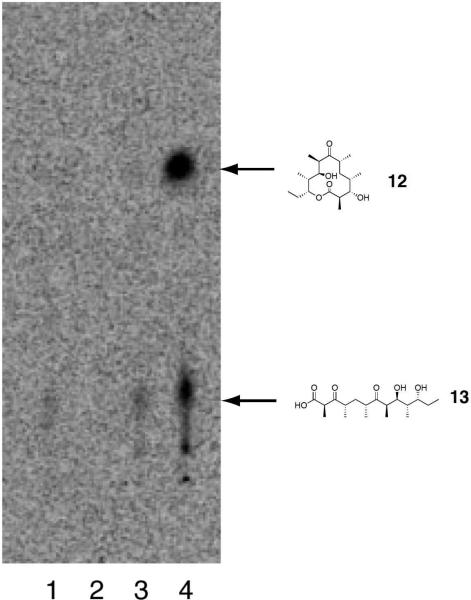
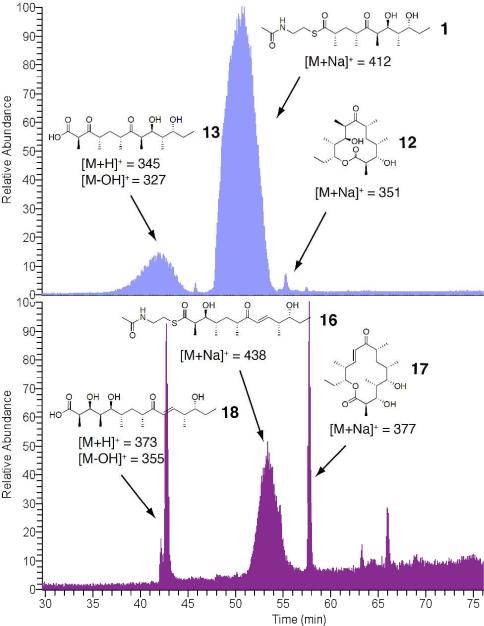
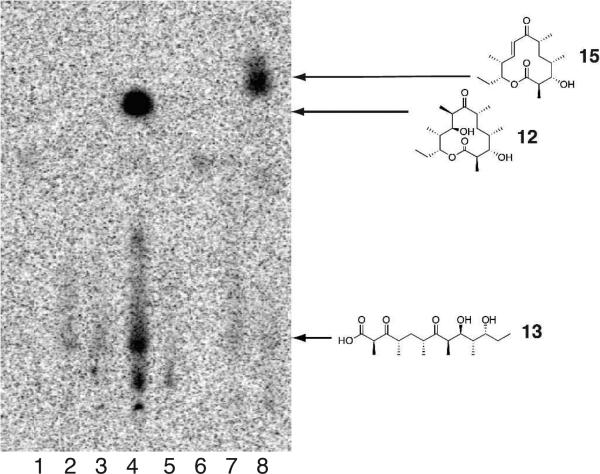
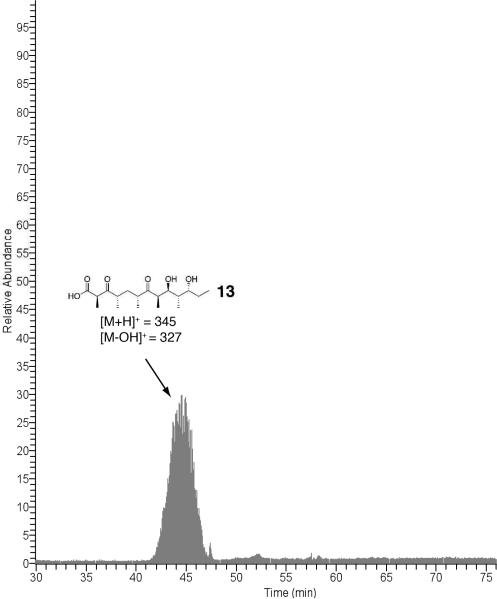
Engineered Ery5, Ery5-TE and Ery6 were incubated with the synthetic DEBS pentaketide SNAC substrate and 2-[14C]-methylmalonyl CoA, and the products were visualized by radio-TLC. In addition, duplicate reactions were performed utilizing unlabeled methylmalonyl CoA, and reaction products were analyzed and confirmed by LC-MS/MS. As demonstrated previously in vivo,25 the Ery5-TE fusion protein also generates an unnatural 12-membered ring macrolactone (12) in vitro (Figure 3 and 4; Scheme 5). This confirms that DEBS TE is capable of producing 12-membered macrocycles, similar to previous observations using PikAIII-TE. However, macrolactone 12 occurs with significant formation of linear hydrolysis products. Interestingly, in the absence of NADPH cofactor the reaction showed little production of the predicted 3-oxo macrocyclic derivative (12a), but instead converted intermediates to an unreduced hexaketide seco-acid 13. This suggests the possibility of a critical hydrogen-bonding interaction in the DEBS TE active site involving the β-position of the substrate. Thus, in comparison to the Pik TE where reduction of the enone functionality in the substrate to the allylic C-7 alcohol abrogated cyclization,26 subtle functional group changes can also significantly affect the ability of DEBS TE to catalyze macrocyclization. Also of note was the formation of some 13 even when NADPH was present in excess with Ery5-TE. This suggests a kinetic competition between reduction of the ACP-bound hexaketide by the Ery5 ketoreductase (KR) domain followed by TE-mediated macrolactonization, or premature transfer of unreduced hexaketide to the TE active site prior to reduction resulting in hydrolytic release. Seco-acid 13 was not previously reported from in vivo studies using an engineered DEBS1-DEBS2-M5-TE system,25 suggesting that its formation in vitro might be caused by suboptimal enzymatic processing from the unnatural Ery5-TE fusion protein.
Figure 3.
Radio-TLC of reaction products for Ery5-TE incubated with DEBS pentaketide SNAC. Assay conditions are described in the Experimental Section. Lane 1: No substrate (negative control); Lane 2: No enzyme (negative control); Lane 3: Ery5-TE w/o NADPH; Lane 4: Ery5-TE w/ NADPH.
Figure 4.
LC-MS chromatogram in selective ion mode (SIM) of Ery5-TE incubated with DEBS pentaketide SNAC. Top: Ery5-TE (green); Middle: Ery5-TE w/o NADPH (red); Bottom: No enzyme control (blue)
Scheme 5.
Illustration of in vitro enzymatic reactions for cognate and non-cognate pentaketide/module 5 pairs from the Pik and DEBS PKS. The putative products are macrolactone 12 and 10-DML (15).
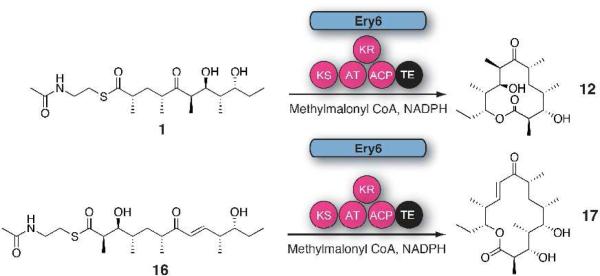
Next, further probing of the DEBS monomodules was carried out in Ery5 lacking the terminal thioesterase fusion and the final module Ery6. Radioassay of Ery5 and Ery6 showed that both modules appeared to generate low, but detectable levels of product when diffusively loaded with the DEBS pentaketide (Figure 5) but required further structural confirmation to confirm their identity (vide infra). A low level or lack of product formation was expected since Ery5 does not have a thioesterase domain to catalyze chain release, and the pentaketide is not the native substrate for Ery6. Since the total synthesis of the DEBS hexaketide chain elongation intermediate proved problematic due to its facile degradation, Ery6 could not be evaluated with its native substrate at this time. As expected, the pairing of Ery5-Ery6 did not generate 6-DEB, since the engineered monomodules are now physically distinct and lack native docking domains27 (unlike in monomodules PikAIII and PikAIV) to mediate polyketide chain transfer between the separated polypeptides. LC-MS/MS analysis confirmed that despite lacking a TE domain, detectable amounts of unreduced hexaketide seco-acid 13 are released from Ery5 presumably through adventitious hydrolysis of the intermediate from the Ery5 ACP. Addition to the reaction mixture of either the DEBS or Pik TE in trans with Ery5, however, did not restore production of 12. This contrasts with the pikromycin system where Pik TE is able to function in trans to catalyze formation of 10-DML from PikAIII both in vivo28 and in vitro.29 Remarkably, we observed that Ery6 was able to accept the DEBS pentaketide resulting in low levels of macrolactone 12 and hydrolysis product 13 (Scheme 6; Figure 6). This demonstrates an unexpected flexibility in this module toward incoming substrates bearing non-cognate functionality and chain length.
Figure 5.
Radio-TLC Analysis of Ery5 and Ery6 with DEBS pentaketide SNAC. Lane 1: Ery5 + Ery6; Lane 2: Ery5; Lane 3: Ery6; Lane 4: Ery5-TE (positive control)
Scheme 6.
Interrogation of Ery6 with non-native DEBS pentaketide and Pik hexaketide (16) substrates.
Figure 6.
SIM LC-MS chromatogram of Ery6 reactions. Top: Ery6 w/ DEBS pentaketide SNAC (light blue) Bottom: Ery6 w/ Pik hexaketide SNAC (purple).
Cross reactivity between DEBS and Pik systems
In order to probe the substrate specificity of the DEBS and Pik late modules, both DEBS pentaketide (1) and Pik pentaketide (14) substrates30 were assessed for their ability to be loaded, extended, processed and cyclized in unnatural substrate/PKS combinations (e.g. DEBS pentaketide SNAC against PikAIII-TE, Pik pentaketide SNAC against Ery5-TE) (Scheme 5). Radio-TLC of the reaction products did not show detectable levels of macrolactones 12 or 10-DML (15) relative to controls with either PikAIII-TE or Ery5-TE in reactions containing the respective non-native pentaketide substrate (Figure 7). LC-MS/MS analysis of the reactions also did not provide detectable levels of these macrolactones, instead showing the major products to be the pentaketide seco-acids, most likely arising from hydrolysis of the parent SNAC compounds by the terminal thioesterase domains. With PikAIII-TE, this hydrolytic activity is especially pronounced. PikAIII-TE incubated with the DEBS pentaketide SNAC revealed low levels of hexaketide hydrolysis product 13 by LC-MS/MS, indicating that some substrate can be accepted and extended by the enzyme, albeit very inefficiently. To assess the influence of the TE domain in these cross-PKS assays, non-cognate modules/substrates were incubated together, but native TEs were added in trans to determine if turnover could be enhanced. Ery5 was thus incubated with Pik pentaketide and the Pik TE, and PikAIII was incubated with the DEBS pentaketide and the DEBS TE. Neither reaction, however, showed detectable levels of macrolactones by radio-TLC or LC-MS/MS.
Figure 7.
Radio-TLC of non-cognate pairing of Pik/DEBS pentaketide SNACs incubated with Ery5-TE and PikAIII-TE. Lane 1: No enzyme w/ Pik pentaketide (negative control); Lane 2: Ery5-TE w/ Pik pentaketide (No NADPH); Lane 3: Ery5-TE w/ Pik pentaketide; Lane 4: Ery5-TE w/ DEBS pentaketide (positive control); Lane 5: No enzyme w/ DEBS pentaketide (negative control); Lane 6: PikAIII-TE w/ DEBS pentaketide (No NADPH); Lane 7: PikAIII-TE w/ DEBS pentaketide; Lane 8: PikAIII-TE w/ Pik pentaketide (positive control)
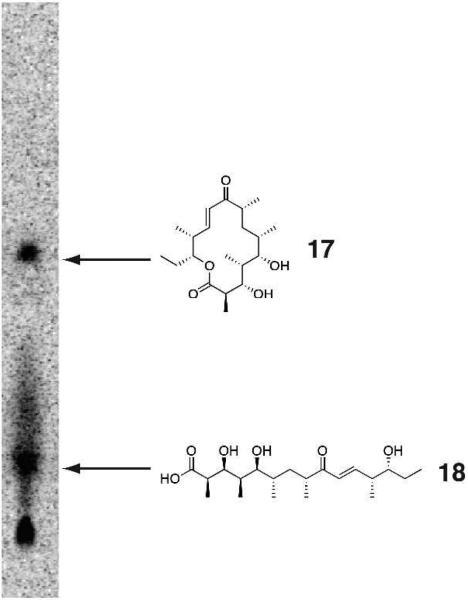
Unlike the experiments involving interrogation of non-cognate pentaketide/module 5 pairs, the module 6 PKSs from Pik and DEBS demonstrated some flexibility toward non-native substrates. Ery6 was able to accept, extend, process and cyclize the non-native Pik hexaketide31 (Scheme 6), though not the Pik pentaketide. When Ery6 was incubated with Pik hexaketide (16), both 3-hydroxy-narbonolide (17) and hydrolyzed linear heptaketide (18) were observed in the radio-TLC (Figure 8) and LC-MS/MS assay (Figure 6). In this case, no competition between ketoreduction and cyclization was detected. Since DEBS hexaketide currently remains unavailable, PikAIV tolerance could not be evaluated in an analogous experiment. Interestingly, when the DEBS pentaketide was incubated with PikAIV, detectable levels of hexaketide seco-acid 13 were generated, though the predicted cyclized product 12a was absent. This observation conforms to the current hypothesis regarding ring-formation requirements of the Pik TE, where a rigid enone at C7-C9 was revealed to be essential for cyclization of the Pik hexaketide intermediate by the thioesterase.26 As had been observed previously in vitro,16 the Pik pentaketide was not accepted by PikAIV to produce 3-oxo-10-DML.
Figure 8.
Radio-TLC of Ery6 incubated with Pik hexaketide SNAC.
Finally, bimodular pairing of PikAIII-PikAIV did not result in production of predicted macrolactones 12 or 3-oxo-6-DEB when incubated with the DEBS pentaketide. This is consistent with the rigid molecular specificity exhibited by PikAIII toward the DEBS pentaketide. Nonetheless, hydrolyzed hexaketide 13 was observed in this bimodular context (Figure 9), the result of direct loading, extension and hydrolysis on the more flexible PikAIV monomodule.
Figure 9.
SIM LC-MS chromatogram for DEBS pentaketide SNAC incubated with PikAIII-PikAIV.
Discussion
Synthesis of the DEBS pentaketide revealed some of the inherent challenges present in the total synthesis of linear polyketides, which often suffer from facile degradation pathways. This was seen in the final deprotection step of the pentaketide, where the intermediate readily yielded to acid-catalyzed hemiketalization and dehydration under most conditions. The synthesis thus required the sparsely used DMBOM group, which enabled mild deprotection conditions and provided the necessary compatibility with the earlier aldol step. In addition, similar degradation pathways thwarted current efforts toward the desired DEBS hexaketide intermediate. These challenges highlight an important, yet often under-appreciated aspect of the PKS catalytic mechanism, whereby the linear polyketide chain elongation intermediates remain covalently linked to the enzyme complex throughout the PKS extension process, rather than being diffusively off-loaded after each catalytic cycle. This mechanism protects the chemically sensitive and transient intermediates against numerous potential degradation pathways until they are released as more stable products.
Analysis of the catalytic abilities of the dissected DEBS monomodules revealed distinctive profiles relative to their naturally occurring counterparts in the Pik system. From these studies, the macrocyclization activity of the DEBS and Pik TEs was revealed to be a key issue, especially in the formation of 12-membered ring products. In Pik, the ability to form 12-membered macrocycles is well evolved, giving nearly exclusive formation of 10-DML or 3-oxo-10-DML (when NADPH cofactor is excluded) when presented with the natural hexaketide substrate. However, with the DEBS TE, the ability to generate the analogous macrolactones 12 and 12a competes significantly with hydrolysis activity. This inefficiency appears to stem from incomplete ketoreduction by the KR of Ery5, followed by subsequent difficulty in cyclizing the resultant 3-oxo hexaketide derivative by DEBS TE. It appears that the unnatural TE fusion facilitates facile cyclization/hydrolysis of unreduced hexaketide prior to reduction by the KR. The reason for inefficient KR function is unclear, as it has not been reported in any previous studies performed in vitro12,13 or in vivo25 with DEBS module 5. In this case, the interplay of multiple factors could cause premature cyclization/hydrolysis of the acyl chain from Ery5-TE, though structural issues related to the ACP-TE fusion appear likely to be strong contributors. Since the TE fusion and linker region are unnatural in this engineered monomodule, this perturbation might result in premature transfer of extended hexaketide to the TE active site prior to complete processing by the ketoreductase. This possibly results from a decrease in the rate of Ery5 modular catalytic events, an increase in the rate of intermediate transfer to the thioesterase from the Ery5 ACP, or a combination of both. Similar competition between reduction and cyclization has also been reported previously with the analogous engineered PikAIII-TE, though this occurred only when the module was incubated with diketides14 rather than native substrates. With native Pik pentaketide, no 3-oxo-10-DML was observed with PikAIII-TE when NADPH was included.16 Thus, unlike Ery5-TE, incomplete reduction in PikAIII-TE is likely a substrate-driven process rather than a result of enzyme structural elements that perturb its function.
The differing cyclization activities of the Pik and DEBS TEs suggest unique substrate binding modes within their active sites. The oxidation state at the β-position is important for TE-mediated cyclization in DEBS, but not in Pik. Thus, it can be reasoned that the β-hydroxy group in the extended DEBS hexaketide acts as a hydrogen-bond donor in the TE active site, creating an interaction that is vital for efficient cyclization. This would not be surprising, as the TE has evolved to generate its sole product 6-DEB from the native heptaketide intermediate, which also contains a β-hydroxy group. Abrogation of this interaction by replacement with a keto group could result in attenuated cyclization. It has been proposed from modeling of 6-DEB into the active site of the DEBS TE crystal structure that the 3-position hydroxyl participates in a hydrogen-bond with Asn-180 and the backbone carbonyl of Tyr-171,32 which is consistent with this observation. This contrasts sharply with the hypothesized binding mode for Pik TE, which has been shown from structural studies using affinity-label probes to have minimal active site hydrogen-bonding interactions with incoming substrates.33 Here, the oxidation state at the β-position does not have a critical effect on macrolactonization, as Pik TE efficiently cyclizes both a hexaketide containing a β-hydroxy and a heptaketide containing a β-keto group to give 10-DML and narbonolide, respectively.16
While there is evidence that Pik TE has developed direct protein-protein interactions with PikAIII29 to enable formation of 10-DML from this monomodule, Ery5 and the DEBS TE appear to be unable to engage in a similar type of molecular recognition. Providing DEBS TE or Pik TE in trans to substrate-bound Ery5 does not enhance release of macrolactone products. Similarly, excised DEBS TE provided in trans also failed to catalyze release of intermediates from PikAIII, strongly suggesting that PikAIII and Pik TE are capable of engaging in a unique type of molecular interaction.
When PKS substrate specificity is considered, both Pik and DEBS module 5 appear to have a strong preference for their native pentaketide chain-elongation intermediates. Both modules (with engineered TE domains) are capable of efficiently accepting and processing their respective pentaketide substrates and cyclizing them to 12-membered ring macrolactones. However, when incubated with the pentaketide from the reciprocal system, neither module was tolerant toward the non-native substrate, despite high structural similarity that differs only at C6-C7 of the respective polyketide chains. These results suggest that even small functional changes distal to the enzyme acyl-thioester linkage are capable of precluding efficient PKS activity. This contrasts with earlier precursor-directed biosynthesis studies in DEBS, where distal functional changes from unnatural starter units were well-tolerated by downstream modules and incorporated into 6-DEB analogues.34 These modifications at the terminus of the chain elongation intermediate, however, are located at a position more remote than those in the substrates from the current studies. Further analysis is thus required to determine whether the substrate-loading event, chain-elongation/processing/termination or all of these steps represent potential roadblocks to catalysis. Previously, it has been shown that the PKS channeling mechanism plays a key role in substrate loading, and that diffusive loading of an acyl-SNAC may not reflect the authentic molecular recognition features of an individual module. In those studies, inherently poor diketide model substrates that could not be accepted by diffusive loading were accepted by PKS modules if presented as appropriate upstream ACP-bound intermediates.13 Nonetheless, these suboptimal substrates were still extended and cyclized (as triketide latones) at much lower rates than optimized diketide model compounds. Since these current studies have relied on diffusive loading, it might be informative to assess the contributions of channeling to the specificity of the substrate-loading event with native chain elongation intermediates.
In contrast to Ery5, Ery6 appears to have significantly increased flexibility toward non-native substrates. Ery6 was capable of accepting, extending and cyclizing the non-cognate DEBS pentaketide and Pik hexaketide substrates, though not the Pik pentaketide intermediate. This was surprising as the DEBS pentaketide and Pik hexaketide not only have differing chain lengths (10 and 12 carbons, respectively), but also differ in their substitution and stereochemistry at the proximal α and β positions as well as at more distal positions. The fact that the Pik pentaketide was not accepted also suggests a key recognition feature at C6-C7 in Ery6, as these are the only chemically unique positions compared to DEBS pentaketide. It is possible that the rigid double bond is structurally untenable for Ery6; however, the Pik hexaketide also contains an alkene, although its location is shifted to the C8-C9 position. Thus, since the presence of the enone alone cannot explain poor activity, the possibility remains that the position of the enone is a key consideration. To a lesser degree than Ery6, PikAIV also demonstrated flexibility in its ability to accommodate the DEBS pentaketide, catalyzing extension to the hydrolyzed linear hexaketide seco-acid 13; however, the Pik TE could not cyclize the pendant DEBS hexaketide intermediate to the expected macrolactone 12a in this reaction. At the same time, the failure of PikAIV to accept the Pik pentaketide indicates that this substrate may not be well tolerated outside of its native module PikAIII.
Taken together, the substrate selectivity profiles that have emerged from these studies for the late modules from the DEBS and Pik PKSs suggest rigidity in their fifth modules with pentaketide substrates, yet tolerance in their sixth and final module. Module 5 in these PKSs may act as stringent molecular gatekeepers, especially for diffusive loading of the KS, as module 5 must mediate intermodular transfer of intermediates from the upstream module 4, and promiscuous loading of the exposed KS active site could result in competing enzymatic pathways or domain inactivation. Module 6, on the other hand, appears to lack this level of stringency. In the native bimodular context of DEBS3, the Ery6 KS accepts chain-elongation intermediates in an intramodular fashion and is likely inaccessible to diffusive loading. Thus, increased flexibility would not be detrimental to overall modular efficiency and substrate stringency would be unnecessary. Due to its monomodular architecture, PikAIV also contains an accessible KS active site and must accept intermediates through an intermodular transfer. Thus, we might expect that PikAIV would exhibit a similar level of molecular specificity as PikAIII or Ery5. Nonetheless, PikAIV is capable of accepting complex non-native intermediates, though more rigorous interrogation is necessary to fully assess its specificity.
Based on this comparative analysis, the late modules in the DEBS and Pik polyketide synthases have evolved unique specificity profiles and catalytic capacities. Specific insights have been gained toward understanding key recognition features necessary for TE-mediated cyclization in these systems, which is vital for efficient formation of new macrolactone scaffolds utilizing TE domains as biocatalysts. This new information also provides an additional basis for future bioengineering efforts involving a range of PKS modules. Due to their promiscuity, both Pik and DEBS module 6, especially Ery6, appear to be excellent candidates for engineering of new biosynthetic pathways. Future efforts will seek to assess the kinetic parameters of the flux through the DEBS modules with native chain-elongation intermediates, including the contribution of channeling to molecular specificity. Concurrently, continued analysis of substrate tolerance in modules from both systems will be assessed using analogues that encompass a variety of stereochemical, structural and functional group variations.
Experimental Section
Materials and General Procedures. Substrate Synthesis
Commercially available reagents were purchased from Sigma-Aldrich, Acros Organics or Fluka, or prepared from established literature procedures. RuCl3·H2O was obtained from Strem Chemicals, Inc. The pikromycin pentaketide and hexaketide SNAC substrates were prepared as previously described.16 Anhydrous solvents were obtained either using an MBRAUN MB-SPS solvent purification system or purchased in septum-sealed bottles from Acros Organics (AcroSeal™) or EMD (DriSolv®). Diisoproylamine and N, N-diisopropylethylamine were distilled over CaH2 and stored under argon. Alkyllithiums were titrated prior to use with diphenylacetic acid. Air- and moisture-sensitive reactions were carried out under an inert argon atmosphere in flame-dried glassware. Flash chromatography was carried out using Merck Silica Gel 60 (230-400 mesh) and thin layer chromatography was performed on Merck TLC plates pre-coated with Silica Gel 60 F254. 1H and 13C NMR spectra were obtained on either Varian MR400 or Inova 500 spectrometers. Proton chemical shifts are reported in ppm relative to TMS with the residual solvent peak as an internal standard. Proton NMR data is reported as follows: chemical shift (δ ppm), multiplicity, coupling constant (Hz) and integration. High-resolution mass spectra were obtained on a Waters Micromass AutoSpec Ultima Magnetic Sector mass spectrometer.
Iodide 4
A suspension of (R)-(-)-3-bromo-2-methyl-1-propanol (4.995 g, 32.64 mmol) and NaI (19.571 g, 130.57 mmol) in 60 mL acetone was brought to reflux at 70 °C. After 18 h, the reaction mixture was diluted with 50 mL H2O and the volatiles were removed en vacuo. The mixture was then extracted with 3 × 50 mL CH2Cl2, and the combined organics were washed with 20 mL saturated Na2S2O3, dried over MgSO4, filtered and concentrated.
The crude iodide was re-dissolved in 100 mL CH2Cl2 and cooled to 0 °C. Imidazole (2.445 g, 35.91 mmol) and TBSCl (5.412 g, 35.91 mmol) were then added sequentially. A white precipitate formed immediately, and the reaction was monitored by TLC. After 1 h, the reaction was filtered and concentrated. The crude mixture was re-suspended in 100 mL pentane and filtered, rinsing the filter with an additional 50 mL pentane. The filtrate was concentrated and the crude oil was purified by flash chromatography (100% hexanes) to give 8.358 g (81.5%) of colorless oil. 1H NMR (CDCl3, 399.5 MHz) δ 3.53 (dd, J = 10.0, 5.0 Hz, 1H), 3.40 (dd, J = 10.0, 6.9 Hz, 1H), 3.30 (dd, J = 9.5, 5.2 Hz, 1H), 3.24 (dd, J = 9.5, 5.6 Hz, 1H), 1.79 – 1.53 (m, 1H), 0.95 (d, J = 6.7 Hz, 3H), 0.90 (s, 9H), 0.06 (s, 3H), 0.06 (s, 3H); 13C NMR (CDCl3, 100.5 MHz) δ −5.4, 13.7, 17.2, 18.2, 25.9, 37.4, 66.7; HRMS CI+ (m/z): 315.0656 (Predicted [M+H]+ for C14H24OSiI is 315.0641).
Amide 5
n-Buli (2.35 M in hexanes, 33.58 mL, 78.91 mmol) was added dropwise at −78 °C to a stirring suspension of flame-dried LiCl (12.948 g, 305.45 mmol) and diisopropylamine (11.871 mL, 84.00 mmol) in 50 mL THF. The reaction was warmed to 0 °C briefly for 5 min, then re-cooled to −78°C. (S,S)-(+)-pseudoephedrine propionamide (9.013 g, 40.73 mmol) in 100 mL THF was added dropwise by cannula, and the reaction was stirred for 1 h at −78 °C, 30 min at 0 °C, and 5 min at room temperature. Iodide 4 (8.000 g, 25.45 mmol) in 10 mL THF was added dropwise at 0 °C, then the reaction was allowed to warm to room temperature and stirred for 22 h. The reaction was quenched with 100 mL saturated NH4Cl and diluted with 100 mL EtOAc. The layers were separated and the aqueous layer was extracted with 2 × 100 mL EtOAc. The combined organics were dried over MgSO4, filtered and concentrated. The crude oil was purified by flash chromatography (30% EtOAc/Hexanes) to give 8.994 g (86.7%) of colorless solid. 1H NMR (CDCl3, 399.5 MHz) δ 7.43 – 7.10 (m, 5H), 4.64 – 4.51 (m, 2H), 4.34 (s, 1H), 3.43 (dd, J = 9.8, 4.8 Hz, 1H), 3.36 (dd, J = 9.8, 5.9 Hz, 1H), 2.83 (s, 3H), 2.80 – 2.64 (m, 1H), 1.70 – 1.60 (m, 1H), 1.58 – 1.47 (m, 1H), 1.13 (d, J = 7.1 Hz, 3H), 1.20 – 1.04 (m, 1H), 1.07 (d, J = 6.7 Hz, 3H), 0.86 (s, 9H), 0.81 (d, J = 6.6 Hz, 3H), 0.01 (s, 6H); 13C NMR (CDCl3, 100.5 MHz) δ −5.5, −5.4, 14.4, 17.3, 17.5, 18.3, 25.9, 33.1, 34.1, 37.6, 67.9, 76.5, 76.7, 77.0, 77.3, 126.2, 126.9, 127.4, 128.3, 128.7, 142.6, 179.1; HRMS ESI+ (m/z): 430.2740 (Predicted [M+Na]+ for C23H41NO3Si is 430.2753). As expected from literature precedent,19 5 was isolated as a mixture of amide rotamers. The reported NMR data only denotes peaks arising from the major rotamer.
Ketone 6
To a stirring suspension of amide 5 (1.866 g, 4.58 mmol) in 50 mL THF was added EtLi (0.46 M in 90/10 benzene:cyclohexane, 22.89 mL, 10.53 mmol) at −78 °C. The reaction was stirred 10 min, then warmed to 0 °C and stirred an additional 30 min. Subsequently, 1 equivalent of diisopropylamine was added to scavenge excess EtLi and the mixture stirred for 15 min. The reaction was quenched with 20% AcOH/Et2O, diluted with H2O and EtOAc, and the layers were separated. The aqueous was extracted with 1 × 50 mL EtOAc, and the combined organics were dried over MgSO4, filtered and concentrated. The crude oil was purified by flash chromatography (2% EtOAc/Hexanes) to give 1.101 g (88.3%) of colorless oil. 1H NMR (CDCl3, 399.5 MHz) δ 3.39 (dd, J = 10.3, 6.4 Hz, 1H), 3.35 (dd, J = 10.2, 6.5 Hz, 1H), 2.70 – 2.60 (m, 1H), 2.52 – 2.35 (m, 2H), 1.77 (ddd, J = 13.8, 7.9, 6.1 Hz, 1H), 1.60 – 1.44 (m, 2H), 1.06 (d, J = 6.9 Hz, 3H), 1.02 (t, J = 7.3 Hz, 3H), 0.87 (s, 9H), 0.86 (d, J = 6.4 Hz, 3H), 0.01 (s, 6H); 13C NMR (CDCl3, 100.5 MHz) δ −5.5, −5.5, 7.7, 17.1, 17.3, 18.2, 25.8, 33.6, 33.6, 36.9, 43.9, 67.9, 215.2; HRMS ESI+ (m/z): 295.2076 (Predicted [M+Na]+ for C15H32O2Si is 295.2069).
Keto acid 2
Ketone 6 (1.081 g, 3.97 mmol) was dissolved in 20 mL 1:1:2 CCl4/CH3CN/H2O and then RuCl3·H2O (0.082 g, 0.397 mmol) and NaIO4 (4.242 g, 19.84 mmol) were added sequentially. The reaction was brought to reflux at 70 °C and heated for 16 h overnight. The mixture was cooled to room temperature, diluted with 20 mL of CH3CN, and filtered through a plug of Celite. The plug was washed with an additional 100 mL of CH3CN and the filtrate volatiles were removed en vacuo. The mixture was taken up in 50 mL EtOAc and extracted with 3 × 50 mL half saturated NaHCO3. The combined aqueous portion was then back extracted with 1 × 50 mL Et2O. The aqueous layer was then carefully acidified with dropwise addition of concentrated HCl to pH 2, then extracted with 4 × 50 mL CH2Cl2. The organic extracts were dried over MgSO4, filtered and concentrated to give 0.564 g (82.6%) of colorless oil. 1H NMR (CDCl3, 499.9 MHz) δ 11.86 (br s, 1H), 2.62 – 2.54 (m, 1H), 2.51 – 2.37 (m, 3H), 2.03 (ddd, J = 14.0, 8.9, 6.4 Hz, 1H), 1.32 (ddd, J = 13.7, 7.7, 5.7 Hz, 1H), 1.14 (d, J = 7.0 Hz, 3H), 1.05 (d, J = 7.0 Hz, 3H), 0.98 (t, J = 7.3 Hz, 3H); 13C NMR (CDCl3, 125.7 MHz) δ 7.7, 16.5, 17.5, 34.0, 36.1, 37.2, 43.7, 182.5, 214.6; HRMS ESI+ (m/z): 195.1002 (Predicted [M+Na]+ for C9H16O3 is 195.0997).
Oxazolidinone 8c
To a solution of Evans aldol product 7 (2.000 g, 6.87 mmol), TBAI (0.254 g, 0.687 mmol), and DIPEA (5.98 mL, 34.32 mmol) in 50 mL CH2Cl2 was added DMBOMCl35 (7.437 g, 34.32 mmol) in 20 mL CH2Cl2 dropwise at 0 °C. The reaction was warmed to room temperature and stirred for 16 h. The reaction was quenched with 50 mL H2O and the layers were separated. The aqueous was extracted with 2 × 25 mL CH2Cl2, and the combined organics were dried over MgSO4, filtered and concentrated. The crude oil was purified by flash chromatography (20% to 30% EtOAc/Hexanes) to give 3.106 g (95.9%) of pale yellow oil that slowly solidified. 1H NMR (CDCl3, 399.5 MHz) δ 7.32 – 7.16 (m, 3H), 7.14 – 7.06 (m, 3H), 6.88 – 6.80 (m, 2H), 6.73 (d, J = 7.9 Hz, 1H), 4.73 (d, J = 7.6 Hz, 1H), 4.71 (d, J = 7.9 Hz, 1H), 4.50 (ABq, νA = 31.7, νB = 11.5 Hz, 2H), 4.42 – 4.33 (m, 1H), 3.99 (dd, J = 9.0, 2.0 Hz, 1H), 3.96 (dd, J = 6.9, 3.9 Hz, 1H), 3.90 – 3.82 (m, 2H), 3.82 (s, 3H), 3.78 (s, 3H), 3.23 (dd, J = 13.3, 3.1 Hz, 1H), 2.67 (dd, J = 13.3, 9.9 Hz, 1H), 1.63 (qd, J = 7.7, 4.6 Hz, 2H), 1.23 (d, J = 7.0 Hz, 3H), 0.96 (t, J = 7.5 Hz, 3H); 13C NMR (CDCl3, 100.5 MHz) δ 10.1, 10.8, 25.6, 37.6, 41.2, 55.8, 66.0, 69.8, 80.1, 94.3, 110.7, 110.8, 119.9, 127.2, 128.8, 129.3, 130.5, 135.4, 148.5, 148.9, 153.3, 174.9; HRMS ESI+ (m/z): 494.2160 (Predicted [M+Na]+ for C26H33NO7 is 494.2155).
Alcohol 9c
LiBH4 (2.0 M in THF, 3.83 mL, 7.66 mmol) was added dropwise to a solution of oxazolidinone 8 (3.010 g, 6.38 mmol) and MeOH (0.341 mL, 7.66 mmol) in 30 mL MTBE at 0 °C. The reaction was stirred for 20 min, then warmed to room temperature and monitored by TLC. After 3 h, the reaction was quenched with 30 mL saturated Na/K tartrate and stirred until the layers became clear. The layers were separated and the aqueous was extracted with 2 × 25 mL EtOAc. The combined organics were dried over MgSO4, filtered and concentrated. The crude oil was purified by flash chromatography (30% EtOAc/Hexanes) to give 1.716 g (90.1%) of pale yellow oil. 1H NMR (CDCl3, 399.5 MHz) δ 6.91 – 6.75 (m, 3H), 4.75 (ABq, νA = 17.4, νB = 6.9 Hz, 2H), 4.55 (ABq, νA = 31.9, νB = 11.5 Hz, 2H), 3.85 (s, 3H), 3.83 (s, 3H), 3.71 – 3.58 (m, 1H), 3.58 – 3.39 (m, 1H), 2.69 (br s, 1H), 2.06 – 1.86 (m, 1H), 1.68 – 1.54 (m, 1H), 1.54 – 1.41 (m, 0H), 0.90 (t, J = 7.4 Hz, 3H), 0.82 (d, J = 7.0 Hz, 3H); 13C NMR (CDCl3, 100.5 MHz) δ 10.5, 10.8, 24.1, 37.6, 55.8, 55.9, 65.3, 69.9, 81.2, 94.3, 110.9, 111.1, 120.4, 130.0, 148.7, 149.0; HRMS ESI+ (m/z): 321.1672 (Predicted [M+Na]+ for C16H26O5 is 321.1678).
Aldehyde 3c
To a suspension of alcohol 9 (1.251 g, 4.19 mmol) and NaHCO3 (1.761 g, 20.96 mmol) in 40 mL CH2Cl2 was added Dess-Martin periodinane (2.133 g, 5.03 mmol) at 0 °C. The reaction was stirred 5 min, then warmed to room temperature and monitored by TLC. After 1 h, the reaction was quenched by addition of 20 mL of half saturated Na2S2O3 and stirred until the layers became clear. The layers were separated and the aqueous was extracted with 2 × 25 mL CH2Cl2. The combined organics were dried over MgSO4, filtered and concentrated. The crude oil was purified by flash chromatography (20% EtOAc/Hexanes) to give 0.941 g (75.7%) of pale yellow oil. 1H NMR (CDCl3, 399.5 MHz) δ 9.78 (s, 1H), 7.10 – 6.41 (m, 1H), 4.75 (ABq, νA =18.1, νB = 7.2 Hz, 2H), 4.47 (ABq, νA = νB = 11.4 Hz, 2H), 4.01 (td, J = 6.8, 3.4 Hz, 1H), 3.87 (s, 3H), 3.85 (s, 3H), 2.56 (qd, J = 7.0, 3.4 Hz, 1H), 1.73 – 1.64 (m, 1H), 1.63 – 1.50 (m, 1H), 1.11 (d, J = 7.0 Hz, 3H), 0.94 (t, J = 7.4 Hz, 3H); 13C NMR (CDCl3, 100.5 MHz) δ 7.6, 10.3, 24.8, 49.4, 55.8, 55.9, 69.7, 78.6, 93.8, 110.9, 111.3, 120.5, 130.1, 148.7, 149.0, 204.5; HRMS ESI+ (m/z): 319.1521 (Predicted [M+Na]+ for C16H24O5 is 319.1527).
DMBOM-protected DEBS pentaketide SNAC 11
LiHMDS (1.0 M in THF, 4.85 mL, 4.85 mmol) was added dropwise to a stirring solution of keto acid 2 (0.348 g, 2.03 mmol) and flame-dried LiCl (0.343 g, 8.10 mmol) in 10 mL THF at −78 °C. The reaction was stirred for 1 h, then warmed to 0 °C for 30 min. The reaction was then re-cooled to −78 °C, and the aldehyde 3c (0.901 g, 3.04 mmol) in 5 mL THF was added dropwise. Following addition, the reaction was stirred for 90 min, then quenched with 20 mL saturated NH4Cl. The mixture was diluted with 20 mL EtOAc, then extracted with 3 × 25 mL half saturated NaHCO3. The aqueous extracts were back extracted with 1 × 25 mL Et2O, then carefully acidified to pH 2 with 1 N HCl. The aqueous layer was then extracted with 4 × 25 mL CH2Cl2. The combined organics were dried over MgSO4, filtered and concentrated. The crude oil (containing a ~7:1 anti-Felkin/Felkin mixture of seco-acids by 1H NMR) was carried on to the next step without further purification.
The crude acid was dissolved in 20 mL CH2Cl2, followed by addition of HATU (1.152 g, 3.03 mmol), DIPEA (1.06 mL, 6.06 mmol) and HSNAC (0.258 mL, 2.43 mmol). The reaction became clear, and the dark yellow solution was stirred for 18 h at room temperature. The reaction was quenched with 20 mL saturated NaHCO3, and the resulting layers were separated. The aqueous layer was then extracted with 2 × 25 mL CH2Cl2. The combined organics were dried over MgSO4, filtered and concentrated. The crude oil was partially purified by flash chromatography (2% MeOH/CH2Cl2), then purified by preparative HPLC on a Phenomenex Luna C18(2) column (5 μm, 21.2 × 250 mm) using a CH3CN/H2O + 0.1% TFA gradient (10% to 100%, 60 min, 10 ml/min). The desired compound eluted between 45.5 and 46.5 (detecting at 254 nm) min to give 0.168 g (14.6%) of colorless oil. 1H NMR (CDCl3, 399.5 MHz) δ 6.90 – 6.66 (m, 1H), 6.05 (br s, 1H), 4.78 (ABq, νA = 23.0, νB = 6.8 Hz, 2H), 4.55 (ABq, νA = νB = 11.5 Hz, 2H), 3.95 (dd, J = 9.5, 2.5 Hz, 1H), 3.89 – 3.85 (m, 1H), 3.87 (s, 3H), 3.85 (s, 3H), 3.51 – 3.30 (m, 2H), 3.10 – 2.89 (m, 2H), 2.82 – 2.73 (m, 2H), 2.69 – 2.62 (m, 1H), 2.09 (ddd, J = 14.5, 8.6, 6.2 Hz, 1H), 1.97 (s, 3H), 1.82 – 1.65 (m, 2H), 1.56 – 1.44 (m, 1H), 1.37 – 1.27 (m, 1H), 1.16 (d, J = 6.8 Hz, 3H), 1.08 (d, J = 7.0 Hz, 3H), 1.06 (d, J = 6.8 Hz, 3H), 0.91 (t, J = 7.4 Hz, 3H), 0.83 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100.5 MHz) δ 8.6, 10.3, 10.7, 16.8, 18.6, 22.8, 24.6, 28.2, 36.8, 37.7, 39.8, 42.0, 46.4, 46.4, 55.8, 55.9, 69.8, 72.0, 80.6, 94.6, 110.9, 111.1, 120.4, 130.0, 148.6, 149.0, 171.4, 203.7, 217.7; HRMS ESI+ (m/z): 592.2933 (Predicted [M+Na]+ for C29H47NO8S is 592.2920).
DEBS pentaketide SNAC 1
DDQ (0.018 g, 0.079 mmol) was added to 11 (0.009 g, 0.016. mmol) in a biphasic mixture of 4:1 CH2Cl2/0.5 M phosphate buffer, pH 7.0 at 0 °C. The reaction was warmed to room temperature and monitored by TLC. The reaction was quenched with 5 mL saturated NaHCO3, and diluted with 10 mL CH2Cl2. The layers were separated and the aqueous was extracted with 2 × 10 mL CH2Cl2. The combined organics were dried over MgSO4, filtered and concentrated. The crude material was resuspended in 1 mL CH3CN, run through a 0.22 μm hydrophobic syringe filter (Millipore), and then purified by preparative HPLC on a Phenomenex Luna C18(2) column (5 μm, 21.2 × 250 mm) using a CH3CN/H2O gradient (10% to 100%, 60 min, 10 ml/min). The desired compound eluted at 33 and 37.5 min (detecting at 240 nm) to give 2.4 mg (39%) of colorless oil as a ~2:1 equilibrium mixture of open linear pentaketide and closed hemiketal. 1H NMR (CDCl3, 399.5 MHz) δ 5.91 (br s, 0.35H), 5.85 (br s, 0.65H), 3.91 (dt, J = 8.8, 2.8 Hz, 0.65H), 3.87 – 3.73 (m, 1.35H), 3.58 – 3.27 (m, 3H), 3.10 – 2.97 (m, 2H), 2.85 (ddd, J = 14.2, 7.1, 2.9 Hz, 1H), 2.81 – 2.61 (m, 2H), 2.12 (ddd, J = 14.4, 8.7, 5.8 Hz, 1H), 1.97 (s, 2H), 1.96 (s, 1H), 1.90 – 1.76 (m, 2H), 1.73 (ddd, J = 9.0, 7.0, 2.0 Hz, 0.65H), 1.64-1.50 (m, 1.65H), 1.48 – 1.31 (m, 2H), 1.23 (d, J = 7.0 Hz, 1H), 1.20 (d, J = 6.9 Hz, 2H), 1.14 (d, J = 7.2 Hz, 2H), 1.11 (d, J = 6.9 Hz, 2H), 0.98 (t, J = 7.4 Hz, 2H), 0.96 (d, J = 5.8 Hz, 1H), 0.95 (d, J = 6.5 Hz, 1H), 0.89 (t, J = 7.4 Hz, 1H), 0.84 (d, J = 7.0 Hz, 2H), 0.82 (d, J = 6.7 Hz, 1H); 13C NMR (CDCl3, 100.5 MHz) δ 4.5, 9.5, 10.4, 11.0, 11.0, 15.0, 16.3, 16.4, 18.6, 19.8, 23.2, 25.2, 26.4, 28.4, 28.5, 29.7, 34.7, 35.9, 36.3, 37.9, 38.9, 42.7, 45.6, 46.3, 47.4, 71.8, 73.0, 73.6, 74.4, 76.7, 100.4, 170.4, 203.6, 218.5; HRMS ESI+ (m/z): 412.2119 (Predicted [M+Na]+ for C19H35NO5S is 412.2134).
Supplementary Material
ACKNOWLEDGMENT
This research was supported by NIH grant GM076477 and the Hans W. Vahlteich Professorship (to D.H.S.).
REFERENCES
- 1.Staunton J, Weissman KJ. Nat. Prod. Rep. 2001;18:380–416. doi: 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]
- 2.Donadio S, McAlpine JB, Sheldon PJ, Jackson M, Katz L. Proc. Natl. Acad. Sci. USA. 1993;90:7119–23. doi: 10.1073/pnas.90.15.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDaniel R, Kao CM, Fu H, Hevezi P, Gustafsson C, Betlach M, Ashley G, Cane DE, Khosla C. J. Am. Chem. Soc. 1997;119:4309–4310. [Google Scholar]
- 4.Tang L, Fu H, McDaniel R. Chem. Biol. 2000;7:77–84. doi: 10.1016/s1074-5521(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 5.Yoon YJ, Beck BJ, Kim BS, Kang HY, Reynolds KA, Sherman DH. Chem. Biol. 2002;9:203–14. doi: 10.1016/s1074-5521(02)00095-9. [DOI] [PubMed] [Google Scholar]
- 6.Menzella HG, Reid R, Carney JR, Chandran SS, Reisinger SJ, Patel KG, Hopwood DA, Santi DV. Nat. Biotechnol. 2005;23:1171–6. doi: 10.1038/nbt1128. [DOI] [PubMed] [Google Scholar]
- 7.McDaniel R, Thamchaipenet A, Gustafsson C, Fu H, Betlach M, Betlach M, Ashley G. Proc. Natl. Acad. Sci. USA. 1999;96:5890–5890. doi: 10.1073/pnas.96.5.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes J, Haydock SF, Roberts GA, Bevitt DJ, Leadlay PF. Nature. 1990;348:176–8. doi: 10.1038/348176a0. [DOI] [PubMed] [Google Scholar]
- 9.Donadio S, Staver MJ, McAlpine JB, Swanson SJ, Katz L. Science. 1991;252:675–9. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- 10.Xue Y, Zhao L, Liu HW, Sherman DH. Proc. Natl. Acad. Sci. USA. 1998;95:12111–6. doi: 10.1073/pnas.95.21.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gokhale RS, Tsuji SY, Cane DE, Khosla C. Science. 1999;284:482–5. doi: 10.1126/science.284.5413.482. [DOI] [PubMed] [Google Scholar]
- 12.Wu N, Kudo F, Cane DE, Khosla C. J. Am. Chem. Soc. 2000;122:4847–4852. [Google Scholar]
- 13.Wu N, Tsuji SY, Cane DE, Khosla C. J. Am. Chem. Soc. 2001;123:6465–74. doi: 10.1021/ja010219t. [DOI] [PubMed] [Google Scholar]
- 14.Beck BJ, Aldrich CC, Fecik RA, Reynolds KA, Sherman DH. J. Am. Chem. Soc. 2003;125:12551–12557. doi: 10.1021/ja034841s. [DOI] [PubMed] [Google Scholar]
- 15.Yin Y, Lu H, Khosla C, Cane DE. J. Am. Chem. Soc. 2003;125:5671–6. doi: 10.1021/ja034574q. [DOI] [PubMed] [Google Scholar]
- 16.Aldrich CC, Beck BJ, Fecik RA, Sherman DH. J. Am. Chem. Soc. 2005;127:8441–8452. doi: 10.1021/ja042592h. [DOI] [PubMed] [Google Scholar]
- 17.Masamune S, Ellingboe JW, Choy W. J. Am. Chem. Soc. 1982;104:5526–5528. [Google Scholar]
- 18.Martin SF, Lee WC, Pacofsky GJ, Gist RP, Mulhern TA. J. Am. Chem. Soc. 1994;116:4674–4688. [Google Scholar]
- 19.Myers AG, Yang BH, Chen H, McKinstry L, Kopecky DJ, Gleason JL. J. Am. Chem. Soc. 1997;119:6496–6511. [Google Scholar]
- 20.Amide 5 was isolated as a single diastereomer. The 2,4-syn diastereomer could be confirmed by reductive removal of the pseudoephedrine auxiliary (see ref. 19) and TBS deprotection to give the meso 1,5-diol.
- 21.Carlsen PHJ, Katsuki T, Martin VS, Sharpless KB. J. Org. Chem. 1981;46:3936–3938. [Google Scholar]
- 22.Crimmins MT, King BW, Tabet EA, Chaudhary K. J. Org. Chem. 2001;66:894–902. doi: 10.1021/jo001387r. [DOI] [PubMed] [Google Scholar]
- 23.Pilli RA, de Andrade CKZ, Souto CRO, de Meijere A. J. Org. Chem. 1998;63:7811–7819. [Google Scholar]
- 24.The anti-Felkin syn diastereomer could be confirmed by conversion and correlation to a known lactone derivative: Crimmins MT, Slade DJ. Org. Lett. 2006;8:2191–2194. doi: 10.1021/ol0607241.
- 25.Kao CM, Luo GL, Katz L, Cane DE, Khosla C. J. Am. Chem. Soc. 1995;117:9105–9106. [Google Scholar]
- 26.Aldrich CC, Venkatraman L, Sherman DH, Fecik RA. J. Am. Chem. Soc. 2005;127:8910–8911. doi: 10.1021/ja0504340. [DOI] [PubMed] [Google Scholar]
- 27.Buchholz TJ, Geders TW, Bartley FE, 3rd, Reynolds KA, Smith JL, Sherman DH. ACS Chem. Biol. 2009;4:41–52. doi: 10.1021/cb8002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue Y, Sherman DH. Nature. 2000;403:571–5. doi: 10.1038/35000624. [DOI] [PubMed] [Google Scholar]
- 29.Kittendorf JD, Beck BJ, Buchholz TJ, Seufert W, Sherman DH. Chem. Biol. 2007;14:944–54. doi: 10.1016/j.chembiol.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Pik pentaketide was synthesized as previously described. See reference 16.
- 31.The Pik hexaketide was synthesized from degradation of 10-DML as previously described. See reference 16.
- 32.Tsai SC, Miercke LJW, Krucinski J, Gokhale R, Chen JCH, Foster PG, Cane DE, Khosla C, Stroud RM. Proc. Natl. Acad. Sci. USA. 2001;98:14808–14813. doi: 10.1073/pnas.011399198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akey DL, Kittendorf JD, Giraldes JW, Fecik RA, Sherman DH, Smith JL. Nat. Chem. Biol. 2006;2:537–542. doi: 10.1038/nchembio824. [DOI] [PubMed] [Google Scholar]
- 34.Jacobsen JR, Hutchinson CR, Cane DE, Khosla C. Science. 1997;277:367–369. doi: 10.1126/science.277.5324.367. [DOI] [PubMed] [Google Scholar]
- 35.Prepared as previously described: Trost BM, Frederiksen MU, Papillon JPN, Harrington PE, Shin S, Shireman BT. J. Am. Chem. Soc. 2005;127:3666–3667. doi: 10.1021/ja042435i.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.