Abstract
In utero hematopoietic cell transplantation (IUHCT) has been shown in the murine model to achieve low levels of allogeneic chimerism and associated donor specific tolerance permissive for minimal conditioning postnatal hematopoietic stem cell transplants (HSCT). In this pilot study, we investigate IUHCT in the canine leukocyte adhesion deficiency (CLAD) model. Haploidentical IUHCT resulted in stable low level donor cell chimerism in all dogs that could be analyzed by sensitive detection methodology (4 of 10) through 18 months of follow up. In the two CLAD recipients, low level chimerism resulted in amelioration and complete reversal of the CLAD phenotype respectively. Six recipients of IUHCT (5 carriers and 1 CLAD) subsequently received postnatal HSCT from the same haploidentical prenatal donor following minimal conditioning with 10 mg/kg Busulfan. Chimerism in 2 of 5 CLAD carriers that received HSCT increased from <1% pre-HSCT to sustained levels of 35 – 45%. Control animals receiving postnatal haploidentical HSCT without IUHCT had no detectable donor chimerism. These results demonstrate that haploidentical IUHCT in the CLAD model; 1) can result in low level donor chimerism that in CLAD dogs can prevent the lethal phenotype; and 2) can result in donor specific tolerance that can facilitate postnatal minimal conditioning HSCT.
Keywords: in utero transplantation, fetus, canine, leukocyte adhesion deficiency, minimal conditioning transplant
Introduction
In utero hematopoietic cell transplantation (IUHCT) is a non-myeloablative approach that can result in low-level mixed hematopoietic chimerism with associated donor specific tolerance.1-5 We have previously shown in the murine model that donor specific tolerance (DST) created by IUHCT can facilitate postnatal enhancement of donor chimerism using a variety of minimal conditioning regimens.2,5,6 This represents a potential clinical strategy for the treatment of disorders that can be prenatally diagnosed, and can be cured by postnatal hematopoietic stem cell transplantation (HSCT). In this study, we hypothesized that a canine model of IUHCT could be developed that would be useful in the pre-clinical translation of strategies developed in the murine model.
The canine model has been used extensively in the pre-clinical testing of postnatal HSCT regimens and has been a reliable predictor of clinical results.7,8 For instance, many strategies to prevent or treat GVHD were first evaluated in the canine model prior to their use in humans.7-13 The canine model also offers biologic and practical advantages specific to the evaluation of IUHCT. The ontogeny of the canine immune system appears relatively similar to that of humans14, and from a technical perspective, the canine pregnancy allows ultrasound guided injection of pups prior to immune maturation.15-17 Finally, the canine model offers the advantage of the availability of disease models that are analogous to human disorders.18-22 One such disease model that we utilize in this study is canine leukocyte adhesion deficiency (CLAD), an autosomal recessive genetic immunodeficiency disease that is the canine equivalent of human leukocyte adhesion deficiency (LAD)-1. Both LAD-1 and CLAD result from mutations in the leukocyte integrin CD18 which ablate the ability of leukocytes to adhere to the vessel wall and migrate to sites of infection.23-25 As a result, children and dogs with LAD suffer recurrent life threatening bacterial infections.26 In the absence of successful HSCT, CLAD dogs have a 100% mortality by 6 months of age.27 However, recent studies have demonstrated that even low levels of donor cell engraftment following minimal conditioning matched littermate HSCT can reverse the lethal disease phenotype in CLAD.24,27-29
In this study, we report our initial experience with IUHCT in the CLAD model. We conclude that IUHCT can result in stable low level donor cell engraftment without evidence of GVHD in both CLAD and CLAD carrier dogs. Furthermore IUHCT can induce DST in the canine model with the subsequent ability to enhance donor chimerism in engrafted animals by postnatal minimal conditioning HSCT from the same donor.
Materials and Methods
Animals
Dogs were housed in the National Institutes of Health (NIH) facilities in Bethesda, MD, and Poolesville, MD, in accordance with NIH guidelines and in the Laboratory Animal Facility of the Abramson Research Center at The Children's Hospital of Philadelphia. These facilities are approved by the American Association for Accreditation of Laboratory Animal Care (AAALAC). The experimental protocols were approved by the Institutional Animal Care and Use Committee at The Children's Hospital of Philadelphia and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Breeding
In all cases male or female CLAD carrier animals were bred to reproductively intact CLAD animals that had previously received minimal conditioning HSC from a DLA-matched littermate donor as previously described.30
BM harvest and CD34+ cell enrichment
Prior to IUHCT or HSCT, BM was harvested from parental CLAD carrier donors and CD34 enriched as previously described.24,28 Since BM was harvested from the CLAD carrier parent, either the mother or the father, for both in utero and postnatal transplantations, the donor cells expressed the CD18/CD11 cell surface antigen. In all cases, the donor for postnatal HSCT in recipients of a previous IUHCT was the same as the initial donor for the in utero transplantation.
CD34+ cell enrichment was performed using the anti-canine CD34 antibody 1H6 (Fred Hutchinson Cancer Research Center, Seattle, WA), anti-mouse IgG1 microbeads and the AutoMACS sorting system (Miltenyi Biotec, Auburn, CA).31
CD26 inhibition
The three amino acid peptide Diprotin A (Peptides International, Louisville, KY) was used to inhibit CD26 activity on donor cells prior to IUHCT, postnatal HSCT or in vitro migration assays. CD34+ enriched cells resuspended in ACD-A solution were incubated with 5mM Diprotin A at room temperature for 15 minutes. After incubation, the cells were washed once (centrifuge @ 400g × 20 minutes) in ACD-A solution. The cells were recounted and either used for an in vitro migration assay or combined with the CD34- cell population as described below prior to transplantation.
In utero hematopoietic cell transplantation
Prior to undergoing an IUHCT, all pregnant dogs underwent trans-abdominal ultrasound (Acuson Sequoia Ultrasound, Siemens, Malvern, PA) to confirm pregnancy and determine the number of fetuses. After BM harvesting and cellular processing as described above, the CD34+ enriched cell population and CD34- cell population were assessed for CD3 composition by flow cytometry. A portion of the CD34- cell population was then added back to the entire CD34+ population such that 1.4-2.4% of the final donor cell inoculums were CD3+ cells. Prior to injection, the final donor inoculums were re-suspended in normal saline with 1% heat inactivated autologous donor serum.
Gestational age was determined by retrograde calculation assuming birth on gestational day 63. Briefly, after induction of general anesthesia and sterile abdominal preparation, a transabdominal ultrasound using a 7 mHz echocardiography probe was used to identify the fetuses and their peritoneal cavities. A 22 gauge, 3.5 inch, amniocentesis needle (Becton Dickenson, Franklin Lakes, NJ) was used to inject the donor cells into the fetal peritoneum in a total volume of 200μL. The amniocentesis needle was withdrawn out of the fetal peritoneum and was then used to inject 10μg of Vancomycin antibiotic in 200μL of normal saline into the uterine cavity. A repeat ultrasound was performed after all fetuses had been injected to confirm post procedure fetal viability.
Postnatal hematopoietic cell transplantation
Six recipients of IUHCT subsequently underwent a haploidentical postnatal HSCT with minor modifications from a minimal conditioning regimen described previously for DLA-matched littermate HCT.27 The parent used as the prenatal donor was also used as the postnatal donor. Control dogs received postnatal haploidentical parental BM transplants using the same conditioning regimen without IUHCT. The minimal conditioning regimen consisted of Busulfan (Busulfex; ESP Pharma, Edison, NJ) 10 mg/kg given over 2 hours on day -2. On the day prior to transplant (day -1), BM was harvested and processed as described above from the parental donor. Following isolation of the CD34+ enriched and CD34- cell populations, the CD34- cell population was combined with the entire CD34+ cell population such that the final donor cell inoculum contained no more than 5.5 × 106 CD3+ cells per kg recipient weight. Prior to recombining the two cell populations the CD34+ enriched population was subjected to CD26 inhibition as described above. To reduce the risk of GVHD, recipients were given a shortened course of immunosuppression post transplant consisting of 1) Cyclosporine A (CsA, Sandimmune; Novartis, East Hanover, NJ) 15 mg/kg BID from the night of day 0 to day 35, 7.5 mg/kg BID from day 36 to day 52, 5 mg/kg BID from day 53 to day 57 and 2) Mycophenolic Acid Mofetil (MMF, Cellcept; Roche, Nutley, NJ) 10 mg/kg BID from the night of day 0 to day 28.
Flow cytometry
The percentage and number of cells in the BM harvest expressing the CD34 antigen and the CD3 antigen were determined by flow cytometry prior to IUHCT or postnatal BM transplants. In two separate reactions, 1 × 106 BM cells were stained with a phycoerythrin (PE)-conjugated anti-canine CD34 antibody (1H6) (Pharmingen, San Diego, CA) and a PE-conjugated anti-canine CD3 antibody (Serotec, Raleigh, NC) and subsequently analyzed by flow cytometry on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ).
Peripheral blood (PB) of recipients of IUHCT was analyzed by flow cytometry at 1 month of age for the expression of the CD18 antigen to determine if they were CLAD carriers (CD18+) or CLAD affected dogs. Cells were stained with anti-CD18 mAb as previously described.32 CLAD dogs underwent monthly CD18 PB analysis up to 18 months of age.
DNA chimerism analysis using SRY PCR and SRY TaqMan Real Time PCR
Donor cell engraftment was assessed in female recipients of male donors by polymerase chain reaction (PCR) for the male specific SRY gene as previously described.33 Briefly, DNA was extracted from the PB and amplified with SRY specific primers (Forward: 5′-CTCGCGATCAAAGGCGCAAGAT-3′; Reverse: 5′-TTCGGCTTCTGTAAGCATTTTC-3′) on an Eppendorf Mastercycler (Eppendorf, Hamburg, Germany) by denaturing for 94°C for 1 minute 30 seconds followed by 35 cycles of 94°C for 15 seconds, 58°C for 15 seconds, 72°C for 20 seconds followed by a final extension of 72°C for 10 minutes. Products were run on a 1.5% agarose gel and visualized by ethidium bromide staining.
Chimerism in female recipients of male donor BM was quantified using a TaqMan SRY Q-PCR protocol on an Applied Biosystems 7500 real time PCR machine as previously described.34 Briefly, triplicate reactions were prepared using 2X real-time PCR master mix, forward primer: CCCCATGAACGCATTCTTG, reverse primer: CTGATCTCTGAGTTTTGCATTTGG, and 5′ FAM conjugated probe: TCTCGCGATCAAAGG (ABI, Foster City, CA). Experimental samples were quantified by comparison to a standard curve derived from mixing known quantities of male with female DNA. In constructing the standard curve, the assay was determined to be sensitive enough to detect 0.01% male DNA in female DNA.
DNA chimerism analysis using PCR for DNA microsatellite repeat markers
Chimerism in CLAD carriers who had received a BM transplant (either IUHCT or a postnatal BM transplant) from a female donor or male recipients of a male donor could not be assessed by flow cytometry for the CD18 antigen or by PCR for the SRY gene. In these recipients, the percentage of donor chimerism in peripheral blood leukocytes was determined by using DNA microsatellite repeat markers that distinguished donor and recipient DNA contribution as previously described.29
CLAD clinical phenotype analysis
CLAD dogs were monitored on a daily basis for evidence of the CLAD phenotype beginning at 3 weeks of life until the end of the study. Seven distinct clinical areas (Temperature, Comfort, Movement, Appearance, Behavior, Interactive Behavior, Vocalization) were evaluated and ranked on a scale of zero to four with the sum of the scores representing the clinical score.
Mixed lymphocyte reaction
In vitro mixed lymphocyte reactions (MLR) were performed to assess the reactivity of recipient peripheral blood mononuclear cells to donor peripheral blood mononuclear cells. Briefly, PB was diluted in clinical grade normal saline and layered over NycoPrep 1.077A (Griener Bio-Onc Inc, Longwood, FL). Cells were centrifuged at 800g for 30 minutes at room temperature. The mononuclear cell layer was harvested, washed twice (centrifuge @ 400g × 15 minutes @ 4°C) with PBS with 0.1% BSA and the cells were subsequently counted. Cells to be used as stimulators were irradiated at 20Gy and cells to be used as responders were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) as per the manufactures instructions. Irradiated stimulator and CFSE labeled responder cells were resuspended in RPMI 1640 with 10% heat inactivated fetal calf serum (FCS) and 1000μL 2-mercaptoethanol at a concentration of 2 × 105 cells per 200μL. Responder and stimulator cells were mixed at a 1:1 ratio in 96 well tissue culture plates in a total volume of 400μL per well. Cells were incubated at 37°C with 5% CO2 and harvested 5 and 7 days after initial mixing for analysis of responder cell division by flow cytometry for CFSE as previously described.35
In vitro migration of BM cells with or without CD26 inhibition
Chemotaxis assays were performed on 24-well Transwell plates (6.5 mm diameter, 5 μm pore size polycarbonate membrane) (Costar, Cambridge, MA) to assess the effect of CD26 inhibition on SDF-1α induced migration as previously described with minor modifications.36 BM cells were harvested with or without CD34+ cell enrichment and either received CD26 inhibition as described above or were unmanipulated. 5 × 105 inhibited or non-inhibited cells were added to the upper wells in 200μL IMDM with 20% FBS and 1 mL of the same media with either 0 ng/mL, 50 ng/mL, 100 ng/mL or 200 ng/mL of recombinant human/rhesus macaque/feline CXCL12/SDF-1α (R & D Systems, Minneapolis, MN) was added to the bottom well. The cells were incubated at 37°C in 5% CO2 for four hours after which the number and thus percent of cells that migrated to the bottom well was calculated. All conditions were performed in triplicate.
GVHD analysis
Dogs were evaluated for any clinical signs of GVHD including anorexia, conjuctival or skin erythema, diarrhea, and weight loss. At the time of blood draw for chimerism analysis, the bilirubin and liver function tests of recipients of a postnatal BM transplant were assessed for elevations consistent with GVHD. Additionally, the bilirubin and LFTs of all recipients of IUHCT were assessed at 3 months of age. Finally, histologic analysis of the liver, skin and intestine of dogs which died in the neonatal period was performed to assess for evidence of GVHD.
Statistical Analysis
All data, where applicable, is represented as the mean ± the standard error of the mean (SEM). Statistical comparisons between groups were performed with the Student t test for two samples assuming unequal variances. A 2-tailed P ≤ 0.05 was considered significant.
Results
IUHCT
We injected a total of 15 fetuses from three pregnant dogs. The details of gestational age, number of fetuses per female, and doses and composition of the cellular graft are summarized in Table 1. The overall perinatal mortality of 33% (5 out of 15 injected fetuses died in the perinatal period) was related to post-natal mortality rather than in utero death. Four of the five deaths occurred within the first two days of life. Although, the exact cause of postnatal death remains unclear, maternal neglect contributed to at least one death. Histologic analysis of the skin, intestine and liver of the diseased pups did not demonstrate any evidence of GVHD and no sites of infection were apparent on tissue examination. Analysis of engraftment could not be reliably performed due to the condition of the hematopoietic tissues after death.
Table 1. Summary of IUHCT.
Pregnant females were injected at the indicated gestational age (GA). GA at injection was calculated retrospectively assuming birth at gestational day 63. All fetuses present in the moms were injected.
| Mother | GA (days) | Donor source | # CD34 cells/kg | # CD3 cells/kg | % CD3 cells | # fetus injected | Survival to weaning |
|---|---|---|---|---|---|---|---|
| Tonic | 40 | Mother | 4.8 × 108 | 7.8 × 106 | 1.4% | 6 | 3 |
| Dancer | 50 | Father | 1.7 × 108 | 5.7 × 106 | 2.4% | 7 | 5 |
| Vixen | 37 | Father | 2.2 × 109 | 5.9 × 106 | 1.9% | 2 | 2 |
Chimerism in the CLAD Carrier Dogs Following IUHCT
Eight of the 10 surviving injected fetuses were CLAD carriers (Table 2) in which analysis of chimerism by CD18 expression following IUHCT could not be performed. Instead, chimerism analysis was performed by PCR for DNA microsatellite repeat markers in all recipients as well as by PCR and quantitative TaqMan PCR for the male specific SRY gene in female recipients (Ella and Bonnie) of male donors. Evaluation of all CLAD carrier recipients by PCR analysis for DNA microsatellite repeat markers, which has a sensitivity of approximately 5%, did not demonstrate detectable chimerism in any of the dogs prior to postnatal HSCT. However, using the more sensitive PCR analysis for the SRY gene in female recipients of a male donor, chimerism was able to be detected in both Ella and Bonnie at all time points evaluated. Quantifying the donor chimerism in Ella by TaqMan PCR for the SRY gene confirmed that donor cell engraftment had occurred, albeit, at very low levels (0.1 to 0.5%). Importantly, no SRY positive cells were detected by this assay in female puppies that did not undergoe IUHCT (n = 4) or female recipients of female donors after IUHCT (n = 2) arguing against any transplacental acquisition of male microchimerism.
Table 2. Geneology of surviving pups.
| Mother | Pup | Sex | Genotype |
|---|---|---|---|
| Tonic | Baku | male | carrier |
| Kojin | female | carrier | |
| Benton | female | carrier | |
| Dancer | Miles | male | carrier |
| Louie | male | carrier | |
| Ella | female | carrier | |
| Duke | male | CLAD | |
| Billie | female | CLAD | |
| Vixen | Bonnie | female | carrier |
| Clyde | male | carrier |
Chimerism in the CLAD Dog Following IUHCT
Two of the dogs, Duke and Billie, from Dancer's litter were CLAD affected in whom analysis for PB chimerism could be performed by flow cytometric analysis of CD18 expression. This analysis demonstrated that both dogs had stable low levels of donor cell engraftment at all time points analyzed following IUHCT (Figure 1). Billie's analysis extends up to 18 months of life, the latest time point assessed. Engraftment analysis following IUHCT in Duke stops at 7 months of life because at that time he received a postnatal same donor transplant. We elected to transplant Duke once a baseline efficacy of IUHCT had been established because of low grade infections and inability to wean from antibiotics. Multilineage donor engraftment was demonstrated in both dogs in the T cell, B cell, neutrophil, and monocyte lineages at the time points assessed (Figure 1B and 1C). Finally, Billie was a female recipient of a male donor and thus donor cell engraftment was confirmed by PCR for the SRY gene. As demonstrated in Figure 2, engraftment levels determined by TaqMan SRY PCR in Billie corresponded closely to those seen by flow cytometry for CD18 expression.
Figure 1. Chimerism in the CLAD Model Following IUHCT.
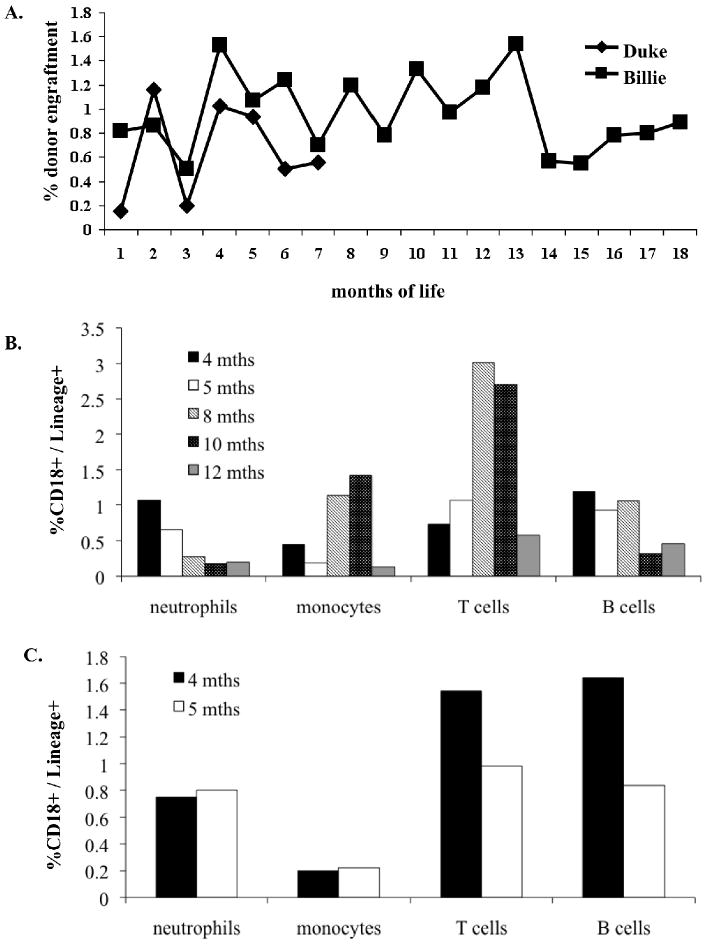
(A) PB was analyzed monthly by flow cytometry for the CD18 antigen in the CLAD dogs Duke and Billie. Duke's chimerism analysis stops at 7 months because he received a postnatal transplant at that time. Mulitlineage Analysis - PB from Billie (B) and Duke (C) was analyzed at the indicated time points by flow cytometry for donor neutrophils, monocytes, B cells and T cells.
Figure 2. Engraftment Following IUHCT in a Female CLAD Recipient: Correlation of Flow Cytometry and TaqMan PCR.
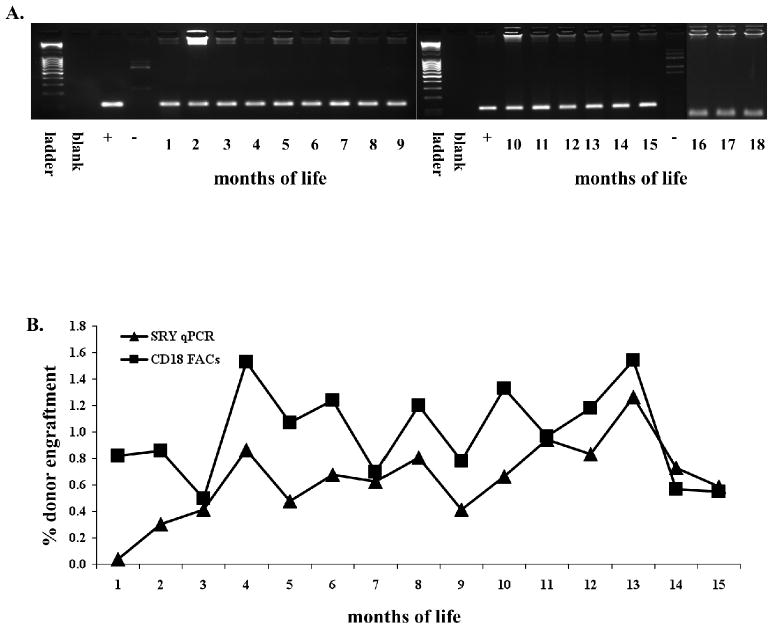
Billie was a female CLAD recipient of male donor BM. Donor engraftment in her was confirmed by PCR for the SRY gene (A). Chimerism was quantified by SRY TaqMan PCR and levels were found to be similar to those determined by flow cytometry for the CD18 antigen (B).
Improvement in the CLAD Phenotype Following IUHCT
In contrast to historical CLAD controls which demonstrate a 100% mortality by 6 months of life, the two chimeric CLAD dogs (Billie and Duke) in the current study were alive and healthy through the length of unmanipulated follow up28 (Figure 3). Duke displayed a mildly affected CLAD phenotype but never required intensive care. At seven months of age Duke underwent a postnatal transplant as discussed below during which time he did not experience any CLAD phenotypic events. He was subsequently euthanized after rejection of his graft and worsening CLAD phenotype. In contrast to Duke, Billie has never displayed any CLAD phenotypic events and has remained completely healthy throughout the time course of this study (18 months). Additionally, in contrast to historical CLAD affected dogs which demonstrate a leukocytosis with WBC counts greater than 50,000, Billie consistently demonstrated normal to slightly elevated WBC counts while Duke's WBC count prior to boosting was moderately elevated but less than 50,000 (Figure 3B).
Figure 3. Clinical Phenotype of Chimeric CLAD Dogs Following IUHCT.
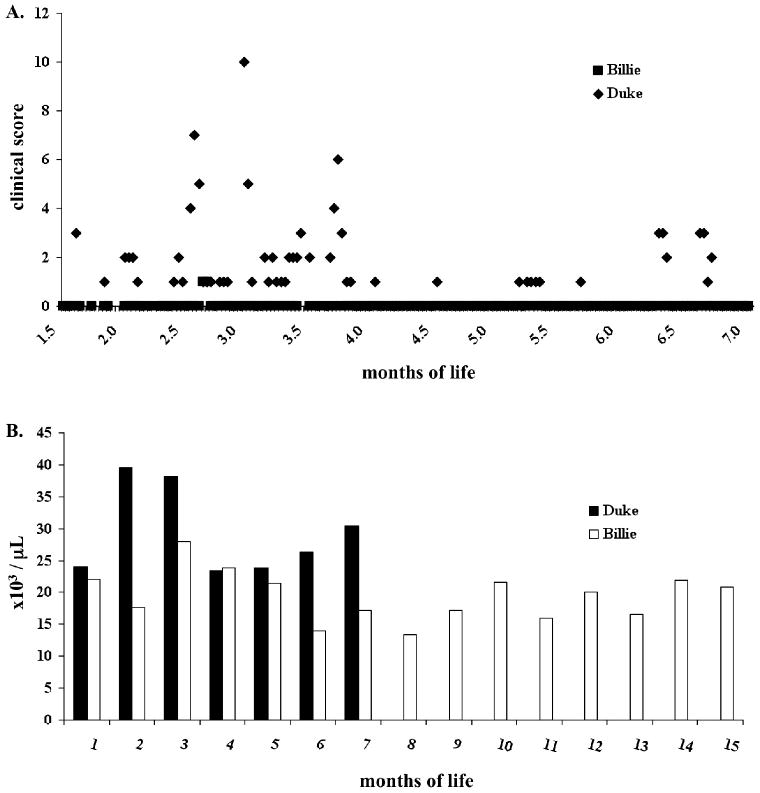
CLAD dogs were monitored and scored using the sum of seven clinical parameters of temperature, comfort, movement, appearance, behavior, interactive behavior, and vocalization where a score ranging from 0 for normal to 4 for maximal abnormality in each category was assigned at each data point. (A). Data is represented up to 7 months of life for Duke at which point he received a postnatal BM transplant. Billie continued to not experience any CLAD clinical events up to the last time point assessed, 18 months of life (not represented on graph). The WBC counts of Billie and Duke were assessed for leukocytosis (>50,000) typical of CLAD affected dogs (B).
GVHD Following IUHCT
None of the dogs demonstrated any clinical signs of GVHD. All dogs demonstrated appropriate weight gain (data not shown) and none demonstrated elevations in their liver enzymes or bilirubin levels. This data argues against the presence of GVHD in any of the recipients of IUHCT.
Mixed Lymphocyte Reaction
In vitro MLRs were performed to assess the reactivity of PB mononuclear cells from Tonic and Dancers' litters to prenatal donor and unrelated third party stimulator cells. The reactivity of responder cells to prenatal donor and third party stimulator cells was standardized to the reactivity of responder cells to autologous stimulator cells. As demonstrated in Figure 4, Baku demonstrated no increased reactivity to prenatal donor cells over response to self. Kojin, Billie, Louie, Miles and Benten demonstrated decreased reactivity to the prenatal donors compared to unrelated third party stimulators but the differences did not reach statistical significance. Ella and Duke, however, demonstrated equal or greater reactivity to the prenatal donor compared to third party unrelated stimulator cell populations.
Figure 4. Mixed Lymphocyte Reaction.
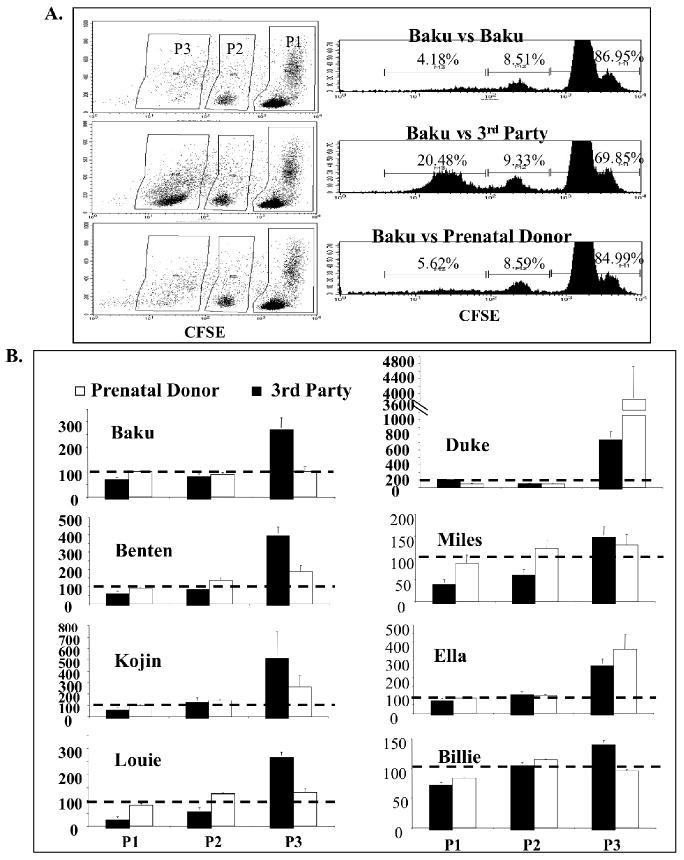
Recipients of IUHCT were tested by in vitro MLR for reactivity against donor cells as compared to reactivity against self and unrelated third party cells. MLR was performed with CFSE staining and increased cell division/reactivity is indicated by decreased intensity of CFSE staining and a shift of cells from population 1 (P1) to population 3 (P3) (A). The reactivity (or % cells in each population) of recipients to self cells was standardized to 100% as indicated by the dotted line and the reactivity of recipients to third party and prenatal donor cells was compared to this standard (B). Thus a bar above the dotted line indicates a larger percentage of cells in that population compared to the percentage of cells in the self reactive negative control assay.
Postnatal Same Donor Haploidentical HSCT in Recipients of IUHCT
Six recipients of IUHCT (5 CLAD carriers and 1 CLAD) received a postnatal HSCT using the same prenatal donor. Recipients were conditioned with a non-myeloablative dose of Busulfan and received a short course of post transplant immunosuppression with Cyclosporine and Mycophenolic Acid Mofetil for GVHD prophylaxis. The age at transplant, total cell dose, and CD34+ and CD3+ cell components of the donor cell inoculums are summarized in Table 3. All recipients of an IUHCT demonstrated a dramatic increase in chimerism levels that peaked from 3-10 weeks post transplant. Two CLAD carriers, Baku and Louie demonstrated a dramatic and substantial increase in donor cell engraftment that has remained stable at donor levels of 35 to 45% (Figure 5). However, the remaining 3 CLAD carrier dogs lost detectable levels (Sensitivity = 5%) of donor cell engraftment by 14 weeks post transplant. Duke, the only CLAD dog that received a postnatal transplant, demonstrated a dramatic increase in chimerism levels that peaked at 6 weeks post transplant. However, his donor cell engraftment dropped to pre-postnatal boosting levels at 8 weeks post transplant. Terry and Cashmere did not receive an IUHCT but only received a postnatal maternal BM transplant and thus served as postnatal transplant controls. These two dogs never demonstrated any detectable levels of donor cell chimerism.
Table 3. Summary of Postnatal Bone Marrow Transplants.
Six dogs received a postnatal BM transplant using the same donor that had been used for their prenatal BM transplant. Two dogs (Terry and Cashimere) did not receive a prenatal BM transplant and only received a postnatal BM transplant in which their mother served as the donor. Tx = transplant.
| Recipient | Age @ Tx (months) | Total Cell Dose | # CD34 cells/kg | # CD3 cells/kg |
|---|---|---|---|---|
| Baku | 4 | 72 × 106 | 8 × 106 | 1 × 106 |
| Duke | 7 | 160 × 106 | 2.6 × 106 | 5.5 × 106 |
| Kojin | 8.5 | 130 × 106 | 7 × 106 | 2.6 × 106 |
| Louie | 10 | 159 × 106 | 5.5 × 106 | 1.2 × 106 |
| Miles | 11 | 126 × 106 | 3.2 × 106 | 4.2 × 106 |
| Benten | 11 | 91 × 106 | 8.6 × 106 | 4.5 × 106 |
| Terry | 4 | 170 × 106 | 2.5 × 106 | 2.3 × 106 |
| Cashimere | 4 | 170 × 106 | 2.5 × 106 | 2.3 × 106 |
Figure 5. Donor Cell Engraftment Following Postnatal “Boosting” BM Transplants.
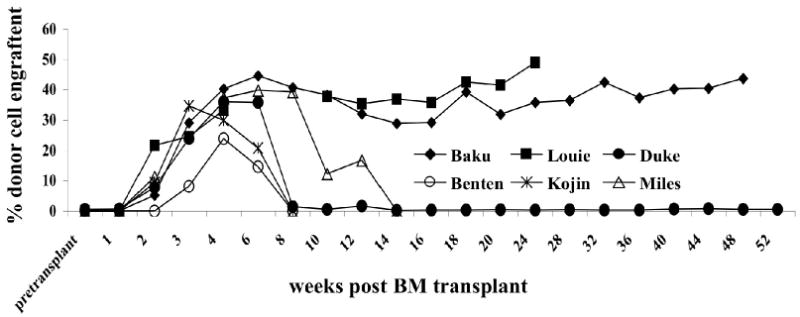
Six dogs received a postnatal BM transplant using the same donor that had been used for their prenatal BM transplant. Two dogs (Terry and Cashimere) did not receive a prenatal BM transplant and only received a postnatal BM transplant in which their mother served as the donor. Terry and Cashimere did not demonstrate any donor cell engraftment at any time point analyzed and are thus not represented on the graph.
Clinical Phenotype and GVHD Following Postnatal Transplant
No dog demonstrated clinical evidence of GVHD. With the exception of a transient peri-transplant weight loss attributed to the decreased appetite associated with the conditioning regimen, all dogs demonstrated appropriate weight gain post transplant (Data not shown).
Duke demonstrated no CLAD phenotypic episodes in the post-transplant period during which time his chimerism levels remained elevated. After the loss of chimerism, Duke again reverted back to a mild CLAD phenotype experiencing clinical events and leukocytosis at the same frequency and intensity that he experienced before the postnatal transplant until he was euthanized at 15 months of age.
CD26 Inhibition and In Vitro Migration Assay
CD26 inhibition has been demonstrated to increase the efficiency and levels of engraftment following IUHCT in a mouse model.37 In the current study we empirically included CD26 inhibition of the CD34+ cell component of the donor cell inoculums prior to IUHCT and postnatal BM transplants. In order to ascertain if CD26 inhibition has the potential to affect canine CD34+ cell migration, an in vitro chemotaxis assay was performed to assess the affect of CD26 inhibition of whole BM and CD34+ enriched BM on SDF-1α induced migration. As demonstrated in Figure 6, CD26 inhibition of both cell populations resulted in an increased percentage of cell migration to an SDF-1α source. The difference in migration between CD26 inhibited and noninhibited cells was most prominent when CD34+ enriched BM cells were analyzed.
Figure 6. Chemotaxis assay to SDF1 alpha gradient after CD26 inhibition.
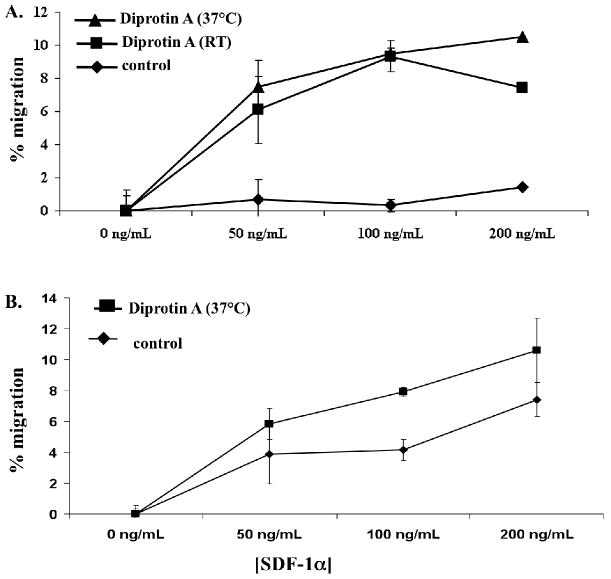
Canine BM derived enriched CD34+ cells (A) and whole BM (B) were subjected to CD26 inhibition by incubation with Diprotin A or were unmanipulated (control) and assessed for SDF-1a mediated chemotaxis following 4 hours of incubation at 37°C in transwell chemotaxis chambers. As indicated in the legend, cells were incubated with Diprotin A at either 37°C or room temperature (RT) for 15 minutes prior to placement in chemotaxis chambers. Migration at all SDF-1a concentrations was assessed in triplicate.
Discussion
IUHCT offers the potential to treat a large number of hematologic genetic disorders without the myeloablative conditioning regimens currently used for haploidentical or mismatched postnatal HSCT transplant protocols. Early success in the sheep model suggested that significant levels of allogeneic engraftment could be relatively easily achieved by IUHCT. However, with the exception of immunodeficiency disorders38-40, this proved not to be the case in humans, and other animal species, where early clinical and experimental studies resulted in limited or no detectable engraftment. Subsequent experimental studies in mice have confirmed that there are both competitive and immunologic barriers to engraftment after IUHCT.37,41,42 Nevertheless, these barriers can be overcome, at least in a percentage of animals, to achieve low levels of mixed hematopoietic chimerism across full MHC barriers, with associated DST. There appears to be a threshold of chimerism required for consistent association of DST in the murine model of around 1%.6 Chimerism levels below this threshold result in inconsistent tolerance, with only a fraction of animals accepting skin grafts or demonstrating enhancement of chimerism after postnatal same donor transplants.3-6 In mice, animals that are tolerant after IUHCT, can be transplanted after birth utilizing a variety of minimal conditioning strategies with enhancement of donor chimerism to levels that would be therapeutic for most target disorders.2,5,6 With this potential clinical strategy in mind, we wished to develop a pre-clinical model where techniques developed in the murine model could be assessed for their applicability to human disease.
In this study, we were able to perform IUHCT in the CLAD model using clinically applicable methodology with similar perinatal mortality to that seen following the natural breeding of dogs.43 IUHCT using haploidentical donors resulted in low levels of mixed hematopoietic chimerism that remained stable for over 18 months. The low levels of chimerism achieved in both CLAD and CLAD carrier dogs reflect the highly competitive engraftment milieu in the canine fetus, which appears to be analogous to that in humans. Remarkably, all dogs that could be analyzed with sensitive methodology (i.e. CD18 or SrY Q-PCR) were engrafted after IUHCT. In the animals where lineage analysis could be performed, donor chimerism was multilineage and durable, supporting the engraftment of hematopoietic stem cells at the time of IUHCT. Consistent with previous postnatal studies in the CLAD model, we demonstrate that even the low levels of engraftment achieved after IUHCT, are adequate to phenotypically ameliorate or correct the disease.
Perhaps the most important observation of this study, was the ability of IUHCT to induce DST in two of the chimeric animals, as evidenced by the ability to enhance engraftment after birth using a non-myeloablative conditioning regimen with sustained high levels of donor chimerism thereafter. This was in distinct contrast to what would be expected for haploidentical transplants using the same conditioning regimen in the absence of tolerance induction by IUHCT. To assess the presence of DST we used CFSE MLR. The one dog, Baku, that demonstrated definitively decreased donor specific reactivity also was successfully boosted, whereas the one dog that demonstrated definitive reactivity to donor cells and also underwent a postnatal transplant (Duke) rapidly lost donor chimerism. The remainder of the dogs who were assessed by MLR had intermediate results. Each of these dogs demonstrated an initial rise in chimerism levels with subsequent loss of engraftment by 14 weeks post HSCT. This pattern of engraftment was distinct from the complete absence of chimerism seen in non-IUHCT control dogs, in which donor chimerism could not be detected at any time after HSCT. These data support our premise that IUHCT was associated with DST, at least in a subset of recipients. However, due to the low number of dogs transplanted in this study, and limitations in the assessment of tolerance in the canine model, we have not absolutely proven that DST resulted from the IUHCT.
Our data in the canine model can be compared and contrasted to results in the murine model. While levels of engraftment in chimeric dogs were generally lower than what can be achieved in mice, the frequency of engraftment appears at least as high, and perhaps higher in the dog, than in the mouse. Sustained chimerism is observed in only around 30% of mice after allogeneic IUHCT, whereas in this study every dog in which sensitive detection methodology could be applied after IUHCT demonstrated sustained chimerism. The canine model appears remarkably similar to the murine model with respect to the correlation of level of chimerism with the apparent association of DST. In the murine model, only approximately 60% of animals with less than 1% chimerism will demonstrate enhancement of chimerism with a Busulfan conditioned same donor HSCT, whereas 100% of animals with greater than 1% chimerism can be boosted.6 In this study where levels of chimerism appear to be equal to or less than 1%, 2 of 6 total dogs and 2 of 5 CLAD carriers that underwent postnatal same donor transplants experienced a sustained increase in levels of donor chimerism. Thus, we would anticipate, based on our murine data, that the achievement of levels of donor chimerism even slightly higher than those in the current study should result in the consistent association of tolerance and allow all recipients of IUHCT to be boosted to potentially therapeutic levels of chimerism after birth.
The primary clinical risk of IUHCT would be anticipated to be GVHD. While the numbers of dogs transplanted in this initial experience were too small to make confident statements regarding the risks of IUHCT in a large animal system, it was encouraging that despite the intentional addition of significant numbers of T-cells, GVHD was not observed. Our protocol for T-cell reconstitution in this study was adapted from studies in the pig model of IUHCT, where multilineage chimerism with DST was achieved after BM was T-cell depleted and unprocessed whole BM was added back to achieve a T-cell concentration of 1.5%44,45. This was in contrast to the strictly lymphoid chimerism that resulted from add back of isolated CD3+ cells at the same CD3+ dose suggesting that other populations in BM besides T-cells may be important in facilitation of HSC engraftment after IUHCT. Earlier studies in the sheep and primate models of IUHCT have utilized similar doses of T-cells to support engraftment.46,47 In the current study, T cell doses as high as 7.8 × 106 CD3+ cells/kg estimated fetal weight were administered without evidence of GVHD but the upper limit of T-cell dose and the safety profile of T-cell dosing with or without other cell populations remains to be defined, Further studies in the canine model are required prior to any conclusions regarding the safety of various T-cell doses after IUHCT can be made.
The results of the current study compare favorably and extend upon the results of Blakemore et al.34,48 who were the first to assess allogeneic IUHCT using haploidentical donor cells in the normal canine model. Similar to our study, they found an overall perinatal mortality rate of 31%. Analysis for donor cell chimerism was performed at a single, short-term time point in the neonatal period on harvested hematopoietic tissues. Their results suggest the presence of low levels (0.01% to <2%) of donor cell chimerism in some of the tissues of some recipients. However, they were unable to demonstrate any evidence of DST following IUHCT by in vitro MLR. Interestingly, there did not appear to be a relationship between T-cell dose and levels of chimerism, and no GVHD was observed, even at high T-cell doses. Beyond the observations that low level chimerism can be achieved without GVHD, direct comparisons between our data and that of Blakemore et al. are difficult due to methodologic differences in the donor cell preparations, donor cell detection methodology, and particularly because of the major difference in length of follow up.
In the current study, we use a protocol of Busulfan conditioning with a short course of post-transplant immunosuppression for GVHD prophylaxis. This regimen has been shown to result in successful engraftment in matched littermate CLAD dogs.27 However, this regimen, as observed in our control animals, and in previous postnatal experience (personal communication Dennis Hickstein) does not allow for successful parental haploidentical engraftment in recipients that did not receive IUHCT. In this study, two of the recipients of an IUHCT demonstrated successful long-term enhancement of engraftment following same donor postnatal haploidentical HSCT. Engraftment levels in these recipients remained stable at 35-45% donor cell chimerism up to the last time point of analysis. The remaining four recipients of IUHCT all experienced an initial rise in chimerism, but subsequently lost measurable engraftment by 14 weeks post transplant. Although the initial rise in engraftment level was encouraging, and distinct from the engraftment pattern seen in the two control dogs that did not undergo IUHCT, we believe the loss of chimerism seen in these recipients reflects an immune rejection. Due to technical limitations of quantifying chimerism in the canine model we were unable to determine the original presence or levels of engraftment in 4 of the 6 recipients of IUHCT that received a postnatal transplant. The sensitivity of the assay used to detect chimerism is 5%, and thus we know that engraftment, if present, occurred at chimerism levels less than 5% in these dogs. Additionally, it is reasonable to assume, based on IUHCT engraftment levels seen in Billie, Ella, Duke, and Bonnie, that chimerism levels were <1%. Thus, it is not surprising that all animals did not show evidence of tolerance. Finally, we believe that the temporary enhancement of chimerism in those dogs in which engraftment following IUHCT could not be determined supports the presence of chimerism, as experience with IUHCT has documented that no tolerance occurs without concomitant donor cell chimerism.
While this study is a valuable first step toward optimization of IUHCT for clinical application, much more work needs to be done. Basic questions such as the optimal timing of transplantation and correlation with specific stages of immune and hematopoietic development require further investigation. Although many questions remain, this study provides encouraging evidence that IUHCT can result in successful engraftment and induction of DST in a preclinical large animal model. While we have demonstrated that the low levels of engraftment achieved by IUHCT can reverse the lethal phenotype of CLAD, we consider the most exciting aspect of the current study to be the apparent finding that IUHCT can in some circumstances induce DST and that this tolerance can provide a platform for postnatal minimal conditioning HSCT with enhancement of donor cell engraftment to clinically relevant levels. We feel that if further enhancement of engraftment in this model with consistent tolerance induction can be achieved, than appropriate clinical trials of IUHCT for the treatment of genetic disorders that can be prenatally diagnosed and treated by mixed hematopoietic chimerism, such as the hemoglobinopathies and selected immunodeficiency disorders could be initiated.
Acknowledgments
This work was supported by grants RO1 HL64715, U54 HL070596-01 from the National Institutes of Health (AWF), a Pennsylvania State Health Research Formula Award (CHOP allocation), and the Greenfield Foundation. WHP and TEH were supported by T32 HD046402 (AWF - Program Director). AWF is also supported by funds from the Ruth and Tristram C. Colket, Jr. Chair of Pediatric Surgery. YCG, TRB, LMT, and DDH were supported by the Center for Cancer Research, National Cancer Intitute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flake AW, Zanjani ED. In utero hematopoietic stem cell transplantation: ontogenic opportunities and biologic barriers. Blood. 1999;94:2179–2191. [PubMed] [Google Scholar]
- 2.Hayashi S, Peranteau WH, Shaaban AF, Flake AW. Complete allogeneic hematopoietic chimerism achieved by a combined strategy of in utero hematopoietic stem cell transplantation and postnatal donor lymphocyte infusion. Blood. 2002;100:804–812. doi: 10.1182/blood-2002-01-0016. [DOI] [PubMed] [Google Scholar]
- 3.Kim HB, Shaaban AF, Milner R, Fichter C, Flake AW. In utero bone marrow transplantation induces donor-specific tolerance by a combination of clonal deletion and clonal anergy. J Pediatr Surg. 1999;34:726–729. doi: 10.1016/s0022-3468(99)90364-0. [DOI] [PubMed] [Google Scholar]
- 4.Kim HB, Shaaban AF, Yang EY, Liechty KW, Flake AW. Microchimerism and tolerance after in utero bone marrow transplantation in mice. J Surg Res. 1998;77:1–5. doi: 10.1006/jsre.1997.5255. [DOI] [PubMed] [Google Scholar]
- 5.Peranteau WH, Hayashi S, Hsieh M, Shaaban AF, Flake AW. High-level allogeneic chimerism achieved by prenatal tolerance induction and postnatal nonmyeloablative bone marrow transplantation. Blood. 2002;100:2225–2234. doi: 10.1182/blood-2002-01-0166. [DOI] [PubMed] [Google Scholar]
- 6.Ashizuka S, Peranteau WH, Hayashi S, Flake AW. Busulfan-conditioned bone marrow transplantation results in high-level allogeneic chimerism in mice made tolerant by in utero hematopoietic cell transplantation. Exp Hematol. 2006;34:359–368. doi: 10.1016/j.exphem.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaconescu R, Storb R. Allogeneic hematopoietic cell transplantation: from experimental biology to clinical care. J Cancer Res Clin Oncol. 2005;131:1–13. doi: 10.1007/s00432-004-0611-6. [DOI] [PubMed] [Google Scholar]
- 8.Storb R. Allogeneic hematopoietic stem cell transplantation--yesterday, today, and tomorrow. Exp Hematol. 2003;31:1–10. doi: 10.1016/s0301-472x(02)01020-2. [DOI] [PubMed] [Google Scholar]
- 9.Kuhr CS, Lupu M, Little MT, Zellmer E, Sale GE, Storb R. RDP58 does not prevent graft-versus-host disease after dog leukocyte antigen-nonidentical canine hematopoietic cell transplantation. Transplantation. 2006;81:1460–1462. doi: 10.1097/01.tp.0000203323.82681.7d. [DOI] [PubMed] [Google Scholar]
- 10.Mielcarek M, Georges GE, Storb R. Denileukin diftitox as prophylaxis against graft-versus-host disease in the canine hematopoietic cell transplantation model. Biol Blood Marrow Transplant. 2006;12:899–904. doi: 10.1016/j.bbmt.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Storb R, Deeg HJ, Raff R, et al. Prevention of graft-versus-host disease. Studies in a canine model. Ann N Y Acad Sci. 1995;770:149–164. doi: 10.1111/j.1749-6632.1995.tb31052.x. [DOI] [PubMed] [Google Scholar]
- 12.Storb R, Thomas ED. Graft-versus-host disease in dog and man: the Seattle experience. Immunol Rev. 1985;88:215–238. doi: 10.1111/j.1600-065x.1985.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 13.Yu C, Seidel K, Nash RA, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581–2587. [PubMed] [Google Scholar]
- 14.Felsburg PJ. Overview of immune system development in the dog: comparison with humans. Hum Exp Toxicol. 2002;21:487–492. doi: 10.1191/0960327102ht286oa. [DOI] [PubMed] [Google Scholar]
- 15.Beccaglia M, Luvoni GC. Comparison of the accuracy of two ultrasonographic measurements in predicting the parturition date in the bitch. J Small Anim Pract. 2006;47:670–673. doi: 10.1111/j.1748-5827.2006.00108.x. [DOI] [PubMed] [Google Scholar]
- 16.Kutzler MA, Yeager AE, Mohammed HO, Meyers-Wallen VN. Accuracy of canine parturition date prediction using fetal measurements obtained by ultrasonography. Theriogenology. 2003;60:1309–1317. doi: 10.1016/s0093-691x(03)00146-8. [DOI] [PubMed] [Google Scholar]
- 17.Luvoni GC, Beccaglia M. The prediction of parturition date in canine pregnancy. Reprod Domest Anim. 2006;41:27–32. doi: 10.1111/j.1439-0531.2006.00641.x. [DOI] [PubMed] [Google Scholar]
- 18.Cerletti M, Negri T, Cozzi F, et al. Dystrophic phenotype of canine X-linked muscular dystrophy is mitigated by adenovirus-mediated utrophin gene transfer. Gene Ther. 2003;10:750–757. doi: 10.1038/sj.gt.3301941. [DOI] [PubMed] [Google Scholar]
- 19.Chuah MK, Schiedner G, Thorrez L, et al. Therapeutic factor VIII levels and negligible toxicity in mouse and dog models of hemophilia A following gene therapy with high-capacity adenoviral vectors. Blood. 2003;101:1734–1743. doi: 10.1182/blood-2002-03-0823. [DOI] [PubMed] [Google Scholar]
- 20.Cooper BJ, Winand NJ, Stedman H, et al. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988;334:154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- 21.Felsburg PJ, Somberg RL, Hartnett BJ, et al. Full immunologic reconstitution following nonconditioned bone marrow transplantation for canine X-linked severe combined immunodeficiency. Blood. 1997;90:3214–3221. [PubMed] [Google Scholar]
- 22.Niemeyer GP, Boudreaux MK, Goodman-Martin SA, Monroe CM, Wilcox DA, Lothrop CD., Jr Correction of a large animal model of type I Glanzmann's thrombasthenia by nonmyeloablative bone marrow transplantation. Exp Hematol. 2003;31:1357–1362. doi: 10.1016/j.exphem.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 24.Creevy KE, Bauer TR, Jr, Tuschong LM, et al. Canine leukocyte adhesion deficiency colony for investigation of novel hematopoietic therapies. Vet Immunol Immunopathol. 2003;94:11–22. doi: 10.1016/s0165-2427(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 25.Kishimoto TK, Hollander N, Roberts TM, Anderson DC, Springer TA. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987;50:193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- 26.Fischer A, Lisowska-Grospierre B, Anderson DC, Springer TA. Leukocyte adhesion deficiency: molecular basis and functional consequences. Immunodefic Rev. 1988;1:39–54. [PubMed] [Google Scholar]
- 27.Sokolic RA, Bauer TR, Gu YC, et al. Nonmyeloablative conditioning with busulfan before matched littermate bone marrow transplantation results in reversal of the disease phenotype in canine leukocyte adhesion deficiency. Biol Blood Marrow Transplant. 2005;11:755–763. doi: 10.1016/j.bbmt.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer TR, Jr, Creevy KE, Gu YC, et al. Very low levels of donor CD18+ neutrophils following allogeneic hematopoietic stem cell transplantation reverse the disease phenotype in canine leukocyte adhesion deficiency. Blood. 2004;103:3582–3589. doi: 10.1182/blood-2003-11-4008. [DOI] [PubMed] [Google Scholar]
- 29.Bauer TR, Jr, Gu YC, Tuschong LM, et al. Nonmyeloablative hematopoietic stem cell transplantation corrects the disease phenotype in the canine model of leukocyte adhesion deficiency. Exp Hematol. 2005;33:706–712. doi: 10.1016/j.exphem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Burkholder TH, Colenda L, Tuschong LM, Starost MF, Bauer TR, Jr, Hickstein DD. Reproductive capability in dogs with canine leukocyte adhesion deficiency treated with nonmyeloablative conditioning prior to allogeneic hematopoietic stem cell transplantation. Blood. 2006;108:1767–1769. doi: 10.1182/blood-2006-02-005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McSweeney PA, Rouleau KA, Wallace PM, et al. Characterization of monoclonal antibodies that recognize canine CD34. Blood. 1998;91:1977–1986. [PubMed] [Google Scholar]
- 32.Bauer TR, Jr, Gu YC, Creevy KE, et al. Leukocyte adhesion deficiency in children and Irish setter dogs. Pediatr Res. 2004;55:363–367. doi: 10.1203/01.PDR.0000111287.74989.1B. [DOI] [PubMed] [Google Scholar]
- 33.Olivier M, Breen M, Binns MM, Lust G. Localization and characterization of nucleotide sequences from the canine Y chromosome. Chromosome Res. 1999;7:223–233. doi: 10.1023/a:1009203500926. [DOI] [PubMed] [Google Scholar]
- 34.Petersen SM, Gendelman M, Murphy KM, et al. Use of T-cell antibodies for donor dosaging in a canine model of in utero hematopoietic stem cell transplantation. Fetal Diagn Ther. 2007;22:175–179. doi: 10.1159/000098711. [DOI] [PubMed] [Google Scholar]
- 35.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 36.Christopherson KW, 2nd, Uralil SE, Porecha NK, Zabriskie RC, Kidd SM, Ramin SM. G-CSF- and GM-CSF-induced upregulation of CD26 peptidase downregulates the functional chemotactic response of CD34+CD38- human cord blood hematopoietic cells. Exp Hematol. 2006;34:1060–1068. doi: 10.1016/j.exphem.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Peranteau WH, Endo M, Adibe OO, Merchant A, Zoltick PW, Flake AW. CD26 inhibition enhances allogeneic donor-cell homing and engraftment after in utero hematopoietic-cell transplantation. Blood. 2006;108:4268–4274. doi: 10.1182/blood-2006-04-018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flake AW, Roncarolo MG, Puck JM, et al. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med. 1996;335:1806–1810. doi: 10.1056/NEJM199612123352404. [DOI] [PubMed] [Google Scholar]
- 39.Touraine JL. In utero transplantation of fetal liver stem cells into human fetuses. J Hematother. 1996;5:195–199. doi: 10.1089/scd.1.1996.5.195. [DOI] [PubMed] [Google Scholar]
- 40.Wengler GS, Lanfranchi A, Frusca T, et al. In-utero transplantation of parental CD34 haematopoietic progenitor cells in a patient with X-linked severe combined immunodeficiency (SCIDXI) Lancet. 1996;348:1484–1487. doi: 10.1016/s0140-6736(96)09392-0. [DOI] [PubMed] [Google Scholar]
- 41.Peranteau WH, Endo M, Adibe OO, Flake AW. Evidence for an immune barrier after in utero hematopoietic-cell transplantation. Blood. 2007;109:1331–1333. doi: 10.1182/blood-2006-04-018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaaban AF, Kim HB, Milner R, Flake AW. A kinetic model for homing and migration of prenatally transplanted marrow. Blood. 1999;94:3251–3257. [PubMed] [Google Scholar]
- 43.van der Beek S, Nielen AL, Schukken YH, Brascamp EW. Evaluation of genetic, common-litter, and within-litter effects on preweaning mortality in a birth cohort of puppies. Am J Vet Res. 1999;60:1106–1110. [PubMed] [Google Scholar]
- 44.Lee PW, Cina RA, Randolph MA, et al. Stable multilineage chimerism across full MHC barriers without graft-versus-host disease following in utero bone marrow transplantation in pigs. Exp Hematol. 2005;33:371–379. doi: 10.1016/j.exphem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Lee PW, Cina RA, Randolph MA, et al. In utero bone marrow transplantation induces kidney allograft tolerance across a full major histocompatibility complex barrier in Swine. Transplantation. 2005;79:1084–1090. doi: 10.1097/01.tp.0000161247.61727.67. [DOI] [PubMed] [Google Scholar]
- 46.Crombleholme TM, Harrison MR, Zanjani ED. In utero transplantation of hematopoietic stem cells in sheep: the role of T cells in engraftment and graft-versus-host disease. J Pediatr Surg. 1990;25:885–892. doi: 10.1016/0022-3468(90)90197-h. [DOI] [PubMed] [Google Scholar]
- 47.Shields LE, Gaur LK, Gough M, Potter J, Sieverkropp A, Andrews RG. In utero hematopoietic stem cell transplantation in nonhuman primates: the role of T cells. Stem Cells. 2003;21:304–314. doi: 10.1634/stemcells.21-3-304. [DOI] [PubMed] [Google Scholar]
- 48.Blakemore K, Hattenburg C, Stetten G, et al. In utero hematopoietic stem cell transplantation with haploidentical donor adult bone marrow in a canine model. Am J Obstet Gynecol. 2004;190:960–973. doi: 10.1016/j.ajog.2004.01.014. [DOI] [PubMed] [Google Scholar]


