Abstract
Neuronal staining techniques have played a crucial role in the analysis of neuronal function. Several different staining techniques have been developed to allow morphological analyses of neurons. Recently diOlistic labeling, in which beads are coated with a lipophilic dye and then ballistically ejected onto brain tissue, has been developed as a useful and simple means to label neurons and glia in their entirety. Although diOlistic labeling provides detailed information on the morphology of neurons, combining this approach with other staining methods is a significant advance. We have developed protocols that result in high quality diOlistically- and retrogradely-labeled or diOlistically-immunohistochemically labeled neurons. These dual-label methods require modification of fixation parameters and the use of detergents for tissue permeabilization, and are readily applicable to a wide range of tracers and antibodies.
Keywords: ballistic, dendritic spine, DiI, diOlistic labeling, dual labeling, neuronal morphology, staining technique, tract tracing
Introduction
Neuronal labeling techniques have played a crucial role in the analysis of neuronal function and networks. More than 100 years ago Santiago Ramon y Cajal deduced several of the fundamental neuronal properties that have become the cornerstone of modern neuroscience based on his observations of the structure of Golgi-impregnated tissue. The Golgi methods have remained the gold standard for the detailed analysis of neuronal morphology over the last century. However, Golgi labeling has some drawbacks, including the black reaction product, which precludes the analysis of other neuronal markers at the light microscopic level. Moreover, analysis of dendritic spines in Golgi-impregnated neurons results in an underestimation of spine density because of the opacity of the dendritic shaft (Feldman and Peters, 1979).
More recently several different approaches to fluorescently label neurons have been developed. Intracellular fluorescent labeling of neurons can be achieved by microinjection of dyes or transfection of fluorescent proteins (Lo et al., 1994, Vecellio et al., 2000, Wallace and Bear, 2004). In addition, lipophilic carbocyanine dyes efficiently label neuronal surfaces (Gan et al., 2000). Confocal microscopic analysis of fluorescently-labeled neurons has improved resolution of dendritic morphology and has been suggested to provide a more accurate quantification of dendritic spines (Gan et al., 2000, Vecellio et al., 2000, Wallace and Bear, 2004). In addition, these fluorescent labeling methods have the advantage that they can be combined with other fluorescent staining methods for phenotypic characterization of the labeled neurons. However, the carboyanine dyes used in diOlistic labeling (“biolistic labeling with fluorophores”) are lipophilic, making it difficult to combine immunohistochemical and diOlistic labeling because of the need for detergents in most immunohistochemical procedures (Elberger and Honig, 1990, Holmqvist et al., 1992).
In order to increase the utility of the diOlistic labeling method, we have developed protocols for combining diOlistic labeling with two other staining methods used to phenotypically characterize neurons. We describe a protocol for dual diOlistic-immunohistochemical labeling and for combining diOlistic labeling with retrograde tracers to characterize the morphology of neurons based on their projection targets.
Materials and Methods
Animals
Adult male and pregnant female Sprague-Dawley rats (Harlan; Indianapolis, IN) were housed on a 12:12 light:dark cycle with food and water available ad libitum. All experiments were conducted in accordance with the National Institutes for Health Guide for the Care and Use of Laboratory Animals and under the oversight of the institutional Animal Care and Use Committees.
Retrograde labeling
Male rats were deeply anesthetized with isoflurane (Henry Schein, Melville, NY) and placed in a stereotaxic frame. A microsyringe was used to pressure inject undiluted green fluorescent latex microspheres (FLMs; Lumafluor Corp., Naples, FL) into several different brain sites, including the ventral tegmental area, substantia nigra, striatum, and medial dorsal thalamic nucleus. Three to seven days later the rats were transcardially perfused with 0.1 M phosphate buffer (23 mM NaH2PO4·H2O, 77 mM Na2HPO4, pH 7.4) followed by either 1.5% or 4% paraformaldehyde in the same buffer. Brains were removed and postfixed for 30 min in the same fixative, and 150 m coronal sections of brain regions containing the retrogradely labeled neuronal somata were cut on a vibrating microtome and collected into PBS for diOlistic labeling (see below). Perfusions and fixations were performed in the absence of sodium chloride and at room temperature, since both salt and hypothermia result in the loss of dendritic spines (Kirov et al., 2004). In a limited number of animals we used the retrograde tracer fluorogold (FG) (Fluorochrome, Denver, CO); in these cases a 5% FG solution was pressure injected into the striatum, the mediodorsal thalamus, or the basolateral amygdala. Animals were perfused 14-21 days later and brain sections diOlistically labeled as described below.
Preparation of organotypic slice cultures
Organotypic slice cultures were prepared from brains of P0 to P2 rat pups according to a modification of the method of Stoppini et al., 1991, as we have described previously (Neely et al., 2007). Cultures were maintained in an incubator at 37°C and 5% CO2 for 4 weeks and the media changed three times weekly. Immediately prior to diOlistic labeling (see below) cultures were fixed with 1.5% or 4% paraformaldehyde in 0.1 M phosphate buffer for 25 min at room temperature and washed in PBS.
Diolistic labeling
All diOlistic labeling was performed immediately after fixation (Gan et al., 2000, Neely et al., 2007). CM-DiI coated tungsten particles (1.3 μm in diameter; BioRad, Hercules, CA) were prepared following the manufacturer's instructions. Briefly, 3 mg of CM-DiI (Invitrogen, Carlsbad, CA) were dissolved into 300 μl of methylene chloride and the solution used to coat tungsten particles. After evaporation of the methylene chloride the coated particles were suspended in 3 ml H2O, sonicated, and introduced into Tefzel tubing (BioRad). CM-DiI-coated tungsten particles were ejected with a gene gun (BioRad; Hercules, CA) that was fitted with a barrel described by O'Brien and collaborators, 2001. A 3.0 μm pore size cell culture insert (Fisher, Pittsburg, PA) was mounted at the tip of the barrel to prevent large aggregates of labeled beads from reaching the tissue. The gene gun was held ∼ 0.5 cm above the brain sections or organotypic cultures and the particles delivered at pressures ranging between 60-140 psi. After diOlistic labeling the tissue was stored in 0.04% paraformaldehyde in phosphate buffered saline for two nights at room temperature in the dark to allow the dye to diffuse throughout the dendritic tree, after which it was postfixed in 4% paraformaldehyde in 0.1 M phosphate buffer for three hours at room temperature and then either mounted in Prolong (Invitrogen) or processed for immunohistochemistry (see below). We used CM-DiI rather than DiI because in pilot experiments we found that CM-DiI extended the time period over which neuronal morphology could be analyzed to at least four weeks, whereas tissue labeled with DiI was best analyzed within several days of labeling.
Combination of diOlistic labeling and immunohistochemistry
Unless otherwise noted diOlistically-labeled tissue was incubated with Tris-buffered saline (TBS) containing 4% horse serum and 0.05% Triton X-100 for 10 min, washed and incubated with one of a number of different antibodies [anti-synaptophysin, Millipore, Temecula, CA; anti-syntaxin, Sigma-Aldrich Co., St. Louis, MO; anti-PSD95, Millipore; anti-vesicular glutamate transporter 1 (VGlut1), MAb-Technologies, Stone Mountain, GA; anti-tyrosine hydroxylase (TH), ImmunoStar, Hudson, WI; anti-parvalbumin, Sigma-Aldrich Co.]; we focused our studies using mouse anti-TH (1:250) or rabbit anti-VGluT1 (1:4000). Sections or cultures were incubated in primary antibodies in TBS containing 4% normal horse serum but without any detergent (e.g., Triton X-100) for two nights at 4°C in the dark. This was followed by incubation in Alexa 488-conjugated secondary antibodies (1:250; Invitrogen) for two nights at 4°C in TBS containing 4% normal horse serum, and then the tissue was mounted in ProLong.
Z-stacks of dendritic segments were acquired with a 63×1.4 NA objective (2.5-4 zoom factor) at 0.5 μm intervals using a LSM Meta confocal laser scanning microscopy system (Carl Zeiss). 3D surface rendering was performed with the Surpass module of Imaris software package (Version 5.5, Bitplane, Saint Paul, MN). Colocalization of signals from diOlistic labeling and immunocytochemistry was determined using the Coloc module of the same software.
Results
Optimization of diOlistic labeling of rat brain sections and organotypic rat brain cultures
We found that the degree of fixation had a major impact on the quality of diOlistic staining. Thus, perfusion with 1.5% paraformaldehyde with a 30 min postfixation in the same fixative proved optimal for rat brain sections. Optimal staining in organotypic cultures was obtained with a 25 min fixation in 1.5 % paraformaldehyde. In contrast, diOlistic labeling of tissue that had been fixed with 4% paraformaldehyde typically resulted in incompletely labeled neuronal processes, with an abundance of DiI-labeled dendritic fragments rather than continuous dendrites (data not shown). In addition, we observed that labeling of tissue that had been stored after fixation but before diOlistic labeling, even for short durations (hours), yielded incomplete labeling of the dendritic trees and increased labeling of glia.
Combination of diOlistic labeling and retrograde tracing
Combining retrograde tracing with FLMs and diOlistic labeling yielded high quality double-labeling (Fig. 1). Cortical neurons retrogradely labeled from the striatum were clearly identified by punctuate green fluorescent staining of the soma (Fig. 1, arrow). Other double-labelled neurons assessed included MSNs retrogradely labelled from the substantia nigra and prefrontal cortex pyramidal cells retrogradely labeled from the ventral tegmental area. We did not observe significant differences in dual diOlistic labeling–retrograde tracing across different brain sites. In addition to FLMs, we also double-labeled diOlistically stained neurons with the retrograde tracer FG. Although the quality of retrograde labeling and number of cells labeled with FG was comparable to the tracing with FLMs, the use of this tracer requires a confocal microscope equipped with a UV laser.
Figure 1. Combination of diolistic labeling with retrograde labeling.
Cortical neurons were retrogradely labeled by injection of green FLMs (green) into the dorsal striatum and diOlistically labeled with CM-DiI (red). Confocal z-stacks of cortical tissue (1-5) were acquired and a projection image rendered (P). One pyramidal cell double labeled for the retrograde tracer (green) and CM-DiI (red) is indicated by an arrow. Scale bar = 20 μm.
Combining diOlistic labeling and immunofluorescence
In preliminary observations we observed that immunocytochemical staining of tissue that was not treated with detergents to permeablize the membrane was possible for some but not all antibodies that we tested. We found that incubation with 0.05% Triton X-100 for 10 min had no effect on the CM-DiI labeling of neurons, but that higher concentrations (0.1 %) of the same detergent resulted in such intensification of the CM-DiI signal in dendritic spines that the morphology of spines was obscured, appearing more “blob-like” than mature spiny protrusions, while the labeling of the dendritic shaft appeared unaffected. Concentrations of Triton X-100 greater than 0.1 %, or detergent incubation times longer than 10 min resulted in a general loss of the CM-DiI signal from the whole surface of the neurons.
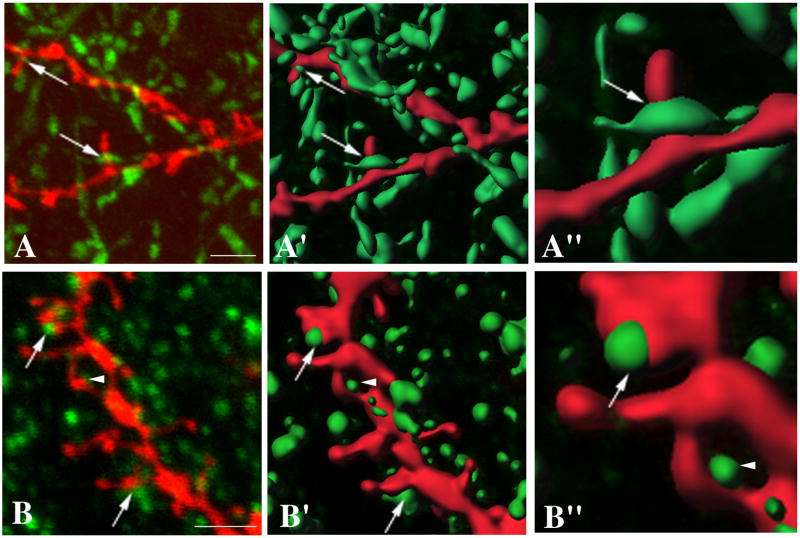
Using a 10 min incubation in 0.05% Triton X-100 treatment, we dual-stained organotypic slice cultures comprised of cortex, striatum, and substantia nigra to assess the relationship between dopaminergic (TH-ir) and glutamatergic (VGluT1-ir) afferents and diOlistically-labeled MSN dendrites. We observed frequent apposition of TH-ir axons and the necks of dendritic spines (Fig. 2A) and colocalization of fluorescent signals from VGluT1-ir terminals and CM-DiI labeled spine heads (Fig. 2B). Using the same protocol we also succeeded to immunostain diOlistically labeled tissue for syntaxin, synaptophysin, PSD-95 and were able to identify diOlistically labeled parvalbumin-ir cortical inteneurons (data not shown).
Figure 2. Combination of diolistic labeling with immunofluorescence.
A. MSNs in the striatum of triple organotypic cultures were diOlistically labeled with CM-DiI (red) and the TH-fibers visualized by immunofluorescence (green). Areas of apposition between TH-ir afferents and the necks of MSN spines (arrows) were determined using Bitplane software. B. Organotypic triple cultures were labeled with CM-DiI (red) and immunocytochemically stained using anti-VGluT1 antibodies (green). Areas of apposition between MSNs dendritic spine heads and VGluT1-ir terminals were determined using Bitplane software (arrows). A VGluT1-ir presynaptic terminal that appeared close, but did not colocalize with the CM-DiI labeled dendritic spine is indicated by an arrow head. A′,B′, A″, B″. Three-dimensional reconstructions are rendered using Bitplane software. Scale bar = 2.5 μm in A,A′ and B,B′. A″ and B″ show 2.5-fold magnified examples of TH-ir and VGlut1-ir appositions with CM-diI labeled MSNs spines respectively.
Discussion
Optimization of diOlistic labeling protocol
DiOlistic labeling allows for the staining of many neurons in live or fixed tissue of young and adult animals, including postmortem human brain (Gan et al., 2000, Grutzendler et al., 2003), and has gained widespread use for the assessment of neuronal morphology due to the efficiency of the procedure and the high quality of neuronal labeling. However, the usefulness of diOlistic labeling is limited to some degree by the lipophilic nature of the carbocyanine dyes. In particular, combining diOlistic labeling with immunohistochemical staining has proven difficult (Elberger and Honig, 1990, Holmqvist et al., 1992).
We found that mild fixation optimized diOlistic labeling of neurons, while higher concentrations of aldehydes or longer fixation times compromised the quality of neuronal labeling. We observed this effect of fixation on the quality of diOlistic labeling across different regions of adult rat, mouse, and rabbit brain as well as in organotypic cultures. Similar findings on the use of fixatives have been described for labeling of rat cortex (Kim et al., 2007), while other investigators have reported that for mice and human tissue short duration fixation with 4% paraformaldehyde yields best results (Gan et al., 2000, Grutzendler et al., 2003). These somewhat conflicting reports suggest that one must avoid overfixation by modifying either the duration of exposure to fixatives or by decreasing the concentration of the fixative, or both. We also found that storage of fixed tissue for even just a few hours at 4°C before diOlistic labeling decreases the quality of diOlistic labeling.
Combination of diOlistic labeling and neuronal retrograde tracing
DiOlistic labeling provides detailed information concerning the morphology of neurons, but additional information concerning the phenotype of the labeled cells is often of considerable value. For example, cortical pyramidal cells that give rise to subcortical projections differ markedly in their projection targets, which in turn can correlate with their morphology or firing patterns (Reiner et al., 2003, Gao and Zheng, 2004, Hattox and Nelson, 2007).
To expand the uses of diOlistic labeling we developed a method that combines retrograde tracing and diOlistic labeling. To the best of our knowledge, this is the first report of such double labeling. We examined both FLMs and FG as retrograde tracers for use in combination with diOlistic labeling. While the two tracers yielded similar results, we favor FMLs because of the distinct punctuate labeling of FLMs and their resistance to photobleaching; in contrast, FG photobleaches quickly. The emission wavelength of green FLMs shows no overlap with that of CM-DiI. Moreover, the examination of green FLM-labeled cells is done with a conventional argon laser of a confocal microscope, while FG requires a UV laser, which is not standard on most confocal miscroscopes. Although FLMs must be deposited by pressure injections, while FG can be iontophoretically deposited to yield very small injection sites, FLMs do not migrate far from the injection site and typically result in small discrete deposits. We have not examined the usefulness of other retrograde tracers for combination with diOlistic labeling, such as fluorophore-conjugated cholera toxin B or horseradish peroxidase. However, we see no reason that similar approaches cannot be combined with other fluorescent tracers, including additional retrograde as well as anterograde tract tracers.
Combination of diOlistic labeling and immunocytochemistry
The most common phenotypic characterization of neurons is based on defining a cell's expression of various proteins and peptides. Previous ultrastructural studies have documented a characteristic relationship between the spines of striatal MSNs and two different afferents to these striatal neurons (Bouyer et al., 1984, Freund et al., 1984). We determined if the relationship between these neuronal elements was recapitulated in organotypic slice co-cultures, using diOlistic labeling to reveal the spines of MSN dendrites, and immunohistochemistry to show afferents derived from the cerebral cortex (VGluT1-ir) and substantia nigra (TH-ir).
We first had to overcome the limitations imposed by diOlistic labeling for subsequent immunohistochemical staining. Early attempts to combine diOlistic labeling with immunohistochemistry led to the conclusion that the method was not feasible except in cases in which the antibody did not require the use of detergents (Elberger and Honig, 1990, Holmqvist et al., 1992, Grutzendler et al., 2003, Moolman et al., 2004). More recently, Lee et al. (2006) combined diOlistic labeling with immunohistochemical detection of GFP in BAC transgenic mice, and determined that diOlistic labeling was retained if sections were incubated in a low (0.01%) concentration of Triton X-100.
In our hands incubation with 0.01 % Triton X-100 did not yield sufficient permeabilization to optimize the quality of staining for some of the antibodies we examined. In the quest to determine optimal permeabilization conditions we observed that the use of CM-DiI rather than DiI permitted the use of somewhat greater concentrations of detergent. Therefore, in addition to limiting the concentration of the fixative, we systematically varied the Triton X-100 concentration and duration of exposure, and found that incubation of diOlistically-labeled tissue with 0.05% Triton X-100 for 10 min or less did not significantly change the quality of CM-DiI stained dendrites. This was observed in both cortical and striatal sites and is therefore probably independent of the brain region analyzed. We did not explore other detergents, such as digitonin, Nonidet P40, or Tween 20 (Kozorovitskiy et al., 2006, Matsubayashi et al., 2008).
Certain antibodies we used (such as the mouse anti-TH) did not require any membrane permeabilization for optimal staining, but several other antibodies (including VGluT1) produced poor staining in the absence of detergents. We were able to compensate for the limited membrane permeabilization to some degree by increasing the antibody concentrations and incubation times that are normally employed when detergent use is not limited. These observations underscore the need to optimize conditions for each antibody used in diOlistic-immunohistochemical double labeling. By optimizing conditions and adhering to the fixation and membrane permeabilization conditions noted above, we were able to determine that in organotypic cultures glutamatergic and dopaminergic terminals form appositions on MSN spine head and neck, respectively, as is the case in vivo (Bouyer et al., 1984, Freund et al., 1984). The application of recently reported methods for quantification of dendritic spines and appositions between spines and presynaptic terminals further enhances the power of diOlistic-immunocytochemical double labeling (Shen et al., 2008, Wierenga et al., 2008). The high degree of resolution afforded by the procedure we have outlined is a significant improvement over previous reports on combined diOlistic-immunohistochemical labeling.
Conclusions
DiOlistic labeling can be combined with retrograde tract tracing or immunohistochemical localization, thereby significantly expanding the utility of the method. The ability to perform such dual staining allows investigators to phenotypically characterize diOlistically-labeled cells. The methods described are simple and do not significantly increase either costs or effort, and most importantly do not compromise the quality of staining obtained using diOlistic labeling, retrograde tract tracing, or immunohistochemistry alone.
Acknowledgments
This work was supported by National Institutes of Health [R01 MH077298 (to AYD) and PO1 NIH NS44282 (to MDN and AYD)] and the National Parkinson Foundation Center of Excellence at Vanderbilt. We thank Dr. Pat Levitt (Vanderbilt University) and his laboratory for intellectual and technical support. Imaging costs were subsidized through NICHD core grant P30HD15052 to the Vanderbilt Kennedy Center. Dr. Karl Kandler (Univ. of Pittsburgh) also provided invaluable advice and assistance to GDS in establishing this method. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institute of Neurological Disorders and Stroke of the National Institutes of Health, or the National Parkinson Foundation.
Abbreviations
- FLMs
fluorescent latex microspheres
- FG
Fluorogold
- -ir
immunoreactive
- MSN
medium spiny neuron
- TH
tyrosine hydroxylase
- VGluT1
vesicular glutamate transporter 1
- TBS
Tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M. Diana Neely, Department of Psychiatry and Vanderbilt Kennedy Center for Research on Human Development, 3066 VPH, Vanderbilt University Medical Center, 1601 23rd Avenue South, Nashville, TN 37212, (615) 322 4260, (615) 322 1901 (fax).
Gregg D Stanwood, Email: gregg.stanwood@vanderbilt.edu, Department of Pharmacology and Vanderbilt Kennedy Center for Research on Human Development, 8405 MRBIV, Vanderbilt University Medical Center, Nashville, TN 37232, (615) 936-3861, (615) 936-2202 (fax)
Ariel Y. Deutch, Email: ariel.deutch@vanderbilt.edu, Department of Psychiatry, Pharmacology and Vanderbilt Kennedy Center for Research on Human Development, 3066 VPH, Vanderbilt University Medical Center, 1601 23rd Avenue South, Nashville, TN 37212, (615) 327 7080, (615) 322 1901 (fax).
References
- Bouyer JJ, Park DH, Joh TH, Pickel VM. Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res. 1984;302:267–275. doi: 10.1016/0006-8993(84)90239-7. [DOI] [PubMed] [Google Scholar]
- Elberger AJ, Honig MG. Double-labeling of tissue containing the carbocyanine dye DiI for immunocytochemistry. J Histochem Cytochem. 1990;38:735–739. doi: 10.1177/38.5.2110209. [DOI] [PubMed] [Google Scholar]
- Feldman ML, Peters A. A technique for estimating total spine numbers on Golgi-impregnated dendrites. J Comp Neurol. 1979;188:527–542. doi: 10.1002/cne.901880403. [DOI] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron. 2000;27:219–225. doi: 10.1016/s0896-6273(00)00031-3. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Zheng ZH. Target-specific differences in somatodendritic morphology of layer V pyramidal neurons in rat motor cortex. J Comp Neurol. 2004;476:174–185. doi: 10.1002/cne.20224. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Tsai J, Gan WB. Rapid labeling of neuronal populations by ballistic delivery of fluorescent dyes. Methods. 2003;30:79–85. doi: 10.1016/s1046-2023(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- Holmqvist BI, Ostholm T, Ekstrom P. DiI tracing in combination with immunocytochemistry for analysis of connectivities and chemoarchitectonics of specific neural systems in a teleost, the Atlantic salmon. J Neurosci Methods. 1992;42:45–63. doi: 10.1016/0165-0270(92)90134-y. [DOI] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Labeling of dendritic spines with the carbocyanine dye DiI for confocal microscopic imaging in lightly fixed cortical slices. J Neurosci Methods. 2007;162:237–243. doi: 10.1016/j.jneumeth.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SA, Petrak LJ, Fiala JC, Harris KM. Dendritic spines disappear with chilling but proliferate excessively upon rewarming of mature hippocampus. Neuroscience. 2004;127:69–80. doi: 10.1016/j.neuroscience.2004.04.053. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo DC, McAllister AK, Katz LC. Neuronal transfection in brain slices using particle-mediated gene transfer. Neuron. 1994;13:1263–1268. doi: 10.1016/0896-6273(94)90412-x. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Iwai L, Kawasaki H. Fluorescent double-labeling with carbocyanine neuronal tracing and immunohistochemistry using a cholesterol-specific detergent digitonin. J Neurosci Methods. 2008;174:71–81. doi: 10.1016/j.jneumeth.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML. Dendrite and dendritic spine alterations in Alzheimer models. J Neurocytol. 2004;33:377–387. doi: 10.1023/B:NEUR.0000044197.83514.64. [DOI] [PubMed] [Google Scholar]
- Neely MD, Schmidt DE, Deutch AY. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neuroscience. 2007;149:457–464. doi: 10.1016/j.neuroscience.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Holt M, Whiteside G, Lummis SCR, Hastings MH. Modifications to the hand-held Gene Gun: improvements for in vitro Biolistic transfection of organotypic neuronal tissue. J Neurosci Methods. 2001;112:57–64. doi: 10.1016/s0165-0270(01)00457-5. [DOI] [PubMed] [Google Scholar]
- Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei WL. Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intrastriatal terminals in rats. J Comp Neurol. 2003;457:420–440. doi: 10.1002/cne.10541. [DOI] [PubMed] [Google Scholar]
- Shen H, Sesack S, Toda S, Kalivas P. Automated quantification of dendritic spine density and spine head diameter in medium spiny neurons of the nucleus accumbens. Brain Struct Funct. 2008;213:149–157. doi: 10.1007/s00429-008-0184-2. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Vecellio M, Schwaller B, Meyer M, Hunziker W, Celio MR. Alterations in Purkinje cell spines of calbindin D-28 k and parvalbumin knock-out mice. Eur J Neurosci. 2000;12:945–954. doi: 10.1046/j.1460-9568.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- Wallace W, Bear MF. A Morphological Correlate of Synaptic Scaling in Visual Cortex. J Neurosci. 2004;24:6928–6938. doi: 10.1523/JNEUROSCI.1110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Becker N, Bonhoeffer T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat Neurosci. 2008;11:1044–1052. doi: 10.1038/nn.2180. [DOI] [PubMed] [Google Scholar]