Abstract
Clinical composite tissue allotransplantation can adequately reconstruct defects that are not possible by other means. However, immunosuppressant toxicity limits the use of these techniques. Clinical attempts to reduce the amount of immunosuppression required by induction of an immunologically permissive state have so far been unsuccessful. The aim of this study was to induce tolerance in a preclinical large animal model. Donor hematopoietic stcm cell (HSC) engraftment was induced by T-cell depletion, irradiation, and a short course of cyclosporine administered to the recipient, along with a hematopoietic cell infusion from a single haplotype major histocompatibility complex (MHC) mismatched donor. Skin was then allotransplanted from the donor. Both primarily vascularized skin flaps and secondarily vascularized conventional skin grafts were allotransplanted to investigate if the mode of transplantation affected outcome. Control animals received the skin allotransplants without conditioning. Tolerance was defined as no evidence of rejection at 90 days following transplantation. Conventional skin grafts only achieved prolonged survival (<65 days) in HSC-engrafted animals (P < .01). In contrast, there was indefinite skin flap survival with the achievement of tolerance in HSC-engrafted animals; this was confirmed on histology with donor-specific unresponsiveness on MLR and CML. Furthermore, a conventional skin donor graft subsequently applied to an animal tolerant to a skin flap was not rejected and did not trigger skin flap rejection. To our knowledge, this is the first time skin tolerance has been achieved across a MHC barrier in a large animal model. This is a significant step toward thc goal of clinical skin tolerance induction.
THE BENEFITS of clinical composite tissue allotransplantation are becoming clearer in that it is possible to restore damaged parts of the body, such as hand or face, to a level of form and function that has not been possible by other means. However, there are significant risks associated with the potential side effects from the immunosuppression required to prevent rejection of the allotransplanted tissue. Some investigators have attempted to reduce the amount of immunosuppression and the concomitant side effects while still avoiding rejection by inducing a more immunologically permissive state. Based on some experimental and clinical success in organ transplantation, both recipient T-cell depletion and donor bone marrow infusion have been tried in composite tissue allotransplantation. However, neither approach has been effective in achieving a state in which less immunosuppression is required. One reason that these techniques may have been ineffective when inducing a more immunologically permissive state is that skin, which has previously been demonstrated to be the most susceptible tissue in a composite tissue allotransplant to rejection, is a component of many composite tissue allotransplants.1
One method for inducing a more immunologically permissive state that has been used in our laboratory combines partial T-cell depletion (with immunotoxin and low-dose irradiation) with a hematopoietic cell infusion and a very brief course of immunosuppression. A key element in the achievement of tolerance by this approach appears to be donor hematopoietic stem cell (HSC) engraftment in the recipient.2
In this study, we used partial T-cell depletion and low-dose irradiation with a hematopoietic cell infusion and a very brief course of immunosuppression to produce donor HSC engraftment in the recipient before allotransplanting skin from the hematopoietic cell donor. Both primarily vascularized skin flaps and secondarily vascularized conventional skin grafts were allotransplanted. These 2 modes of skin transplantation were used because previous work has suggested that the mode of transplantation may affect the susceptibility of skin to rejection.3 This has been supplemented by work we have carried out (Horner et al, in press, JPRAS) that indicates there are significant differences in the immune response depending on the method of skin transplantation.
METHODS
MGH MHC-defined miniature swine were used to control the MHC barrier for transplantation. There were 2 experimental groups (groups 1 and 2; see Table 1). Both groups underwent donor HSC engraftment induction. Group 1 (n = 2) received a conventional skin allograft from the HSC donor; group 2 (n = 2) received a primarily vascularized skin flap. Control groups received conventional skin allografts (n = 2; group 3) and primarily vascularized skin flaps (n = 2; group 4) without conditioning.
Table 1.
Experimental Groups and Skin Transplant Survival
| Group No. | Animal No. | Haplotype Mismatch | 1°/2° Skin Transplant† | HSC Engraftment | Skin Survival (Postoperative Day) |
|---|---|---|---|---|---|
| 1 | 13476 | CD→AD | 2° | Yes | Rejected (d55) |
| 16626 | AC→AD | 2°* | Yes | Rejected (d64) | |
| 2 | 17468 | AC→AD | 1° | Yes | Survival (>d46) |
| 17469 | AC→AD | 1° | Yes | Survival (>d300) | |
| 3 | 17476 | DD→AC | 2° | No | Rejected (d8) |
| 17506 | DD→AC | 2° | No | Rejected (d7) | |
| 4 | 17519 | AC→AD | 1° | No | Rejected (d6) |
| 17520 | AC→AD | 1°* | No | Rejected (d6) |
Topical 0.1% FK506 ointment applied daily in attempt to delay rejection.
1° refers to primarily vascularized skin flap; 2° refers to skin grafts that are secondarily vascularized after placement.
Donor and recipient animals differed by a single MHC haplotype. Donor animals were given pig stem cell factor and interleukin (IL)-3 to mobilize the bone marrow progenitor cells. The animals were then leukopheresed to collect the mobilized hematopoietic cells. Recipient animals received 100 cGy total body irradiation, T-cell depletion, and donor hematopoietic cell infusion, with a 45-day course of oral cyclosporine cover for the peri-infusion period. Animals received a subsequent donor leukocyte infusion with the aim of increasing the level of engraftment.
HSC engraftment was defined as presence of donor-derived bone marrow colony-forming units (CFUs) over 14 weeks following transplantation. Bone marrow was biopsied and cultured for CFUs. The presence of donor CFUs was detected using polymerase chain reaction (PCR) analysis and Southern blot confirmation with donor MHC specific primers.
Conventional split thickness skin allografts were harvested using a dermatome and secured to a freshly prepared dermal bed on the flank of the recipient in groups 1 and 3. Topical 0.1 % FK506 ointment was applied to some of the skin grafts (see Table 1). Skin flap allotransplants were harvested from the knee area on the hind limb of the donor, based on the sapheno-femoral vessels, with direct closure of the donor site. The skin flap was then placed on the jaw area of the recipient in groups 2 and 4, with end-to-end anastomosis to the internal carotid and jugular vessels.
Tolerance was defined as showing no evidence of rejection of the transplant at 90 days following transplantation and normal immune responses to nondonor alloantigens. Tolerance was assessed by direct observation, histologically, and in vitro by MLR and CML.
RESULTS
Survival of Conventional Donor Skin Allografts is Only Prolonged in HSC-Engrafted Animals, Even With Topical Immunosuppression
Two HSC-engrafted animals (group 1) transplanted across a single-haplotype MHC barrier received a conventional skin allograft from the original HSC donor. The transplanted skin survived significantly longer (t test: P < .01) in HSC-engrafted animals (D55, D64) than the control animals (D7, D8; group 3), but was eventually rejected.
One experimental and 1 control animal also received topical FK506 applied daily to the graft from the day of transplantation with only mild prolongation of the experimental animal’s skin allograft survival (rejection at day 64 vs day 55).
Tolerance to Primarily Vascularized Donor Skin Allotransplanted Flaps is Achieved in HSC-Engrafted Animals
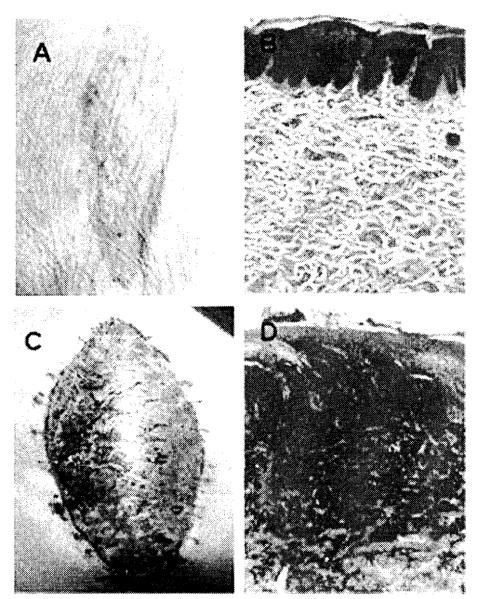
Two HSC-engrafted animals (group 2) received a primarily vascularized skin flap allograft transplanted across a single-haplotype MHC barrier from the HSC donor. Animal 17468 unfortunately died of an unrelated infection at day 46; at the time of death, there was no evidence of rejection macroscopically or histologically. Animal 17469 achieved tolerance to its skin flap allotransplant, with no evidence of rejection macroscopically and histologically (Fig 1A and 1B), and donor-specific tolerance on MLR (Fig 2) and CML at >300 days post– allotransplantation. In contrast, the control animals (group 4) rejected their skin flap allografts at 6 days posttransplantation (Fig 1C and 1D).
Fig 1.
Tolerance to a vascularized skin flap allotransplant following induction of donor HSC engraftment. Animal 17469 was tolerant to transplanted vascularized skin flap allotransplant across a MHC barrier following induction of donor HSC engraftment. At day 265 after skin flap transplantation there was no evidence of rejection macroscopically (A) and histological examination of a biopsy specimen revealed a viable skin flap with no inflammatory cell infiltrate (B). Animal 17519 received a vascularized skin flap allotransplant across a MHC barrier without prior conditioning. By day 6 there was evidence of full rejection of the flap both macroscopically (C) and on histological examination (D).
Fig 2.
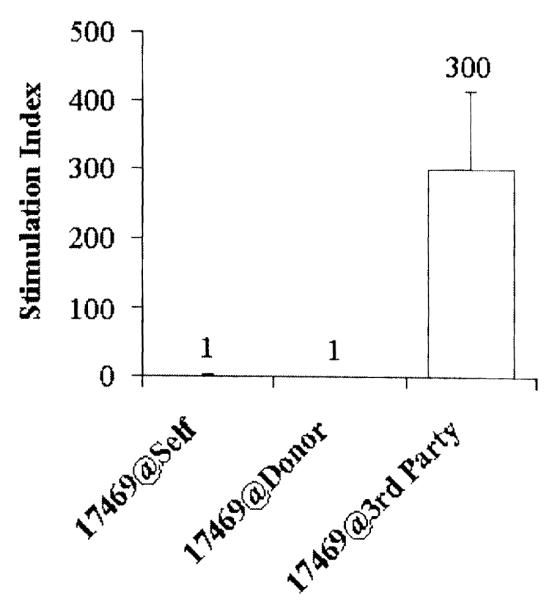
Tolerance was confirmed in vitro with donor-specific unresponsiveness on MLR.
Tolerance to a Skin Graft is Achieved if Placed Following Attainment of Primarily Vascularized Skin Flap Tolerance
Animal 17469 received a conventional skin graft allotransplant from the original HSC donor 124 days following skin flap allografting. Tolerance was not broken to the skin flap. Furthermore, tolerance was achieved to the skin graft, with no evidence of rejection at 200 days following transplantation.
DISCUSSION
Induction of clinical tolerance is the ultimate goal of composite tissue allotransplantation research. Since Medawar and his colleagues opened the field of modern transplantation with the induction of skin tolerance in a mouse model, several models have successfully achieved skin allograft tolerance in rodents.4 However, there has only been limited success in large animal models with tolerance achieved only across non-MHC minor-mismatched barriers,5-7 but not across a MHC barrier.
To our knowledge, this is the first time skin tolerance has been achieved across a MHC barrier in a large animal model. These findings suggest 3 principles. First, the achievement of skin tolerance only to a primarily vascularized skin flap allotransplant but not a conventional skin graft indicates that, via this approach, tolerance can be only be achieved if the skin is presented in an immunologically permissive way. Second, the achievement of tolerance also indicates that the role of putative skin-specific antigens is not as important as it has sometimes been assumed because they do not prevent the achievement of a tolerant state when a vascularized skin flap is transplanted. Third, the inability to break tolerance with a subsequently applied skin allograft indicates that once achieved, tolerance is quite stable.
Although the achievement of skin tolerance in a large animal model as described here is a significant step toward the goal of clinical skin tolerance induction, this approach is not directly clinically applicable. The delay in this protocol between induction of HSC engraftment and skin allotransplantation would not be possible clinically with a brain-dead donor. Possibilities to overcome this problem, including simultaneous HSC engraftment and transplantation, and delayed HSC engraftment, are currently being examined.
REFERENCES
- 1.Lee WP, Yaremchuk MJ, Pan YC, et al. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg. 1991;87:401. doi: 10.1097/00006534-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Horner BM, Cina RA, Wikiel KJ, et al. Predictors of organ allograft tolerance following hematopoietic cell transplantation. Am J Transplant. 2006;6:2894. doi: 10.1111/j.1600-6143.2006.01563.x. [DOI] [PubMed] [Google Scholar]
- 3.Steinmuller D. The enigma of skin allograft rejection. Transplant Rev. 1998;12:42. [Google Scholar]
- 4.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance to foreign cells. Nature. 1953;172:603. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 5.Tillson M, Niemeyer GP, Welch JA, et al. Hematopoietic chimerism induces renal and skin allograft tolerance in DLA-identical dogs. Exp Hematol. 2006;34:1759. doi: 10.1016/j.exphem.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Mathes DW, Randolph MA, Solari MG, et al. Split tolerance to a composite tissue allograft in a swine model. Transplantation. 2003;75:25. doi: 10.1097/00007890-200301150-00005. [DOI] [PubMed] [Google Scholar]
- 7.Yunusov MY, Kuhr CS, Georges GE, et al. Partial donor-specific tolerance to delayed skin grafts after rejection of hematopoietic cell graft. Transplantation. 2006;82:629. doi: 10.1097/01.tp.0000229449.09622.28. [DOI] [PubMed] [Google Scholar]