Abstract
Epidemiological studies suggest that obesity increases the risk of developing several cancers, including melanoma. Obesity increases the expression of angiogenic factors, such as leptin, that may contribute to tumor growth. However, a direct cause and effect relationship between obesity and tumor growth has not been clearly established and the role of leptin in accelerating tumor growth is unclear. Our objective in the present study was to examine the rate of melanoma tumor growth in lean and obese mice with leptin deficiency or high levels of plasma leptin. We injected 1 × 106 B16F10 melanoma cells subcutaneously into lean wild type (WT), obese melanocortin receptor 4 knockout (MC4R−/−), which have high leptin levels, obese leptin-deficient(ob −/−), pair fed lean ob−/−, and lean ob+/− mice. Mean body weights were 29.7 ± 0.3 g (WT), 46.3 ± 1.9 g (MC4R−/−), 63.7 ± 0.9 g (ob−/−), 30.5 ± 1.0 g (pair fed ob−/−) and 31.6 ± 1.7 g (ob+/−). Tumors were much larger in the obese leptin deficientob−/− (5.1 ± 0.9 g) and obese MC4R−/− (5.1 ± 0.7 g) than in lean WT (1.9 ± 0.3 g) and ob+/− (2.8 ± 0.7 g) mice. prevention of obesity by pair feeding ob−/− mice dramatically reduced tumor weight (0.95 ± 0.2 g) to a level that was significantly lower than in WT mice of the same weight. Tumor VEGF levels were the highest in the obese mouse tumors (p < 0.05), regardless of the host leptin levels. Except for the lean ob+/−, MC4R−/− and ob−/− melanomas had the highest VEGF receptor 1 and VEGF receptor 2 protein expression (p < 0.01 and p < 0.05), respectively. These results indicate that obesity markedly increases melanoma tumor growth rate by mechanisms that may involve upregulation of VEGF pathways. although tumor growth does not require host leptin, melanoma tumor growth may be accelerated by leptin.
Keywords: obesity, cancer, melanoma, leptin, angiogenesis, VEGF
Introduction
Obesity has increased dramatically in the United States in the past three decades; approximately 32% of adults are obese with a body mass index (BMI) greater than 30 kg/m2 and 65% of the adult population is overweight with a BMI greater than 25 kg/m2.1 The same trends have been noted in children.2 Currently, 18.8% of children in the 6–11 y old range are overweight or obese and it is likely that many of these overweight children will become obese adults.
Epidemiological studies have shown that there is a strong, positive correlation between BMI and the incidence of several types of cancer, such as colon, breast, ovarian, prostate, renal cell carcinoma and melanoma. Calle et al.3 concluded that obesity may account for 50% of cancer deaths in women aged 50 y or older. However, there have been few studies that have demonstrated a direct cause and effect relationship between obesity and cancer. Moreover, the epidemiological evidence suggests that the response to obesity is not the same for all tumors. Consequently, the mechanisms by which obesity may influence carcinogenesis are poorly understood.
Adipose tissue secretes several cytokines (i.e., adipokines) that are believed to promote inflammation, cell proliferation and angiogenesis. Leptin, an adipokine that is produced in proportion to the mass of adipose tissue and which circulates in the blood, has been suggested to link obesity with tumor growth.4–6 Leptin stimulates cell proliferation in several tumor cell lines, enhances endothelial cell migration in vitro, and has been suggested to be an angiogenic factor.7,8 Thus, leptin has several effects that could contribute to tumor growth. However, most of the studies supporting leptin’s role in promoting cell proliferation and angiogenesis have been conducted in vitro and have involved the use of large, pharmacological concentrations of leptin to demonstrate the effects.
The few published animal studies attempting to determine whether leptin and obesity promote tumor growth have produced mixed results. Some studies support the hypothesis that the absence of leptin signaling attenuates mammary tumor growth in mice.9–11 For example, Gonzalez et al. demonstrated that mouse mammary tumor growth was dramatically reduced when mice were treated with a leptin receptor antagonist,11 suggesting that leptin signaling may be necessary for certain types of breast tumor growth. In apparent contradiction to these studies, obese Zucker rats, which have a mutation in the leptin receptor, developed more mammary tumors at an earlier age than lean Zucker rats after exposure to the carcinogen, 7,12-dimethylbenzanthracene.12 Thus, the role of leptin in promoting tumor growth is still unclear, and there have been no previous studies, to our knowledge, that have investigated the role of obesity and leptin in contributing to melanoma tumor growth.
We designed the present study to test the hypothesis that obesity promotes melanoma growth and to examine leptin’s contribution to tumor growth in this model. We compared melanoma tumor growth in lean C57BL6/J mice with tumor growth in two genetically obese mouse models on the same genetic background: (1) the ob−/− mouse which has a mutation in the leptin gene and is leptin deficient; (2) the melanocortin 4 receptor knock out (MC4R−/−) mouse, which has high levels of leptin because of its large amount of adipose tissue. We also studied tumor growth in lean ob+/− mice and in leptin deficient ob−/− mice, in which obesity was prevented by controlling the amount of food they ate to match the weight of the WT mice. This permitted us to separate the effect of leptin deficiency from that of obesity.
Results
Obesity promotes melanoma tumor growth
Body weights for each group of mice are shown in Table 1. Pair feeding ob−/− mice, beginning at weaning, resulted in body weights that were not significantly different from WT mice (30.5 g for the pair fed ob−/− vs. 29.7 g for WT). Palpable tumors appeared in all but the food restricted group by experimental day 10. In the pair fed ob−/− mice, tumor latency was increased and melanomas were not apparent until experimental day 13. Palpable tumors appeared 1–2 d earlier in ob−/− and MC4R−/− groups than other groups.
Table 1.
Biological characteristics of mice used in this study
| Genotype | Body weight (g) | Body length (cm) | Tumor weight (g) | Plasma leptin (ng/mL) | Tumor leptin (ng/mg) | Plasma VEGF (pg/mL) | Plasma glucose (mg/dL) |
|---|---|---|---|---|---|---|---|
| Ob+/− | 31.6 ± 1.7 | 9.3 ± 0.1 | 2.8 ± 0.7 | 2.9 ± 0.5 | 0.8 ± 0.4 | 72 ± 12 | 215 ± 20 |
| Ob−/− | 63.7 ± 0.9* | 10.2 ± 0.1 | 5.1 ± 0.8* | BD | 0.4 ± 0.1* | 64 ± 15 | 299 ± 3* |
| Ob−/− (pair-fed) | 30.5 ± 1.0 | 8.5 ± 0.1 | 0.9 ± 0.2* | BD | 0.2 ± 0.1* | 84 ± 4* | 332 ± 27* |
| WT | 29.7 ± 0.3 | 9.6 ± 0.1 | 1.9 ± 0.3 | 2.4 ± 0.5 | 1.1 ± 0.2 | 50 ± 11 | 251 ± 28 |
| MC4R−/− | 46.3 ± 1.9* | 9.8 ± 0.1 | 5.2 ± 0.7* | 37.9 ± 3* | 4.4 ± 1.1* | 114 ± 15* | 231 ± 53 |
p < 0.01, compared to WT; BD, below detectable limits of the assay.
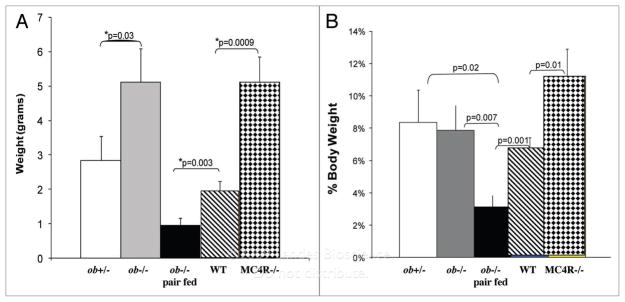
Mice were sacrificed after 17 d and tumors were weighed after dissecting away skin and fat. Tumors grew significantly larger in both groups of obese mice compared to the lean groups of mice (Fig. 1A). Both MC4R−/− and ob−/− had mean tumor weights that were 2.5 times heavier than those from non-obese WT and ob+/− mice. Ob−/− mice that were pair fed the same amount of food as lean WT mice to prevent the development of obesity had tumors that were less than 20% the size of obese ob−/− and obese MC4R−/− mice (Fig. 1A). Thus, energy restriction markedly attenuates melanoma tumor growth.
Figure 1.
Obesity increases melanoma growth. (a) Mean tumor weights. after 17 days, mice were euthanized, tumors were excised, freed of skin and weighed. The mice groups are as follows: lean ob+/− mice (ob+/−), n = 7; obese leptin-deficient mice(ob−/−), n = 10; pair fed lean ob−/− mice (ob−/− pair fed), n = 11; lean wild type mice (WT), n = 14; and obese melanocortin receptor 4 knockout mice (MC4R−/−), n = 10. (B) Tumor weight expressed as a percentage of body weight. Mean tumor weights were divided by the mean body weights for each group of mice.
Plasma glucose levels in the different groups of mice are shown in Table 1. The pair fed ob−/− mice had the highest plasma glucose of all the mice used in the study. However, pair fed ob−/− mice had smaller tumors than even WT mice. There were no significant differences in plasma glucose levels between WT, ob+/− and MC4R−/− mice although ob−/− mice had higher plasma glucose levels than WT mice.
Role of leptin in melanoma growth
When tumor weight was expressed as a percentage of body weight, melanoma tumors from MC4R−/− mice, which had high levels of leptin, were significantly larger than tumors from other groups of mice (Fig. 1B). Pair feeding of leptin deficient ob−/− mice resulted in body weights that were not different from those of WT mice (30.5 g for the pair fed ob−/− vs. 29.7 g for WT). However, melanomas in pair-fed ob−/− mice were only half the size of those from lean WT. These observations indicate that while leptin is not required for melanoma growth, leptin deficiency greatly attenuates tumor growth while increased levels of leptin may modestly increase tumor growth.
Tumors express leptin receptors but not leptin
Leptin has been proposed to act in an autocrine or paracrine manner on endothelial cells to promote angiogenesis. We measured leptin levels in the plasma and tumors of the different groups of mice, and in the cell culture medium from B16F10 cells to determine whether these melanoma tumors produce leptin. Plasma leptin in obese MC4R−/− mice was 37.9 ng/mL, compared to 2.4 and 2.9 ng/mL in non-obese WT and obese ob+/− mice, respectively (Table 1). These levels are similar to those previously reported for these mice.13,14 In the plasma of ob−/− and pair fed ob−/− mice, leptin was below detectable limits of the assay.
Tumor leptin levels were very low and appeared to reflect host circulating leptin levels, with the highest leptin found in tumors from MC4R−/− mice, followed by WT and ob+/− tumors (Table 1). Leptin was undetectable in the tumors from ob−/− and pair fed ob−/− mice. We were also unable to detect leptin in the medium from cultured B16F10 cells. These observations suggest that very little or no leptin is produced by mouse melanoma tumors.
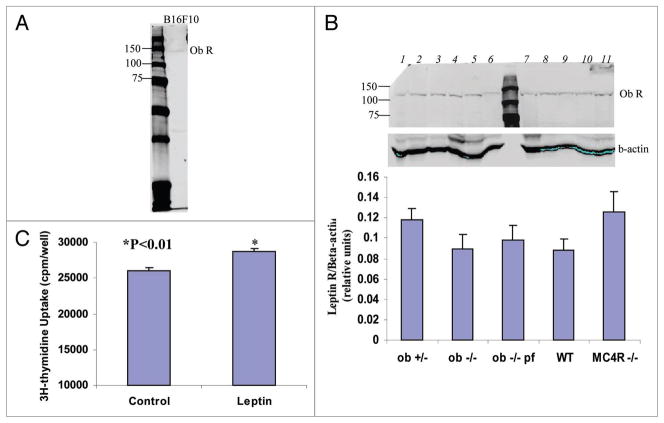
Leptin receptor expression in tumors of the different groups of mice and in B16F10 cells was measured by western blot. The leptin receptor was expressed in all melanoma tumors and in B16F10 cells (Fig. 2A). Leptin receptor expression in the tumors was not different among the five groups of mice (Fig. 2B). 3H-thymidine incorporation assay showed that human leptin (100 ng/ml) slightly (10.4%) but significantly increased the proliferation of cultured B16F10 cells (Fig. 2C). These observations indicate that the lepin receptor in B16F10 cell is functional.
Figure 2.
B16F10 cells and melanoma tumors express the leptin receptor. (a) Leptin receptor (Ob-R) was measured in B16F10 cell lysate by western blot with Ob-R antibody. (B) Melanoma tumor homogenates from mice were made and 40 μg was subjected to western blotting with Ob-R antibody. Lanes 1 and 6: ob+/−, 2 and 11: ob −/−, 8 and 9: ob −/− pair fed, 6 and 10: WT, 3, 4, 5 and 7: MC4R −/−. Leptin receptor expression in the tumors was not different among the five groups of mice. (C) 3H-thymidine incorporation assay showed that human leptin (100 ng/ml) slightly (10.4%) but significantly (p < 0.01) increases the proliferation of cultured B16F10 cells.
Tumors from obese mice are more necrotic
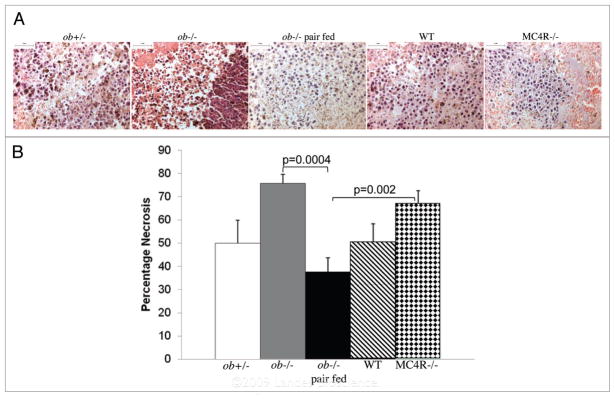
Upon dissection at the end of the study, 70–80% of the tumors taken from obese mice were surrounded by blood and pus, whereas the percentage was less than 43% in the tumors from lean mice. Necrosis was examined in haematoxylin and eosin stained tumor cross sections. Overall, the larger tumors were more necrotic. Ob−/− tumors had the most necrosis and were followed by MC4R−/− tumors (Fig. 3A and B). While WT and ob+/− tumors had similar levels of necrosis, tumors from pair fed ob−/− mice had the least amount of necrosis.
Figure 3.
Tumor necrosis. (a) pictures of heamatoxylin and eosin stained tumor sections. scale bar 50 μm. (B) Larger tumors have the most necrosis. Necrosis was measured in three cross sections 200 μm apart taken from the center of each tumor. sections were examined by two investigators who were blinded to the sample identities. scale bar 100 μm.
Obesity increases expression of VEGF and its receptors
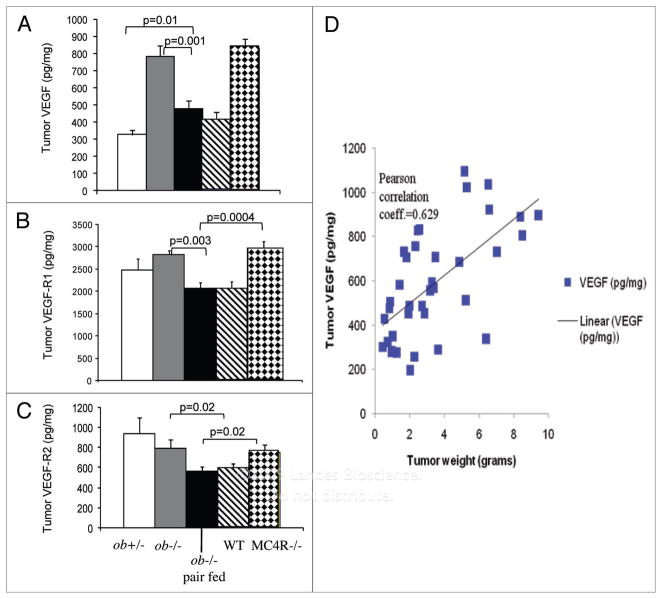
In plasma taken from mice at the time of sacrifice, VEGF was highest in MC4R−/− mice, while WT mice had the lowest VEGF (Fig. 4A). There were no differences in plasma VEGF among ob+/−, ob−/− and pair fed ob−/− mice. However, pair feeding in ob−/− mice significantly increased VEGF production in comparison to WT mice.
Figure 4.
Larger tumors express more VEGF and VEGF-R1. (A) Tumors were coded to prevent indentification by the investigator and homogenized in RIpa buffer. VeGF was measured in protein extracts by eLIsa. (B and C) VeGF-R1 and VeGF-R2 were measured in protein extracts by eLIsa. (D) Correlation between tumor VeGF and tumor weight. s hown are the means ± seM. p < 0.05.
We measured the expression of VEGF and its two receptors, VEGF-R1 and R2, in the melanoma tumors to determine the effects of obesity and leptin on these angiogenic factors. VEGF expression was increased in the tumors of obese leptin deficient ob−/− mice, as well as in tumors of obese MC4R−/− mice. In contrast, lean WT, pair fed ob−/− mice, and ob+/− mice expressed low levels of VEGF protein (Fig. 4B). There were no differences in tumor VEGF levels between tumors obtained from leptin-deficient and leptin-replete mice. Thus, tumor VEGF levels were highly correlated with tumor size regardless of host leptin (Fig. 4C).
The expression of VEGF receptor 1 and 2 was examined in the melanomas by ELISA. Both receptors were proportional to tumor size, except for the ob+/− tumors, which expressed the receptors similarly to obese MC4R−/− and ob−/− tumors (Fig. 4D and E).
Obesity increases tumor blood vessel growth
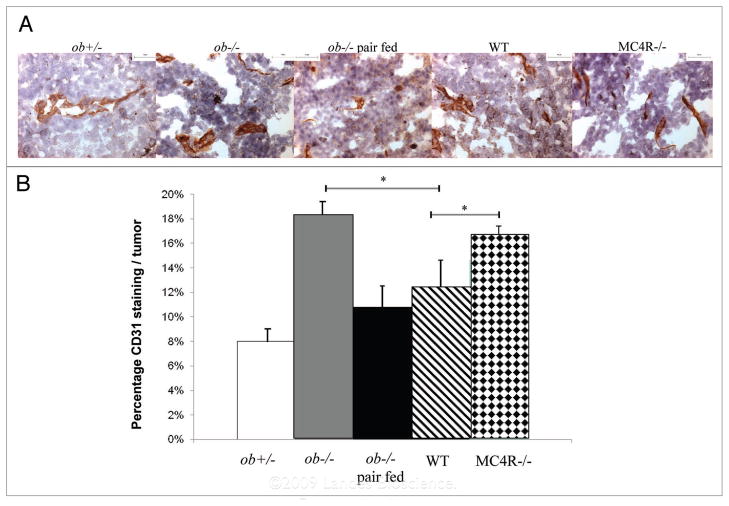
We also examined blood vessels in the mouse melanomas using immunohistochemistry for the endothelial cell marker, CD31. Blood vessels formed in the tumors from all groups of mice and appeared as elongated and punctuated structures throughout the tumor. Nine cross sections from each tumor were examined for CD31 staining. Because the amount of necrosis was different in each group of melanomas, blood vessels were counted in at least twenty randomly obtained images and expressed as the number of CD31-stained areas touching points in a grid overlay out of the total number of points touching the tumor. The highest density of blood vessels in all tumors was seen on the side of the tumor touching the dorsal body wall. CD31 staining was proportional to tumor size. MC4R−/− and ob−/− melanomas appeared to have a highest percentage of CD31 staining followed by WT, ob−/− pair fed, ob−/− and ob+/− mice (Fig. 5), and there was a significant difference between the wild-type and two obese groups, MC4R−/− and ob−/− (p < 0.05).
Figure 5.
MC4R−/− and leptin-deficient obese melanomas have higher vascularity than wild-type, ob+/− and pair fed ob−/− melanomas. (a) Tumors were coded to prevent indentification by the investigator. Frozen sections were cut and fixed in acetone. Nine sections were subjected to immunohistochemistry with a rat CD31 antibody. Images were obtained with a Leica DMRB microscope equipped with a color CCD camera that was operated by Imagepro software. scale bar 50 μm. (B) CD31 staining was measured in at least twenty random images per tumor and reported as the percentage of CD31 stained areas out of the total tumor area. s hown are the means ± se M. *p < 0.05.
Discussion
Important new findings of this study are that (1) obesity promotes melanoma tumor growth, regardless of the presence or absence of leptin; (2) energy restriction greatly attenuates tumor growth in obese mice; (3) leptin, although not essential for melanoma tumor growth, may accelerate tumor growth while leptin deficiency in the absence of obesity attenuates tumor growth; (4) leptin receptors are expressed in mouse melanoma cells and leptin slightly but significantly increases the proliferation of cultured B16F10 cells, as assessed by DNA-synthesis assay. We also found that tissue VEGF levels were much higher in the melanomas from obese mice and that tumor VEGF was independent of host plasma leptin or circulating VEGF levels.
While epidemiological studies suggest a positive correlation between body mass index and the risk of certain cancers (e.g., colon, breast, renal cell and uterine cancers) no clear cause and effect relationship has been previously established.3,15 Until recently, there had been no studies that have examined the relationship between obesity and the incidence of melanoma. Our finding that melanomas from obese MC4R−/− mice with high levels of leptin and obese ob−/− mice with leptin deficiency grew much faster than melanomas from lean WT or lean ob −/+ mice suggests that obesity was a major factor in determining tumor growth even in the absence of leptin. Moreover, restriction of energy intake to prevent obesity in ob−/− mice greatly reduced tumor size to levels below those observed in lean WT mice, further supporting a key role for excess energy intake and obesity in stimulating melanoma tumor growth.
Angiogenesis is required for adipose tissue expansion during weight gain and a direct relationship between obesity and angiogenesis was demonstrated by experiments in which angiogenesis inhibition prevented obesity and caused weight loss in genetically obese mice.16 One mechanism by which obesity has been suggested to contribute to enhanced tumor growth is by promoting angiogenesis. Our finding that plasma VEGF was significantly higher in obese MC4R−/− mice, compared to WT mice, supports a role for enhanced angiogenesis in these mice. However, there were no significant differences in plasma VEGF among obese ob−/− mice with high rates of tumor growth and lean ob+/− and pair fed ob−/− mice with much lower rates of tumor growth.
The use of a pair fed ob −/− group in this study was intended to prevent obesity in the leptin-deficient mice so the impact of leptin deficiency could be assessed independently of increased body weight. Our observation that energy restriction greatly reduced melanoma tumor growth is consistent with previous findings that chronic or intermittent caloric restriction may slow the growth of other types of cancers, such as mammary tumors.17,18 The mechanisms that mediate the effects of food restriction on tumor growth are unclear but likely involve numerous metabolic pathways, including those involved in angiogenesis. The lower rates of melanoma tumor growth observed in pair fed ob−/− mice relative to WT mice of similar body weight, despite higher levels of VEGF in the pair fed ob−/− mice, could result from increased production of antitumorigenic factors during food restriction that inhibit melanoma growth. This observation is surprising and raises important questions for future research.
Our results also suggest that while leptin is not required for melanoma growth, it may play at least a partial role in amplifying tumor growth. Ob −/− mice fed ad libitum were significantly more obese than MC4R−/− mice, but had similar VEGF expression and tumor sizes. If leptin had no effect on tumor growth, we would expect larger tumors in the obese ob−/− mice, since these mice are 38% heavier than the MC4R−/− mice. Moreover, in lean, pair fed leptin deficient ob−/− mice, melanomas were half the size of tumors from lean WT or ob+/− mice, which have similar body weights but higher leptin levels. These observations suggest that leptin deficiency greatly attenuates melanoma tumor growth while high leptin levels may accelerate tumor growth.
The mechanisms for leptin’s effects on tumor growth are still uncertain, although a role for leptin in promoting angiogensis has been previously suggested. Results from one study demonstrated that treatment with leptin induced neovascularization in the corneas of normal rats but not in the corneas of obese Zucker fa/fa rats, which have a mutation in the leptin receptor.19 In addition, leptin has been shown to interact synergistically with fibroblast growth factor 2 (FGF-2) and VEGF, two potent and commonly expressed angiogenic factors, to stimulate angiogenesis in corneal explants.20 In this study, we were unable to detect leptin in the medium from cultured B16F10 cells. These observations suggest that very little or no leptin is produced by mouse melanoma tumors. However, the leptin receptor was expressed in all melanoma tumors and in B16F10 cells. It is possible that leptin can interact with VEGF to promote the growth of melanoma tumors.
There is indirect evidence from clinical studies that leptin may enhance tumor growth by stimulating angiogenesis. For example, leptin expression correlated well with VEGF expression in human gastric cancers and both factors were associated with poor patient prognosis.21 Results from a recent study in mice showed that mammary tumor VEGF, VEGF-R2 and tumor growth were significantly reduced when mice were treated with the leptin receptor antagonist, LPrA2.11 While we found that VEGF and VEGF receptor expression was well correlated with melanoma size, they were not well correlated with leptin levels. Thus, leptin may interact with VEGF to promote growth of some cancers, but not others.
Leptin has been identified in several types of human cancers and may also be linked to poor prognosis. In two studies, leptin and leptin receptor expression were significantly increased in primary and metastatic breast cancer relative to noncancerous tissues in women.22,23 In a clinical study of colorectal cancer, leptin expression was positively correlated with tumor G2 grade.24 Serum leptin and leptin receptor expression in renal cell carcinomas was well correlated with progression-free survival, venous invasion and lymph node metastasis.25 Leptin has also been shown to be expressed in uterine and endometrial cancers.26 However, it is difficult to dissociate the direct effects of leptin from other effects of obesity in these studies; increased adiposity has important metabolic effects and stimulates release of many cytokines, inflammatory mediators and factors other than leptin that could promote tumor growth.
There is very little previous information on the relationship between leptin and melanoma. One epidemiological study reported that high serum leptin was positively correlated with melanoma risk.27 However, to our knowledge there have been no previous studies that have examined leptin or leptin receptor expression in melanoma tumors. Results from the single animal study published found no differences in the incidence of skin papillomas induced by 7,12-dimethylbenz(a)anthracene between obese ob−/− and lean ob+/− mice.28 Our results indicate that while leptin receptors are expressed in melanoma tumors, the melanoma cells do not appear to produce leptin. In leptin deficient ob−/− mice, leptin measured in the tumors was barely detectable, whereas in MC4R−/− mice with high plasma leptin levels, the amount of leptin extracted from the tumors was markedly elevated compared to that of WT and ob+/− tumors. The leptin in these tumors likely derives from host leptin that is sequestered in the tissues from the circulation or from immune cells that infiltrate the tumor.
Epidemiological studies suggest that hyperglycemia and hyperinsulinemia are associated with increased risk for development of cancer.29 In the present study, we found that pair fed ob −/− mice had the smallest tumors in comparison with tumors from the other groups of mice even though they had high plasma glucose levels. These findings suggest that hyperglycemia alone does not increase melanoma tumor growth in this in vivo model. Although we do not have insulin data, we previously reported that MC4R −/− mice had several-fold greater plasma insulin levels than WT mice.13 Additionally, other investigators have published plasma insulin levels in ob −/− mice and found them to be significantly higher than lean wild type mice.30 However, further studies are needed for testing the hypothesis that hyperinsulinemia increases tumor growth in this model of melanoma.
Tumors from obese mice are more necrotic
The observation of increased necrosis in tumors from both groups of obese mice is in line with what is expected in a model such as this. Mice were injected with a large number of cells that grow rapidly. Ten days after injection, nearly all of the mice had palpable tumors and by experimental day 17, some tumors were more than four centimeters in diameter. Most of the necrotic areas were concentrated in the center of the tumors where the diffusion of nutrients from blood vessels would not be reduced. The small percentage of necrosis in pair fed ob −/− tumors suggests that tumor angiogenesis kept pace with tumor growth.
Obesity increases tumor angiogenic factor expression
The expression of VEGF in tumors is well correlated with tumor size and we found that VEGF levels were much higher in the melanomas from obese mice. Tumor VEGF was independent of host plasma leptin levels.
Except for ob+/− melanomas, the expression of VEGF-R1 and R2 was also proportional to the size of the melanoma. A possible explanation for this is that VEGF is very low in ob+/− tumors compared to VEGF in the tumors from other lean groups, which may have caused upregulation of VEGF-R1/R2 expression in an effort to boost VEGF signaling. The body weights, plasma leptin levels, and tumor sizes were similar between ob+/− and WT mice, thus the differences in tumor VEGF receptor expression were surprising.
Materials and Methods
Animals
Mice used in this study were all on the C57BL6/J background and approximately 20 w of age when experiments were begun. Mice were housed individually in shoebox cages in a temperature controlled room kept on a 12 h/12 h light/dark cycle and given regular mouse chow and water ad libitum, except for one group, as described below.
Five groups of mice were used for these experiments: group 1 consisted of wild-type C57BL6/J (n = 14, purchased from the Jackson Laboratory), group 2 was comprised of MC4R−/− mice (n = 10, bred from founder mice obtained from Roger Cone of the Vollum Institute), group 3 consisted of ob−/− mice (n = 10, B6.V-Lepob/ob/J), group 4 consisted of ob+/− (n = 7, B6.V-Lep+/ob/J), and group 5 consisted of ob−/− mice (n = 11, B6.V-Lepob/ob/J) whose food intake controlled, immediately after weaning, so that their weight matched the weight of WT mice to prevent the development of obesity. All ob −/− and ob+/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
Mice were anesthetized with isoflurane and injected subcutaneously on the dorsum with 1 × 106 B16F10 mouse melanoma cells using a syringe fitted with a 21½ gauge needle. Mice were weighed daily and monitored for signs of physical distress for seventeen days after injection of the tumor cells. Upon appearance, tumor size was measured daily with calipers. At the end of the experiment, mice were anesthetized with isoflurane and blood samples were obtained via cardiac puncture. Animals were then perfused with 0.1 M phosphate buffered saline at a rate of 0.8 mL/minute for 5–7 min, until the blood was drained from the kidneys and liver. Tissue samples were collected and frozen in liquid nitrogen or fixed in 10% buffered formalin.
Melanoma tumor cells and proliferation assay
B16F10 cells were purchased from the American Type Culture Collection. These cells were chosen because they are syngenic with the mouse models used in this study. Cells were grown in M199 medium (Gibco/Invitrogen) containing Earle’s salts and L-glutamine supplemented with 10% fetal bovine serum, 1.5 g/L sodium bicarbonate and 1% penicillin/streptomycin. The incubator was maintained at 37°C with 5% CO2. Cells were passaged less than four times before injection into mice. When the monolayer reached about 80% confluence, the cells were washed with PBS and incubated with fresh M199 media with 10% FBS in the absence (vehicle control) and presence of human leptin (100 ng/ml) for 48 h. 3H-thymidine incorporation assay was used to determine the cell proliferation during the last 6 h of incubation as previously described.31
Histology and immunohistochemistry
For hematoxylin and eosin staining, formalin fixed paraffin-embedded tissues were cut into 5 micron sections on a microtome and stained according to standard methods. Three sections 100 μm apart were taken from the center of each tumor and were analyzed using a Leica DMRB microscope (Wetzler, Germany) equipped with a Cool Snap-Pro cf color camera (Media Cybernetics) and ImagePro image analysis software. Images of the entire section were obtained using the 20× objective and then overlaid with a grid of 70 points. Only points that were touching tumor tissue were counted. Points that touched a necrotic area were counted as positive. The analysis was completed by two different investigators who were blinded from the sample identities.
For immunohistochemistry, tumors frozen in liquid nitrogen were cut in half, embedded in Cryostat, cut into 12 micron sections, and placed on Superfrost glass slides (Fisher). Nine sections per tumor were labeled with a rat CD31 antibody (BD Pharmingen). Briefly, tumor sections were fixed in ice-cold acetone for 10 min at −20°C and then air dried for 30 min. After washing 3X for 2 min in 0.1 M phosphate buffered saline (PBS), peroxidase was blocked by incubation in 3% hydrogen peroxide diluted in 100% methanol for 10 min at room temperature (RT). Afterwards, sections were blocked in 5% rabbit serum for 10 min at RT and then incubated overnight at 4°C with anti-CD31 rat polyclonal antibody diluted 1:10 in 5% rabbit serum in a sealed, humidified chamber. Sections were rinsed 3X with 0.1 M PBS plus 0.05% Tween-20 and then incubated with biotinylated anti-rat IgG (Vector) diluted 1:200 in 0.1 M PBS for 1 h at RT. The rinse steps were repeated and then sections were incubated in extravidin peroxidase (Sigma) diluted 1:400 in 0.1 M PBS for 30 min at RT. Nova red (Vector), as the chromagen, was added to the sections and incubated for 6 min, followed by a wash in distilled water. Sections were counterstained with Harris haematoxylin (Sigma), differentiated and mounted in Gelmount (Sigma). Analysis was performed on blinded samples with a Leica DMRB microscope and ImagePro analysis software. CD31 staining was measured by overlaying a grid of 70 points on each image obtained with the ×10 objective and counting the number of points touching CD31 positive areas out of the total number of points touching the tumor.
VEGF and VEGF receptor measurements
Half of each tumor was powdered under liquid nitrogen, weighed and solubilized in RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% sodium dodecylsulfate, 50 mM Tris-HCl, pH 7.4, Pierce). Samples were incubated on ice for 5 min and then vortexed twice for 15 sec. Following a second 5 min incubation on ice, samples were centrifuged at 16,000 ×g for 10 min at 4°C. The protein concentration was measured and approximately 50 μg was used to measure tumor VEGF, VEGF-R1 and VEGF-R2 by ELISA (R&D Systems), according to the manufacturer’s protocol. Samples were measured in two independent tests. The total protein concentration of tissue supernatant was determined using a Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA). The protein levels of VEGF in the tumor tissues were normalized and expressed as picograms per milligram total tissue protein.
Leptin, leptin receptor and blood glucose measurements
Mice were fasted for 6 h prior to sacrificing in order to obtain fasted blood samples. Leptin was measured in plasma and in tumor homogenates by ELISA (R&D Systems) in at least two separate measurements. Leptin was also measured in the media from cultured B16F10 cells. Leptin receptor expression in tumors and in cultured B16F10 cells was measured by western blot. Lysates were prepared as described above and 40 μg were separated by 8% Tris-HCl, SDS-PAGE (Bio-Rad). After transfer to nitrocellulose, membranes were blocked in Odyssey buffer (Li-Cor) for 1 h at RT and incubated in rabbit leptin receptor antibody (H-300, sc-8325, Santa Cruz Biotechnology) diluted 1:100 and mouse anti-beta-actin antibody (ab6276, Abcam) diluted 1:5,000 in Odyssey buffer overnight at 4°C. Membranes were rinsed 5× for 5 min in Tris-buffered saline with 0.05% Tween-20. Infrared tagged secondary antibodies raised in donkey (Rockland) were diluted 1:5,000 and incubated with membranes for 2 h at RT. Membranes were rinsed as above and scanned on a Li-Cor Odyssey infrared scanner. Blood glucose levels were measured using a Reli-On glucose monitor (Hypoguard).
Statistics
The data are expressed as the mean ± SEM. Comparisons between different groups were done by unpaired t-tests and one way ANOVA when appropriate. Statistical significance was accepted at a level of p < 0.05.
Conclusions and Perspectives
Our observations indicate that obesity causes rapid melanoma growth and that energy restriction greatly attenuates tumor growth in obese mice. The mechanisms by which obesity promotes melanoma growth likely involve increased angiogenesis, since tumors from the obese mice have more VEGF and CD31 staining. Leptin deficiency attenuates but does not abolish melanoma tumor growth. Leptin receptors are expressed in mouse melanoma cells, but we found no evidence that melanoma tumors produce significant amounts of leptin. A key factor in obesity induced angiogenesis that may be involved in promoting melanoma growth is inflammation. Obesity is often described as a low level inflammatory state and the literature is replete with studies supporting a central role for macrophages in tumor angiogenesis. During weight gain, adipocytes increase in size, preadipocytes differentiate into mature adipocytes and there is a sudden increase in the metabolic demand of the tissue. Adipocytes respond by secreting inflammatory cytokines that recruit macrophages. Macrophages, in turn, initiate angiogenesis by secreting matrix metalloproteinases to break down the extracellular matrix and basement membranes of nearby blood vessels. Together with adipocytes, macrophages secrete angiogenic factors that promote the proliferation and migration of endothelial cells. Once the new blood vessel “sprouts” are formed, macrophages secrete different cytokines that remodel the stroma to support their growth. The similarities between angiogenesis in obesity and cancer suggest that macrophages are central to both scenarios. Future studies should examine whether inhibition of inflammation in obesity reduces tumor growth. A reduction in inflammation would also provide a potential mechanism by which food restriction could reduce melanoma growth.
Acknowledgments
This work was supported by National Heart, Lung and Blood Institute Grant HL-51971.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CCL. Prevalence and trends in obesity among US adults 1999–2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Stattin P, Lukanova A, Biessy C, Soderberg S, Palmqvist R, Kaaks R, et al. Obesity and colon cancer: does leptin provide a link? International Journal of Cancer. 2004;109:149–52. doi: 10.1002/ijc.11668. [DOI] [PubMed] [Google Scholar]
- 5.Chang S, Hursting SD, Contois JH, Strom SS, Yamamura Y, Babaian RJ, et al. Leptin and prostate cancer. The Prostate. 2001;46:62–7. doi: 10.1002/1097-0045(200101)46:1<62::aid-pros1009>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Saglam K, Aydur E, Yilmaz M, Goktas K. Leptin influences cellular differentiation and progression in prostate cancer. J Urol. 2003;169:1308–11. doi: 10.1097/01.ju.0000055903.18400.25. [DOI] [PubMed] [Google Scholar]
- 7.Fenton JI, Hord NG, Lavigne JA, Perkins SN, Hursting SD. Leptin, insulin-like growth factor-1, and insulin-like growth factor-2 are mitogens in ApcMin/+ but not Apc+/+ colonic epithelial cell lines. Cancer Epidemiol Biomarkers Prev. 2005;14:1646–52. doi: 10.1158/1055-9965.EPI-04-0916. [DOI] [PubMed] [Google Scholar]
- 8.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circulation. 1998;83:1059–66. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 9.Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, et al. Genetically obese MMTV-TGFalpha/Lep (ob) Lep (ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–15. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- 10.Cleary MP, Grande JP, Maihle NJ. Effect of high fat diet on body weight and mammary tumor latency in MMTV-TGFα mice. Int J Obes Relat Metab Disord. 2004;28:956–62. doi: 10.1038/sj.ijo.0802664. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281:26320–8. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 12.Hakkak R, Holley AW, Macleod SL, Simpsom PM, Fuchs GJ, Jo CH, et al. Obesity promotes 7,12 dimethylbenz (a) anthracene-induced mammary tumor development in female zucker rats. Breast Cancer Res. 2005;7:627–33. doi: 10.1186/bcr1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 14.Chung WK, Belfi K, Chua M, Wiley J, Mackintosh R, Nicolson M, et al. Heterozygosity for Lep (ob) or Lep (rdb) affects body composition and leptin homeostasis in adult mice. Am J Physiol. 1998;274:985–90. doi: 10.1152/ajpregu.1998.274.4.R985. [DOI] [PubMed] [Google Scholar]
- 15.Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91:421–30. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA. 2002;99:10730–5. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleary MP, Jacobson MK, Phillips FC, Getzin SC, Grande JP, Maihle NJ. Weight-cycling decreases incidence and increases latency of mammary tumors to a greater extent than does chronic caloric restriction in mouse mammary tumor virus-transforming growth factor-alpha female mice. Cancer Epidemiol Biomarkers Prev. 2002;11:836–43. [PubMed] [Google Scholar]
- 18.Cleary MP, Hu X, Grossmann ME, Juneja SC, Dongan S, Grande JP, et al. Prevention of mammary tumorigenesis by intermittent caloric restriction: does caloric intake during refeeding modulate the response? Exp Biol Med. 2007;232:70–80. [PubMed] [Google Scholar]
- 19.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–6. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 20.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci USA. 2001;98:6390–5. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Huang K, Zhu Z, Chen S, Hu R. Correlation between expression of leptin and clinicopathological features and prognosis in patients with gastric cancer. J Gastroenterol Hepatol. 2007;22:1317–21. doi: 10.1111/j.1440-1746.2007.04941.x. [DOI] [PubMed] [Google Scholar]
- 22.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewka J, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:447–53. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–31. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 24.Koda M, Sulkowska M, Kanczuga-Koda L, Surmacz E, Sulkowski S. Overexpression of the obesity hormone leptin in human colorectal cancer. J Clin Pathol. 2007;60:902–6. doi: 10.1136/jcp.2006.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horiguchi A, Sumitomo M, Asakuma J, Asano A, Zheng R, Nanus DM, et al. Leptin promotes invasiveness of murine renal cancer cells via extracellular signal-regulated kinases and rho dependent pathway. J Urol. 2006;176:1636–41. doi: 10.1016/j.juro.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 26.Koda M, Sulkowska M, Wincewicz A, Kanczuga-Koda L, Musiatowicz B, Szymanska M, et al. Expression of leptin, leptin receptor and hypoxia-inducible factor 1alpha in human endometrial cancer. Ann N Y Acad Sci. 2007;1095:90–8. doi: 10.1196/annals.1397.013. [DOI] [PubMed] [Google Scholar]
- 27.Gogas H, Trakatelli M, Dessypris N, Terzidis A, Katsambas A, Chrousos GP, et al. Melanoma risk in association with serum leptin levels and lifestyle parameters: a case-control study. Ann Oncol. 2008;19:384–9. doi: 10.1093/annonc/mdm464. [DOI] [PubMed] [Google Scholar]
- 28.Ablamunits V, Cohen Y, Brazee IB, Gaetz HP, Vinson C, Klebanov S. Susceptibility to induced and spontaneous carcinogenesis is increased in fatless A-ZIP/F-1 but not in obese ob/ob mice. Cancer Res. 2006;66:8897–902. doi: 10.1158/0008-5472.CAN-05-4679. [DOI] [PubMed] [Google Scholar]
- 29.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 30.Flatt PR, Bailey CJ. Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia. 1981;20:573–7. doi: 10.1007/BF00252768. [DOI] [PubMed] [Google Scholar]
- 31.Gu JW, Bailey AP, Sartin A, Makey I, Brady AL. Ethanol stimulates tumor progression and expression of vascular endothelial growth factor in chick embryos. Cancer. 2005;103:422–31. doi: 10.1002/cncr.20781. [DOI] [PubMed] [Google Scholar]