Abstract
Behavioral analyses are a natural choice for understanding the wide-ranging behavioral consequences of racial stereotyping and prejudice. However, neuroimaging and electrophysiological research has recently considered the neural mechanisms that underlie racial categorization and the activation and application of racial stereotypes and prejudice, revealing exciting new insights. Work reviewed here points to the importance of neural structures previously associated with face processing, semantic knowledge activation, evaluation, and self-regulatory behavioral control, allowing for the specification of a neural model of race processing. We show how research on the neural correlates of race can serve to link otherwise disparate lines of evidence on the neural underpinnings of a broad array of social-cognitive phenomena, and consider implications for effecting change in race relations.
New Frontiers in the Study of Race and Social Cognition
Brain imaging and electrophysiological methods have emerged as important new tools for scholars of race. In particular, research using functional brain imaging (e.g., fMRI) and electrocortical responses (EEG/ERPs) is providing unparalleled access to how race is processed in the brain, as well as new insights on how race influences perceptions and behaviors (see Box 1 for the longer history of studying race relations with physiological measures). Several recent models explicate neural structures involved in particular aspects of social cognition, such as judging mental states [1,2], perceiving faces [3], and activating attitudes [4]. Although such models are critical for understanding specific social-cognitive constructs, it is important to recognize that in vivo social perception draws on multiple, overlapping social cognitive processes. Race perception permits examination of how these and related systems interact to inform judgments and behaviors. Here we review representative recent research investigating the neural systems associated with race processing. On the basis of this work, we sketch an initial model of the neural correlates of race that can serve as a basis for future research aimed at understanding the interacting systems involved in race processing and its downstream cognitive, affective and behavioral consequences.
Box 1. A Brief History of Psychophysiological Responses to Race.
While contemporary social neuroscience research has emphasized neural mechanisms of racial perception, the roots of this more recent work can be traced to numerous early studies that employed peripheral physiological measures. At the time of these initial investigations, researchers were motivated to understand whether post-World War II changes in cultural attitudes that made Caucasian research participants increasingly reluctant to verbally express racial prejudice reflected a true lack of racial antipathy. Physiological measures were used in this endeavor as a way to more covertly assess reactions to race.
In one of the first known efforts of this kind, Rankin and Campbell [49] measured electrodermal responses (i.e., skin conductance) from Caucasian participants as they interacted with either a Caucasian or a Black experimenter. They found significantly larger responses to the Black than to the Caucasian experimenter, suggesting heightened anxiety responses–attributed to more negative attitudes–to Blacks (see also [50]). Others reported similar findings among Caucasian participants for anticipated contact with Blacks [51], as well as for simple visual depictions of Black targets [52,53].
In addition to electrodermal responses, researchers have used electromyographic (EMG) measures to covertly assess reactions to race. EMG provides a sensitive measure of even very small movements of facial muscles associated with negative (i.e., frowning) and positive (i.e., smiling) reactions to stimuli (see [54]), including targets varying by race. In a seminal study of this kind, Vanman, Paul, Ito, and Miller [55] recorded EMG as Caucasian participants imagined cooperative work experiences with Black and Caucasian partners. EMG responses indicated negative covert reactions to Black targets (i.e., heightened activation of the corrugator supercilii muscles, associated with expression of negative affect) despite very positive overt ratings of the Black targets (see also [56]).
Studies using peripheral physiological measures were instrumental in highlighting the relevance of bodily reactions to understanding complex psychological processes (for an in-depth review, see [57]), and thereby laid the foundation for the more recent investigations reviewed in the main text. Contemporary researchers in the burgeoning field of social neuroscience owe a considerable debt to these early pioneers and the knowledge their work produced. Moreover, research in this tradition remains active, as scientists continue to make important theoretical advances using a broad range of psychophysiological measures, including cardiovascular and neuroendocrine responses (e.g. [58,59]).
Race Perception, Categorization, and the Putative Face Processing Network
Racial categorization can occur based on facial features, which means that race perception often begins with the perception of a face. Consequently, this review begins by considering research on the neural structures supporting face processing. Although work on face perception often focused on mechanisms that differentiate faces from non-faces, and on how we retrieve personal identity [e.g., 3], growing research shows that even very basic aspects of face perception are affected by race, and that sensitivity to race occurs in a very fast and seemingly automatic fashion.
Fusiform gyrus and posterior cingulate cortex (PCC)
Race effects have been observed in two brain areas traditionally associated with face perception, the lateral fusiform gyrus and PCC, with greater activity to racial ingroup than outgroup members in both areas [5–8] (but see Box 2 for more equivocal results from electrocortical studies of fusiform responses). Lateral fusiform activity has been linked to encoding of the visual appearance of the face. Modulation of this process by race could reflect greater experience with racial ingroup members, as other research shows an increase in fusiform activity with expertise [9]. Motivation may also play a role, as suggested by a study in which Caucasian participants were told they had been randomly assigned to one of two competing, racially-diverse teams [10]. Participants first learned to recognize members of both teams, then viewed pictures of team members’ faces. Activity in bilateral fusiform gyrus was sensitive to team designation, showing greater activity to own than competing team members regardless of their race. Thus, the motivational significance conferred by status as a fellow team member modulated recruitment of face processing mechanisms; suggesting that prior race effects could reflect inherent motivations to more deeply attend to ingroup members. Regardless of the cause, race effects on fusiform activity have downstream consequences; greater activity in left fusiform to ingroup than outgroup faces also correlates with an ingroup memory advantage [5].
Box 2. Race Effects in the N170 Electrocortical Index of Face Processing.
The N170 ERP component is a negative-going deflection maximal over lateral temporal areas that is larger to faces than non-faces [60]. Its sensitivity to race has been equivocal, with support obtained for three mutually exclusive hypotheses. First, the N170 has been argued to reflect structural face encoding, sensitive only to global features that differentiate faces from non-faces, but not to features of individual faces [60,61]. This perspective implies that race should not modulate N170 responses, a pattern obtained in several studies [62–64].
Other research indicates that N170 amplitude is also increased to non-face stimuli with which participants are expert [65]. This suggests the N170 reflects a more general expertise mechanism sensitive to faces, about which humans are normally expert, and to other stimuli about which individuals are idiosyncratically expert. This perspective predicts that N170 amplitude should be greatest to racial ingroup members because perceivers typically have more experience interacting with ingroup members. This also converges with source localization data implicating the fusiform gyrus in the generation of the N170 [66], and the greater fusiform activity to racial ingroup faces observed in fMRI studies [5,6]. To date, though, only one study has obtained this N170 pattern [14].
Finally, N170 amplitude is increased by manipulation that disrupts the configural processing typically applied to faces [67]. Racial outgroup faces are often less familiar, and may be processed in a less configural manner [68]. Consequently, the prediction can be derived that N170s should be larger to racial outgroup faces, a pattern obtained in several studies [69–71].
These conflicting findings suggest that features other than simple physical/structural differences influence N170 responses to faces. One possibility is that motivational factors that make race more or less salient to perceivers modulate this neural response. Consistent with this view, all studies producing larger N170s to racial outgroups have made identity salient (e.g., by having participants detect when two consecutively presented faces match or trying to remember the faces) whereas none of the studies obtaining other patterns have. Given assumptions that the N170 reflects structural face encoding, and that perceivers typically process racial outgroup faces in a less configural shallower manner (e.g., as reflected in poorer memory for outgroup faces), tasks that require attention to identity may selectively increase recruitment of face processing mechanisms to racial outgroup members. However, further research is needed to clarify these seemingly contradictory patterns of N170 to racial outgroup and ingroup targets.
PCC activity generally is enhanced during retrieval of information about familiar versus unfamiliar individuals [3]. However, enhanced PCC activity to ingroup faces has been obtained with faces that are unknown to the participants, suggesting that a more general sense of group-based familiarity also affects PCC activity. In sum, work examining race effects on face processing both informs understanding of race categorization and suggests that motivational factors (e.g., those relevant to ingroup-outgroup distinctions) importantly influence recruitment of face processing mechanisms, thereby enhancing understanding of this fundamental component of social cognition.
Electrocortical responses to race
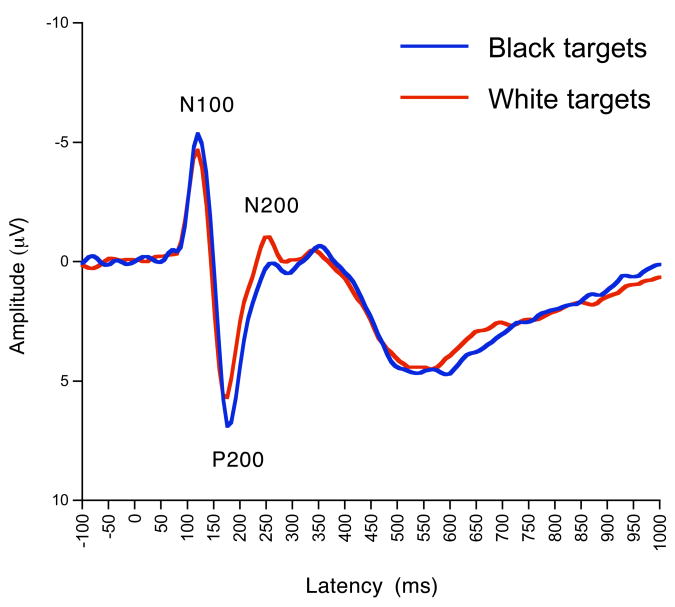
While fMRI has revealed neural structures affected by race during face processing, studies using event-related brain potentials (ERPs) have elucidated mechanistic aspects of race perception and categorization, such as its timecourse and malleability. In an initial investigation, participants viewed pictures of Black and Caucasian individuals (targets) while ERPs were recorded [11]. Modulations as a function of target race occurred as early as the N100 ERP component, peaking 122 ms after face onset. Race effects also were observed in the subsequent P200, N200, and P300 components (see Figure 1). Numerous subsequent investigations have replicated these findings [12–18] (see Table 1). These components are generally sensitive to attentional and categorization processes [19], suggesting that the race effects reflect automatic encoding of and orienting toward racial category information. Importantly, sensitivity to race occurs whether participants are explicitly attending to race, attending to another social dimension (gender), or making person-based, individuating judgments [14], indicating that attention to race is fairly obligatory.
Figure 1.
Grand average ERP waveforms over central midline areas showing racial sensitivity in the N100 (mean peak latency of 122 ms), P200 (mean peak latency of 176 ms), and N200 (mean peak latency of 256 ms) components. As shown here, Caucasian participants typically show larger N100s and P200s to Blacks, but larger N200s to Caucasians. This pattern is reversed for Black participants [12]. Reproduced with permission from [11].
Table 1.
ERP Components Sensitive to Racial Ingroup/Outgroup Status
| ERP Component | Typical Effect | Typical Mean Peak Latency | Studies Showing Effect | Functional Significance |
|---|---|---|---|---|
| N100 | Larger to racial outgroup members | 120 ms |
Dickter & Bartholow (2007) Ito & Kubota (2007) Ito & Urland (2003) Ito & Urland (2005) |
N100 and P200 amplitude generally reflects attentional deployment. In the context of race, the effects suggest early orientation to more novel targets. Effects could be thought of as a form of coarse, rapidly occurring vigilance |
| P200 | Larger to racial outgroup members | 180 ms |
Dickter & Bartholow (2007) Ito & Kubota (2007) Ito & Urland (2003) Ito & Urland (2005) Willadsen-Jensen & Ito (2006) Willadsen-Jensen & Ito (2006) |
|
| N200 | Larger to racial ingroup members | 250 ms |
Dickter & Bartholow (2007) Dickter & Bartholow (in press) Ito & Kubota (2007) Ito, Thompson, & Cacioppo (2007) Ito & Urland (2003) Ito & Urland (2005) James, Johnstone, & Hayward (2001) Willadsen-Jensen & Ito (2006) Willadsen-Jensen & Ito (2008) |
N200 amplitude is also sensitive to attentional deployment. In studies examining person perception, N200s are larger to more familiar individuals (Bentin & Deouell, 2000; Tanaka, Curran, Porter, Weld, & Collins, 2006). Race effects showing orientation of greater attention to racial ingroup members in this component have been interpreted as reflecting the spontaneous direction of deeper levels of attention to more familiar (ingroup) individuals (following initially greater attention to outgroup members in the N100 and P200). The anterior scalp distribution of the N200 is broadly consistent with the role of the MPFC in making mental state inferences. |
| P300 | Larger to targets whose race differs from preceding individuals | 540 ms |
Dickter & Bartholow (2007) Ito, Thompson, & Cacioppo (2007) Ito & Urland (2003) Ito & Urland (2005) Willadsen-Jensen & Ito (2006) Willadsen-Jensen & Ito (2008) |
The P300 has been associated with a broadly-distributed network involving the locus-coeruleus norepinephrine system that responds to motivationally-significant events. Increased P300 amplitude to individuals who differ in race from preceding individuals has been interpreted as reflecting contextual updates along inherently motivationally-relevant dimensions. |
Just as face processing areas appear sensitive to ingroup-outgroup distinctions, effects of race on ERP components reflect distinctions between ingroup and outgroup members. Dickter and Bartholow [12] recorded ERPs as Black and Caucasian perceivers viewed pictures of Black and Caucasian targets in a gender categorization task. Results for Caucasian perceivers replicated previous work (e.g. [11,14,15]), showing larger P200 amplitude to Black than to Caucasian targets and larger N200 amplitude to Caucasian than to Black targets. However, the pattern was reversed among Black perceivers, with larger P200s to Caucasian targets and larger N200s to Black targets. Willadsen-Jensen and Ito [17] reported similar findings with Asian participants. These results, coupled with the fMRI face processing data, suggest that neural differentiation of race operates at the level of broader social distinctions (ingroup vs. outgroup).
Of importance, neural signals reflecting early, spontaneous racial distinctions are related to subsequent race-biased responding. In one study, participants viewed pictures of Caucasian and Black men holding small objects, and had to quickly indicate via key press whether the object was a gun or something innocuous (e.g., cell phone) [20]. Participants whose P200 and N200 ERP responses indicated greater differentiation between Black and Caucasian targets showed more pronounced behavioral bias (i.e., faster responses to armed Blacks than armed Caucasians). Moreover, ERP effects mediated the relation between self-reported endorsement of stereotypes linking Blacks with threat and behavioral expression of racial bias. Thus, the ERP-behavior correlation in this study suggests a link between electrocortical sensitivity to race and application of activated racial stereotypes.
Race Perception, Stereotype Activation, and Behavior
Countless studies have shown that categorizing another’s race (whether intentionally or not) spontaneously activates beliefs linked to their racial category (e.g. [21,22]). These beliefs (i.e., stereotypes) then influence reactions toward and judgments about the individual. To date, few studies have sought to identify brain areas associated with racial stereotyping (see Box 3) but ERPs have been used to investigate rapidly-unfolding neural responses to stereotype activation and violation. Bartholow and colleagues [23] recorded ERPs during a face priming task known to elicit stereotype activation. The P300 component was sensitive to stereotype violations, with counter-stereotypic associations eliciting larger amplitude and longer latency than stereotype-consistent associations. The P300 has been linked to activity in the broadly-distributed locus coeruleus-norepinephrine system, which responds to motivationally-significant events [24]. Stereotype violations challenge existing semantic knowledge and thus are hypothesized to engage this system, reflecting either updating of existing content or a motivated attempt to resolve the inconsistent information.
Box 3. Neural Structures Relevant for Understanding Stereotype Activation.
Although few studies to date have directly investigated the neural underpinnings of racial stereotype activation, consideration of work in related domains suggests brain areas that may be involve in racial stereotyping. Stereotypes can be considered analogous to other constructs stored in semantic memory, and therefore research investigating the neural structures supporting semantic memory is relevant to consider. Several studies have pointed to areas of the medial temporal lobes as important for semantic memory retrieval [47]. Additionally, considerable work implicates areas within PFC as important for both semantic retrieval and for cognitive control, and suggests that semantic knowledge is an important moderator of the extent to which control is implemented [see 48]. As reviewed elsewhere in this article, control-related resources marshaled in PFC are important for regulation of stereotype-based responding. Thus, this work underscores the functional dependence between knowledge of stereotypes and the ability to control responses based on them.
This notion provides a context for interpreting the results of another recent study. investigating the neural correlates of gender stereotype activation and application [72]. Findings indicated an extensive area of right PFC that distinguished trials in which stereotypes were applied from those in which they were not applied. Moreover, activation in this region -- and only this region -- correlated with a behavioral measure of implicit gender stereotyping. These results suggest that stereotype application relies on cognitive processes generally underlying semantic knowledge about categories.
Finally, although not directly associated with stereotype activation, recent work implicates specific anterior temporal lobe regions and connections with MPFC in conceptual knowledge about people. A recent review [73] indicated that the superior temporal sulcus stores semantic representations of functional knowledge about people. Similarly, Mitchell and colleagues [74] reported that person knowledge (as opposed to object knowledge) was associated with activity in superior temporal cortex and MPFC.
Determining whether racial stereotyping is subserved by the same structures identified in these other lines of work awaits future research. However, given the structural similarity between stereotypes and semantic knowledge in general, the conceptual similarity between racial and gender stereotypes, and the consistency with which aspects of social judgment activate these regions, it is reasonable to predict that the superior temporal lobes and MPFC are strong candidates for structures involved in activation and implementation of racial stereotypes.
Subsequent research investigated spontaneous stereotype activation in a task that required no explicit trait inferences [25]. Participants categorized the race of centrally-presented Black and Caucasian faces (targets) flanked on 4 sides by trait words that were either stereotype-consistent (e.g., a Black face with “violent”) or stereotype-inconsistent (e.g., a Black face with “safe”) with respect to the target’s race. Strictly speaking, the flanker words were task-irrelevant and required no attention. However, categorization responses were slower to faces flanked by stereotype-inconsistent words, indicating that participants implicitly activated racial stereotypes associated with the faces and were affected by their congruence with the covertly-attended flankers. Moreover, the amplitude of an ERP component associated with response conflict was enhanced on stereotype-inconsistent trials, indicating that the presence of stereotype-inconsistent information enhanced response conflict during racial categorization. Consistent with this idea, the lateralized readiness potential showed evidence of competing response activations in motor cortex on inconsistent (but not consistent) trials. This work underscores that stereotypic beliefs influence even simple, perceptual judgments about race, and that the extent to which stereotypes influence these perceptual judgments can be determined by whether neural activation of motor responses is controlled. In the next section, we highlight the importance of conflict and control processes in regulating race-based responding.
Race Perception, Evaluation, and Regulation of Intergroup Behavior
In addition to stereotypic beliefs, negative feelings about the group (i.e., prejudice) are often activated following racial categorization. Moreover, these spontaneous evaluations have important implications for behavioral responses. From this perspective, it makes sense to look for links between the neural circuits important for evaluation and those involved in regulation of intergroup behavior. In fact, considerable evidence points to overlap between these systems, particularly involving the amygdala, anterior cingulate cortex (ACC), and dorsolateral and ventrolateral prefrontal cortex (DLPFC & VLPFC) [4].
Amygdala
Consistent with amygdala involvement in the arousal of negative affect [26], numerous studies find greater amygdala activity elicited by racial outgroup than ingroup members [27,6,28–31]. Moreover, indirectly-assessed race bias responses indicating negativity toward Blacks correlate with enhanced amygdala activity to Black than Caucasian faces [32,33]. These effects were initially interpreted as reflecting the implicit activation of racial outgroup bias.
This pattern typically has occurred when participants are engaged in perceptual encoding of faces or making social category judgments. Such conditions maintain the natural salience of race, leading to the implicit activation of prejudice. However, this activation can be attenuated by factors that direct attention away from race or engage the perceiver’s motivation to control bias. Similarly, the amygdala responds flexibly as a function of current goals [34]. Consistent with these findings, amygdala activation to racial group membership is modulated by processing goals. In one study, the tendency toward greater amygdala activity to racial outgroup than ingroup faces was eliminated when participants made either nonsocial judgments or individuation judgments about the individuals [27].
Features of the target individuals also can moderate amygdala activity to race. One study found greater amygdala activity to Black than Caucasian faces among Caucasian participants when the targets were gazing at the perceivers, but not when the targets’ eyes were averted or closed [30]. The authors argued that averted or closed eyes signal a low potential for threat, and so attenuate racial differences in amygdala activity. Also, greater amygdala activity to racial ingroup than outgroup faces has been reported, but only with faces displaying fearful expressions [35]. Together, the results indicate that while amygdala activity is sensitive to more than just outgroup antipathy, it is clearly responsive to evaluative reactions based on race, with the specific nature of those reactions sensitive to a range of contextual features.
ACC and PFC
People often attempt to control or override negative race-based reactions, and several contemporary models of race bias emphasize the role of conflict between responses driven by automatic negative evaluations and those reflecting egalitarian goals in determining the extent of bias expressed toward outgroup members [e.g., 36]. Electrocortical and fMRI research on cognitive control has identified a network involving the ACC (conflict monitoring) and PFC (regulative control) as important for regulating responses under conditions of conflict [37,38]. Findings reviewed in this section converge in suggesting that the same mechanisms are engaged to override racially-biased responses.
In a recent study of Caucasian participants reporting strong motivation to control prejudice [32], greater amygdala activity was elicited by Black than Caucasian faces only when faces were shown too briefly to be consciously detected (for 30 ms) [see also 28]. By contrast, no racial difference in amygdala activity was observed when faces were shown for 525 ms. Instead, Black faces presented for this longer duration elicited greater activity in the ACC and right VLPFC and DLPFC (see Figure 2), presumably reflecting enhanced control over implicit negative evaluations. Other studies similarly have found greater ACC and DLPFC activity when participants need to override responses based on negative associations with racial outgroup members [39,40].
Figure 2.
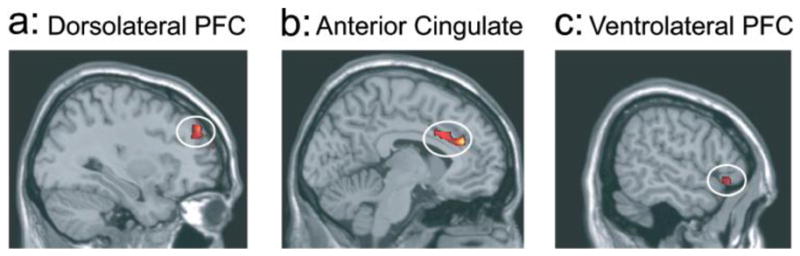
Areas in prefrontal cortex that have been associated with cognitive control are more active when Caucasian participants view Black as compared to Caucasian faces under conditions that allow for more reflective processing. Specifically, areas in the dorsolateral prefrontal cortex (panel A), the anterior cingulate cortex (panel B), and ventrolateral prefrontal cortex (panel C) are more active to Black than Caucasian faces presented above conscious threshold (for 525 ms). This differential activation is not observed when faces are shown below conscious awareness (for 30 ms) which would presumably eliminate the ability to engage more controlled processes. Reproduced with permission from [32].
ACC involvement in the control of racially-stereotypic responding is further supported by research using the error-related negativity (ERN), a response-related ERP component generated in the ACC and thought to reflect conflict [38] and/or distress related to error commission [41]. In several studies, mistakenly categorizing a tool as a gun following a Black face prime elicits larger ERNs than similar errors following Caucasian face primes, particularly among participants highly motivated to control prejudice toward Blacks, underscoring that motivational factors importantly determine how race influences the activation of neural systems for behavioral control [42–44]. Moreover, the amplitude of the race-bias ERN significantly predicts estimates of behavioral control in the task, supporting a role for conflict detection in the control of race-biased responses.
Related work has focused on behavioral control following stereotype activation [23]. Participants completed a go/stop version of a racial priming task in which they had to withhold stereotype-consistent or -inconsistent responses on some trials. The amplitude of the negative slow wave, a frontally-prominent ERP component reflecting implementation of cognitive control, was larger for successfully inhibited stereotype-consistent responses, indicating that more regulatory control was needed to withhold race-biased responses.
A Proposed Model of Race Processing and its Implications for Social Cognition
The research summarized in this review underscores the importance of a number of neural structures for the processing of race and the regulation of race-related responding. There are several points of convergence between the areas identified here and those featured in recent models of the neural foundations of a number of social-cognitive phenomena [1,3,4]. Thus, the study of race perception is intriguing because it underscores the extent to which the neural processes identified in these other models interact, highlighting that theoretically separable aspects of social cognition (e.g., face perception, evaluation, semantic person knowledge) come together in service of processing this higher-order social construct. Figure 3 presents a model of the distributed brain areas identified as important for race perception. Also included are areas associated with other aspects of social cognition but as yet not reported to be sensitive to race, to indicate potential directions for future research.
Figure 3.
A preliminary model of the distributed brain areas involved in race perception. Extant research indicates that racial category membership modulates responses in areas previously associated with face perception (labeled “Face Perception”), inferences about the storage and retrieval of person knowledge (“Person Knowledge”), arousal of affect and evaluation (“Evaluation”), and regulation of behavioral responses (“Behavior Regulation”). This model also includes brain areas for which sensitivity to race has not been widely examined, but are expected based on the involvement of these areas with related aspects of social cognition. These areas are denoted with italics.
Race perception begins with categorization, often based on physical characteristics of faces. ERP research shows that extracting race information begins as early as one-tenth of a second following initial perception of faces, and fMRI and ERP source localization data indicate that neural regions specialized for face processing are sensitive to racial group distinctions. These face processing differences are modulated by motivational factors distinguishing ingroup from outgroup.
Following the quick processing of racial information, networks associated with three interacting processes may be activated. The first involves affective evaluation. To date, differential responses to racial ingroup and outgroup members have been obtained primarily in the amygdala. However, areas such as the orbitofrontal cortex and insula are also involved in evaluative processing [cf 4] and in some cases have shown sensitivity to race [39,45]. Because interracial interactions are so imbued with evaluation–including the retrieval of group-based evaluations and valenced-stereotypical beliefs -- it is proposed that activity in a widely distributed network of brain areas subsuming evaluative processing is important for race perception.
Figure 3 also contains a network of areas supporting processes broadly associated with the storage and retrieval of person knowledge. Of these areas, to date only the PCC has been shown to differentiate as a function of race, but there are strong reasons to expect other areas will as well. The medial prefrontal cortex (MPFC), for instance, repeatedly has been implicated in making inferences about others’ psychological states [1,2]. Although we know of no research directly testing race effects on MPFC activity, MPFC activity increases when making inferences about the mental states of similar compared to dissimilar others [46], suggesting that MPFC activity may increase to racial ingroup relative to outgroup members. Given the consistently larger medial-frontal responses to ingroup versus outgroup members in ERP studies, it could be that these findings reflect a similar phenomenon; future research could examine whether this ERP activity is generated by the MPFC. In addition, superior and medial temporal lobe structures consistently have been associated with semantic knowledge representations about people [47], which form the foundation of stereotypes.
Finally, activation of stereotypical beliefs and evaluations concerning race engage brain systems involved in behavioral regulation, especially the ACC, DLPFC, and VLPFC, to control race-biased responses. Although these areas are distinguished from those associated with evaluation and knowledge activation in Figure 3, they likely operate in conjunction. Indeed, evidence indicates that the ACC serves an evaluative function [38,41] and that areas implicated in semantic person knowledge closely interact with those supporting cognitive control [see 48].
The results reviewed here support both a bottom-up sensitivity to race cues as well as top-down modulation of other social cognitive processes by race. The former is most clearly illustrated by the quickly occurring and so far unmalleable sensitivity to race seen in ERP responses [14]. The latter can be seen in modulation of amygdala sensitivity to fearful faces depending on target race [35]. This demonstrates that race both carries meaning on its own, but can also serve as a contextual influence that moderates ongoing processes in other contexts.
This model also has several implications for considering changes in race relations. ERP research indicating that racial category information is processed in a fairly obligatory manner implies that attempts to get people to not “see” race will be relatively ineffective. Moreover, the model suggests that change occurring at the single level of stereotypical or evaluative associations is unlikely to eliminate racially-biased behavior because biased responses could still occur through processes mediated by other parts of the neural network. However, while the model generally supports the benefit of improving race relations through strategies that target both semantic and evaluative associations, to the degree that behavior regulation have modulatory effects on other processes, interventions that seek to improve behavior regulation capabilities may be effective in at least reducing the expression of bias. It also suggests that while race relations will be affected by race-specific beliefs and feelings, the expression of bias will also be determined by an individual’s general regulatory abilities.
In sum, race is a multifaceted social variable, through which processes such as categorization, knowledge activation, and motivation interact in complex yet subtle ways. This review highlights progress made in understanding the neural basis of race perception. Although this research is still relatively new, there are sufficient converging findings to support the model in Figure 3, providing a foundation from which future research on this important construct can be launched (see Box 4 for outstanding research questions).
Box 4. Outstanding Questions.
Does race influence activity in other brain areas associated with social cognition that as yet have not been widely investigated in the context of race perception, such as the MPFC?
What are the brain mechanisms associated with the storage and retrieval of racial stereotypes? Researchers have examined mechanisms of stereotype activation and regulation of stereotypic responses, as well as structures involved in semantic memory retrieval, but neural structures and/or networks subserving the activation and application of racial stereotypes specifically have yet to be identified.
What are the psychological mechanisms that produce the race effects reviewed here? Some studies [10,75] support a role for motivational factors in driving differential neural responses to ingroup targets, but differences could also derive from the content of group stereotypes (e.g., that members of a given group are threatening) or from differential familiarity with members of ingroups and outgroups.
Most (although not all) studies of race perception have tested the reactions of Caucasian perceivers to Caucasian and Black targets. Only a limited number of studies have investigated reactions from more diverse groups of perceivers to targets representing different racial groups. Consequently, more remains to be learned about how factors such as social and cultural context might influence race perception (for a relevant discussion, see [56]).
Acknowledgments
Preparation of this article was supported by grants from the National Institute of Mental Health (R01MH071257) and the National Institute on Drug Abuse (R01DA024002) to T.A.I., a grant from the National Institute on Alcohol Abuse and Alcoholism (R21AA017282) to B.D.B., and a grant from the National Science Foundation (BCS-0847872) to T.A.I. and B.D.B. We thank Lindsey Newnes for her assistance in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tiffany A. Ito, University of Colorado, Department of Psychology and Neuroscience, 345 UCB, Boulder, CO 80309-0345
Bruce D. Bartholow, Department of Psychological Sciences, 10 McAlester Hall, University of Missouri, Columbia, MO 65211
References
- 1.Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JP. Social psychology as a natural kind. Trends Cogn Sci. 2009;13:246–251. doi: 10.1016/j.tics.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham WA, Zelazo PD. Attitudes and evaluations: A social cognitive neuroscience perspective. Trends Cogn Sci. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Golby AJ, et al. Differential responses in the fusiform region to same-race and other-race faces. Nat Neurosci. 2001;4:845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman MD, et al. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nat Neurosci. 2005;8:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- 7.Lee KU, et al. Distinct processing of facial emotion of own-race versus other-race. Neuroreport. 2008;19:1021–1024. doi: 10.1097/WNR.0b013e3283052df2. [DOI] [PubMed] [Google Scholar]
- 8.Iidaka T, et al. Neural correlates involved in processing happy affect on same race faces. J Psychophysiol. 2008;22:91–99. [Google Scholar]
- 9.Gauthier I, et al. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- 10.Van Bavel JJ, et al. The neural substrates of in-group bias: A functional magnetic resonance imaging investigation. Psychol Sci. 2008;11:1131–1139. doi: 10.1111/j.1467-9280.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 11.Ito TA, Urland GR. Race and gender on the brain: Electrocortical measures of attention to race and gender of multiply categorizable individuals. J Pers Soc Psychol. 2003;85:616–626. doi: 10.1037/0022-3514.85.4.616. [DOI] [PubMed] [Google Scholar]
- 12.Dickter CL, Bartholow BD. Event-related brain potential evidence of ingroup and outgroup attention biases. Soc Cogn Affect Neurosci. 2007;2:189–198. doi: 10.1093/scan/nsm012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito TA, et al. Tracking the timecourse of social perception: The effects of racial cues on event-related brain potentials. Pers Soc Psychol Bull. 2004;30:1267–1280. doi: 10.1177/0146167204264335. [DOI] [PubMed] [Google Scholar]
- 14.Ito TA, Urland GR. The influence of processing objectives on the perception of faces: An ERP study of race and gender perception. Cogn Affect Behav Neurosci. 2005;5:21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- 15.Kubota JT, Ito TA. Multiple cues in social perception: The time course of processing race and facial expression. J Exp Soc Psychol. 2007;43:738–752. doi: 10.1016/j.jesp.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willadsen-Jensen EC, Ito TA. Ambiguity and the time course of racial perception. Soc Cognit. 2006;24:580–606. [Google Scholar]
- 17.Willadsen-Jensen EC, Ito TA. A foot in both worlds: Asian Americans’ perceptions of Asian, White, and racially ambiguous faces. Group Processes and Interpersonal Relations. 2008;11:182–200. [Google Scholar]
- 18.James MS, et al. Event-related potentials, configural encoding, and feature-based encoding in face recognition. J Psychophysiol. 2001;15:275–285. [Google Scholar]
- 19.Fabiani M, et al. Event-related brain potentials: Methods, theory, and applications. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. Cambridge University Press; 2007. pp. 85–119. [Google Scholar]
- 20.Correll J, et al. Event-related potentials and the decision to shoot: The role of threat perception and cognitive control. J Exp Soc Psychol. 2006;42:120–12. [Google Scholar]
- 21.Brewer MC. A dual process model of impression formation. In: Wyer R, Scrull T, editors. Advances in Social Cognition. 1. Erlbaum; 1988. pp. 1–36. [Google Scholar]
- 22.Fiske ST, Neuberg SL. A continuum of impression formation, from category–based to individuating processes: Influences of information and motivation on attention and interpretation. Adv Exp Soc Psychol. 1990;23:1–73. [Google Scholar]
- 23.Bartholow BD, et al. Stereotype activation and control of race bias: Cognitive control of inhibition and its impairment by alcohol. J Pers Soc Psychol. 2006;90:272–287. doi: 10.1037/0022-3514.90.2.272. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwenhuis S, et al. Dicision making, the P3, and the locus coeruleus—norepinephrine system. Psychol Bull. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- 25.Bartholow BD, Dickter CL. A response conflict account of the effects of stereotypes on racial categorization. Soc Cognit. 2008;26:273–291. [Google Scholar]
- 26.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler ME, Fiske ST. Controlling racial prejudice: Social cognitive goals affect amygdala and stereotype activation. Psychol Sci. 2005;16:56–63. doi: 10.1111/j.0956-7976.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 28.Amodio DM, et al. Individual differences in the activation and control of affective race bias as assessed by startle eyeblink responses and self-report. J Pers Soc Psychol. 2003;84:738–753. doi: 10.1037/0022-3514.84.4.738. [DOI] [PubMed] [Google Scholar]
- 29.Hart AJ, et al. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport. 2000;11:2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- 30.Richeson JA, et al. Eye-gaze direction modulates race-related amygdala activity. Group Process Intergroup Relat. 2008;11:233–246. [Google Scholar]
- 31.Ronquillo J, et al. The effects of skin tone on race-related amygdala activity: An fMRI investigation. Soc Cogn Affect Neurosci. 2007;2:39–44. doi: 10.1093/scan/nsl043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham WA, et al. Separable neural components in the processing of black and white faces. Psychol Sci. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- 33.Phelps EA, et al. Performance on indirect measures of race evaluative predicts amygdala activation. J Cogn Neurosci. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham WA, et al. Affective flexibility: Evaluative processing goals shape amygdale activity. Psychol Sci. 2008;19:152–160. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 35.Chiao JY, et al. Cultural specificity in amygdala response to fear faces. J Cogn Neurosci. 2008;20:2167–2174. doi: 10.1162/jocn.2008.20151. [DOI] [PubMed] [Google Scholar]
- 36.Sherman JW, et al. The self-regulation of automatic associations and begavioral impulses. Psychol Rev. 2008;115:314–335. doi: 10.1037/0033-295X.115.2.314. [DOI] [PubMed] [Google Scholar]
- 37.Kerns J, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 38.Yeung N, et al. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- 39.Beer JS, et al. The Quadruple Process model approach to examining the neural underpinnings of prejudice. NeuroImage. 2008;43:775–783. doi: 10.1016/j.neuroimage.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 40.Knutson KM, et al. Neural correlates of automatic beliefs about gender and race. Hum Brain Mapp. 2007;28:915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hajcak G, Foti D. Errors are aversive: Defensive motivation and the error-related negativity. Psychol Sci. 2008;19:103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- 42.Amodio DM, et al. Neural signals for the detection of unintentional race bias. Psychol Sci. 2004;15:88–93. doi: 10.1111/j.0963-7214.2004.01502003.x. [DOI] [PubMed] [Google Scholar]
- 43.Amodio DM, et al. Alternative mechanisms for regulating racial responses according to internal vs. external cues. Soc Cogn Affect Neurosci. 2006;1:26–36. doi: 10.1093/scan/nsl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amodio DM, et al. Individual differences in the regulation of intergroup bias: The role of conflict monitoring and neural signals for control. J Pers Soc Psychol. 2008;94:60–74. doi: 10.1037/0022-3514.94.1.60. [DOI] [PubMed] [Google Scholar]
- 45.Xu X, et al. Do you feel my pain? Racial group membership modulates empathic neural responses. J Neurosci. 2009;29:8525–8529. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell JP, et al. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 47.Hart J, Jr, Kraut MA. Neural basis of semantic memory. Cambridge University Press; 2007. [Google Scholar]
- 48.Wagner AD, et al. Cognitive control, semantic memory, and priming: Contributions from prefrontal cortex. In: Gazzaniga MS, editor. The cognitive neurosciences. 3. MIT Press; 2004. pp. 709–725. [Google Scholar]
- 49.Rankin RE, Campbell DT. Galvanic skin response to Negro and white experimenters. J Abnorm Soc Psychol. 1955;51:30–33. doi: 10.1037/h0041539. [DOI] [PubMed] [Google Scholar]
- 50.Porier GW, Lott AJ. Galvanic skin responses and prejudice. J Pers Soc Psychol. 1967;5:253–259. doi: 10.1037/h0021205. [DOI] [PubMed] [Google Scholar]
- 51.Vander Kolk CJ. Physiological reactions of Black, Puerto Rican, and White students in suggested ethnic encounters. J Soc Psychol. 1978;104:107–114. doi: 10.1080/00224545.1978.9924042. [DOI] [PubMed] [Google Scholar]
- 52.Vidulich RN, Krevanick FW. Racial attitudes and emotional response to visual representations of the Negro. J Soc Psychol. 1966;68:85–93. doi: 10.1080/00224545.1966.9919669. [DOI] [PubMed] [Google Scholar]
- 53.Westie FR, DeFleur M. Autonomic responses and their relationship to race attitudes. J Abnorm Soc Psychol. 1959;58:340–347. doi: 10.1037/h0047847. [DOI] [PubMed] [Google Scholar]
- 54.Cacioppo JT, et al. Electromyographic activity over facial muscle regions can differentiate the valence and intensity of affective reactions. J Pers Soc Psychol. 1986;50:260–268. doi: 10.1037//0022-3514.50.2.260. [DOI] [PubMed] [Google Scholar]
- 55.Vanman EJ, et al. The modern face of prejudice and structural features that moderate the effect of cooperation on affect. J Pers Soc Psychol. 1997;73:941–959. doi: 10.1037//0022-3514.73.5.941. [DOI] [PubMed] [Google Scholar]
- 56.Vrana SR, Rollock D. Physiological response to minimal social encounter: Effects of gender, ethnicity, and social context. Psychophysiology. 1998;35:462–469. [PubMed] [Google Scholar]
- 57.Guglielmi SR. Psychophysiological assessment of prejudice: Past research, current status, and future directions. Pers Soc Psychol Rev. 1999;3:123–157. doi: 10.1207/s15327957pspr0302_3. [DOI] [PubMed] [Google Scholar]
- 58.Mendes WB, et al. Threatened by the unexpected: Challenge and threat during inter-ethnic interactions. J Pers Soc Psychol. 2007;92:698–716. doi: 10.1037/0022-3514.92.4.698. [DOI] [PubMed] [Google Scholar]
- 59.Mendes WB, et al. Why egalitarianism might be good for your health: Physiological thriving during inter-racial interactions. Psychol Sci. 2007;18:991–998. doi: 10.1111/j.1467-9280.2007.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eimer M. Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clin Neurophysiol. 2000;111:694–705. doi: 10.1016/s1388-2457(99)00285-0. [DOI] [PubMed] [Google Scholar]
- 61.Bentin S, Deouell LY. Structural encoding and identification in face processing: ERP evidence for separate mechanisms. Cogn Neuropsychol. 2000;17:35–54. doi: 10.1080/026432900380472. [DOI] [PubMed] [Google Scholar]
- 62.Caldara R, et al. Face versus non-face object perception and the “other-race” effect: A spatio-temporal event-related potential study. Clin Neurophysiol. 2003;114:515–528. doi: 10.1016/s1388-2457(02)00407-8. [DOI] [PubMed] [Google Scholar]
- 63.Caldara R, et al. Event-related potentials and time course of the “other-race” face classification advantage. NeuroReport. 2004;15:905–910. doi: 10.1097/00001756-200404090-00034. [DOI] [PubMed] [Google Scholar]
- 64.James MS, et al. Event-related potentials, configural encoding, and feature-based encoding in face recognition. J Psychophysiol. 2001;15:275–285. [Google Scholar]
- 65.Tanaka JW, Curran T. A neural basis for expert object recognition. Psychol Sci. 2001;12:43–47. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- 66.Herrmann, et al. Source localization of early stages of face processing. Brain Topogr. 2005;18:77–85. doi: 10.1007/s10548-005-0277-7. [DOI] [PubMed] [Google Scholar]
- 67.Halit H, et al. Modulation of event-related potentials by prototypical and atypical faces. NeuroReport. 2000;11:1871–1875. doi: 10.1097/00001756-200006260-00014. [DOI] [PubMed] [Google Scholar]
- 68.Michel C, et al. Holistic processing is finely tuned for faces of one’s own race. Psychol Sci. 2006;17:608–615. doi: 10.1111/j.1467-9280.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- 69.Walker PM, et al. Social contact and other-race face processing in the human brain. Soc Cogn Affect Neurosci. 2008;3:16–25. doi: 10.1093/scan/nsm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herrmann MJ, et al. The other-race effect for face perception: An event-related potential study. J Neural Transm. 2007;114:951–957. doi: 10.1007/s00702-007-0624-9. [DOI] [PubMed] [Google Scholar]
- 71.Stahl J, et al. Expertise and own-race bias in face processing: An event-related potential study. NeuroReport. 2008;19:583–587. doi: 10.1097/WNR.0b013e3282f97b4d. [DOI] [PubMed] [Google Scholar]
- 72.Mitchell JP, et al. Neural correlates of stereotype application. J Cogn Neurosci. 2009;21:594–604. doi: 10.1162/jocn.2009.21033. [DOI] [PubMed] [Google Scholar]
- 73.Moll J, et al. The neural basis of human moral cognition. Nat Rev Neurosci. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- 74.Mitchell JP, et al. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci. 2002;99:15238–15243. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henry AE, et al. Death on the brain: Effects of mortality salience on the neural correlates of ingroup and outgroup categorization. Soc Cogn Affect Neurosci. 2009 doi: 10.1093/scan/nsp041. [DOI] [PMC free article] [PubMed] [Google Scholar]