Abstract
It is increasingly evident that 17β-oestradiol (E2) via a distinct membrane oestrogen receptor (Gq-mER) can rapidly activate kinase pathways to have multiple downstream actions in CNS neurons. We have found that E2 can rapidly reduce the potency of the GABAB receptor agonist baclofen and mu-opioid receptor agonist DAMGO to activate G protein-coupled, inwardly rectifying K+ (GIRK) channels in hypothalamic neurons, thereby increasing the excitability (firing activity) of POMC and dopamine neurons. These effects are mimicked by the membrane impermeant E2-BSA and a new ligand (STX) that is selective for the Gq-mER that does not bind to ERα or ERβ. Both E2 and STX are fully efficacious in attenuating the GABAB response in ERα, ERβ and GPR 30 knockout mice in an ICI 182,780 reversible manner. These findings are further proof that E2 signals through a unique plasma membrane ER. We have characterised the coupling of this Gq-mER to a Gq-mediated activation of phospholipase C leading to the up-regulation of protein kinase Cδ and protein kinase A activity in these neurons, which ultimately alters gene transcription. Finally as proof of principle, we have found that STX, like E2, reduces food intake and body weight gain in ovariectomised females. STX, presumably via the Gq-mER, also regulates gene expression of a number of relevant targets including cation channels and signalling molecules that are critical for regulating (as a prime example) POMC neuronal excitability. Therefore, E2 can activate multiple receptor-mediated pathways to modulate excitability and gene transcription in CNS neurons that are critical for controlling homeostasis and motivated behaviors.
Keywords: hypothalamus, ER, Gq-mER, signaling pathways, gene expression
Introduction
Oestrogen receptors (ER) regulate cellular function through at least two signalling pathways previously broadly classified as “genomic” versus “nongenomic” (1,2). Recently, a more appropriate terminology was suggested. Hammes and Levin suggested “nuclear-initiated steroid signalling” for genomic signalling pathways, and “membrane-initiated steroid signalling” for rapid, nongenomic pathways (3). Under nuclear-initiated signalling, oestrogen (E2) via ERα and ERβ exerts diverse effects on a variety of tissues that involves gene stimulation as well as gene repression (4–9). In general, this “classical” signalling pathway of E2 involves steroid-dependent formation of nuclear oestrogen receptor (ER) homo- or heterodimers and the subsequent binding of this complex with a unique DNA sequence known as an oestrogen response element (ERE), in E2-responsive gene promoters (10–12). There is also compelling evidence that ERα and ERβ can regulate transcription of some of these “oestrogen-responsive” genes by interacting with other DNA-bound transcription factors, such as specificity protein-1 (SP-1) and activator protein 1 (AP-1), rather than binding directly to DNA (12–14).
However, over thirty years ago rapid electrophysiological effects of E2 were documented (15) and at the same time E2 membrane binding sites on endometrial cells were identified (16,17). Subsequently, high affinity binding of [3H]-17β-oestradiol was demonstrated in synaptosomal membranes prepared from the adult rat brain (18). It is now generally accepted that membrane-initiated signalling of E2 in the brain does not require nuclear targeting of ERs (for review (3,19,20)). This type of E2 signalling occurs at the plasma membrane to trigger intracellular signalling events that result in gene transcription and alterations in neuronal activity. New gene transcription results from E2 activation of multiple intracellular kinase cascades including mitogen-activated protein kinases (MAPK), phosphoinositide 3-kinase (PI3K), cAMP-protein kinase (PKA) and protein kinase C (PKC) pathways (21–25). For example, E2 rapidly up-regulates cAMP in hypothalamic neurons by increasing adenylyl cyclase activity (26), which in turn activates PKA. PKA phosphorylates cAMP-responsive element binding protein (CREB) and elicits new gene transcription (27–30). Genes with CRE binding sites are activated rapidly in neurones independent of ER interacting with EREs including genes encoding neurotransmitters such as dopamine, enkephalin, dynorphin and neurotensin (28,29). In this review, we describe a novel, putative membrane ER that is a GPCR functionally characterised in arcuate POMC neurones and how the classical ER and this novel Gq-mER interact through nuclear-initiated and membrane-initiated signalling pathways. Recent data suggests that oestrogenic gene regulation and channel modulation is a multiple-receptor mediated mechanism, which synergistically controls hypothalamic functions during the ovulatory cycle.
Membrane-initiating signalling of E2 on hypothalamic arcuate neurones
Previous reviews have described the acute, membrane-initiated signalling actions of E2 in the brain through multiple signalling pathways (20,31,32) including G-protein-activated pathways (33). At least some of these rapid actions of E2 cannot be attributed to classical nuclear-initiated steroid signalling of ERα or ERβ. There are several potential candidates for novel membrane ERs including ER-X and two G-protein-coupled receptors, GPR30 and Gq-mER (34–39); therefore, it is evident that E2 can rapidly alter cell function through ERα, ERβ and/or novel ERs.
Compelling evidence has been generated in the support of a Gq-linked membrane ER (Gq-mER). Intracellular and whole cell recording from guinea pig and mouse hypothalamic slices have been used to characterise the Gq-mER (26,35,37). In these studies, E2 acts stereospecifically to significantly attenuate the potency of μ-opioid and GABAB agonists in activating an inwardly rectifying K+ conductance within physiologically-relevant concentrations (EC50 = 8 nM) in arcuate POMC neurones (26,35). Oestrogenic modulation of μ-opioid and GABAB agonists potency is mimicked either by stimulation of adenylyl cyclase with forskolin or by direct PKA activation with Sp-cAMP (26,35). Furthermore, selective antagonists of PKA block the effects of E2. The activation of PKA is downstream in a signalling cascade that is initiated by the Gq-mER that is linked to activation of phospholipase C (PLC) and protein kinase C (PKC) (35,37). Importantly, the anti-oestrogens ICI 164,384 and ICI 182,780 block the actions of E2 with sub-nanomolar affinity that is similar to ICI’s affinity (Ki) for ERα (26,40). The SERMs, 4-OH tamoxifen and raloxifene all behave like E2 in mediating this response.
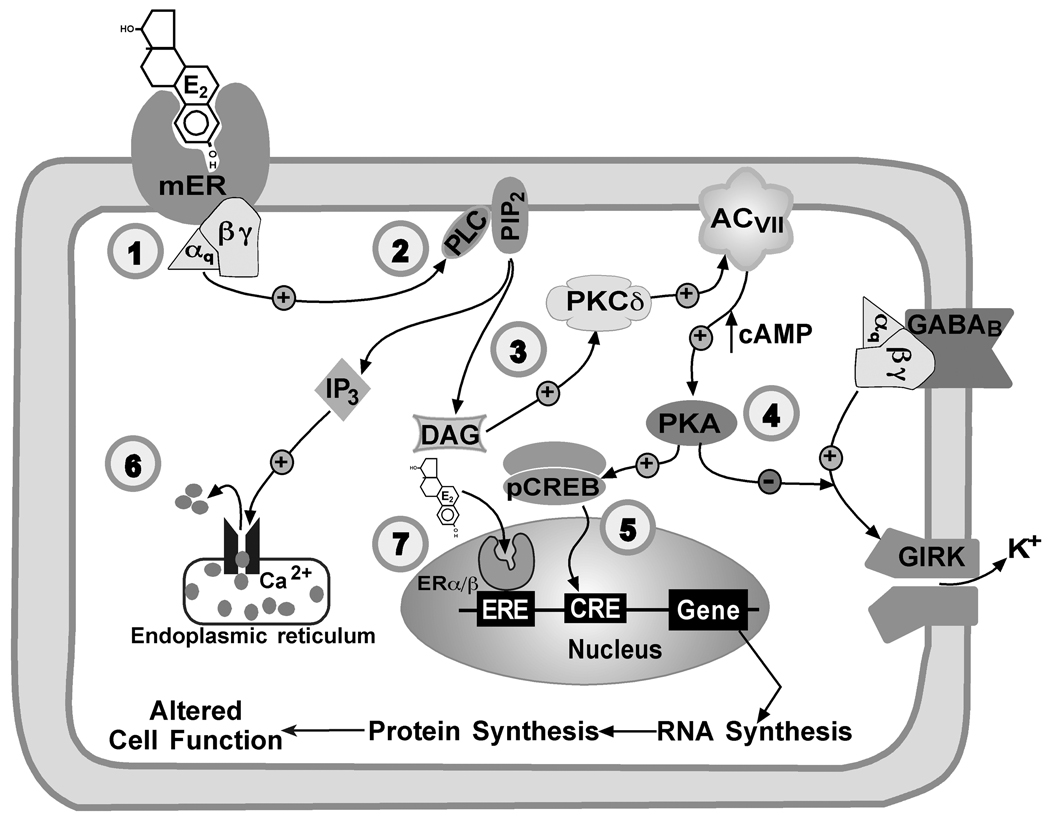
We have formulated the signal transduction pathway for the rapid response to E2 in arcuate neurones. The sequence of events in this model are as follows (See Figure 1): (1) E2 binds to a novel transmembrane oestrogen receptor and activates Gαq; (2) activated Gαq in turn activates PLC which hydrolyzes PIP2 and liberates DAG; (3) free DAG stimulates PKCδ, and PKCδ activates adenylyl cyclase (VII); (4) cAMP levels are elevated and stimulates PKA, which through phosphorylation uncouples the inhibitory GABAB and μ-opioid receptors from activation of GIRK channels (35). Furthermore, activated PKA will phosphorylate CREB (cAMP response element-binding protein) to initiated gene expression via cAMP response element (CRE) (5) and IP3 may release calcium through the IP3 receptor on the endoplasmic reticulum (6). Oestradiol will also bind to nuclear receptors and activate ERE-dependent transcription (7). Other transcriptional pathways that the Gq-mER may activate include the MAPK and calcium-sensitive pathways through PKC and IP3. Recently, we have characterised the signalling of a non-steroidal compound, STX, that specifically targets the G-protein-coupled signalling pathway in both male and female arcuate neurones. In fact, STX has a greater affinity (~20-fold) for the Gq-mER than E2 (37).
Figure 1. Activation of an oestrogen-responsive G-protein-coupled receptor (GPCR) and the associated signalling pathways in arcuate neurons.
(1) The putative Gq-coupled membrane oestrogen receptor (mER) is a G-protein coupled receptor linked to Gαq/11 protein. Oestrogen binding activates the Gαq/11 (2) which in turn activates (+) phospholipase C (PLC) and initiates the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2). PLC hydrolyzes PIP2 into diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). (3) DAG activates protein kinase Cδ (PKCδ) which activates adenylate cyclase VII (AC VII). (4) AC VII increases cAMP production subsequently stimulating protein kinase A (PKA), which through phosphorylation uncouples the inhibitory GABAB and μ-opioid receptors from activation of G-protein coupled inwardly rectifying K+ channels (GIRK) channels. (5) Activation of PKA will also phosphorylate cAMP-response element binding protein (pCREB) and control gene expression through the cAMP response element (CRE). (6) IP3 produced from the hydrolysis of PIP2 activates Ca2+ release from the endoplasmic reticulum that can activate calcium-dependent signalling. (7) Oestradiol will also bind to nuclear receptors and activate oestrogen response element (ERE)-dependent transcription. E2, 17β-oestradiol.
Because many of the rapid effects of E2 can be induced by selective ERα or ERβ ligands, antagonised by the ER antagonist, ICI 182,780, or are lost in animals bearing mutations in ERα and/or ERβ genes (5,41–45), it has been suggested that the membrane-associated ERs might be derived from the same genes as ERα and ERβ (45–48). However, the Gq-mER is not ERα or ERβ, since it is activated by STX, which does not bind to ERα or ERβ (35,37) and has no proliferative effects on reproductive organs (37). STX (and E2) also activates the Gαq signalling pathway in mice lacking either ERα, ERβ or both of these nuclear receptors (37). Our findings indicate that the Gq-mER in hypothalamic arcuate (POMC) neurones is distinct from ERα or ERβ. Definitive characterisation of this Gq-mER awaits cloning of the gene.
Toran-Allerand et al. identified a high-affinity, saturable oestrogen receptor, ER-X, that is associated with caveolar-like microdomains in developing neocortical neurones (49). In organotypic explants of the developing cerebral cortex, E2 induces tyrosine phosphorylation of both ERK1 (extracellular signal-regulated protein kinase 1) and ERK2, an action very similar to a number of growth factors including nerve growth factor (NGF) (34). Interestingly, ER-X also has a distinct pharmacology in that 17α-oestradiol is equipotent as E2 in activating the MAPK/ERK pathway (43,49). However, 17α-oestradiol has no effect on the GABAB response (35), nor on the μ-opioid response, which are both coupled to the same family of GIRK channels in hypothalamic neurones (50). Similarly, Gu and Moss (51) found that 17α-oestradiol did not mimic the actions of E2 in the hippocampus to potentiate the glutamate (kainate)-mediated currents in CA1 pyramidal neurones. Therefore, it appears that the Gq-mER that modulates channel activity in neurones via the PKC-PKA pathway is pharmacologically distinct from the receptor (ER-X) that is coupled to activation of ERK1 and ERK2 to promote growth and survival.
The orphan GPCR, GPR30, is a new oestrogen receptor involved in the rapid actions elicited by E2 in peripheral reproductive tissue (36,52). In transfected breast cancer cells, E2 activates ERK1 and ERK2 independently of ERα or ERβ (38,53). In these cells, E2 activates Gβγ-subunits that promote the release and activation of an epidermal growth factor precursor (proHB-EGF). The active HB-EGF binds to the EGF receptor (ErbB) to facilitate receptor dimerisation and downstream activation of ERK (53–55). GPR30 is localised to endoplasmic reticulum and binds E2 with nanomolar affinity (36). Previously, the expression of GPR30 in the brain including the hypothalamus has been reported (39,56,56–59). In recent studies, GPR30 mRNA was detected in the paraventricular nucleus and supraoptic nucleus (59) and in magnocellular optic tract neurones (60). However, the pharmacology of GPR30 in these neurones has not been characterised. Previously, we have established that E2 is fully efficacious in attenuating the GABAB response in arcuate (POMC) neurones from GPR30 knock-out mice suggesting that GPR30 is not the Gq-mER signalling in hypothalamic POMC neurones (61).
Activation of the Gq-mER in the arcuate nucleus affects energy homeostasis and gene expression
Oestradiol is involved in the regulation of appetite, energy expenditure, body weight, adipose tissue deposition and distribution in females (62–64). Ovariectomy induces an increase in food intake which is reversed with E2 replacement (65–68). In fact, hypo-oestrogenic states are associated with an increase in body weight in many rodent models (37,68–75). The anorectic effects of E2 are partially mediated through actions in the hypothalamus because direct injections of E2 into the paraventricular nucleus of the hypothalamus (PVN) or arcuate/ventromedial nucleus are effective in reducing food intake and body weight (65,66,70). Arcuate POMC neurones through direct synaptic contacts modulate the excitability of hypothalamic neurones that regulate energy homeostasis and are also affected by E2 (76). E2 up-regulates the expression of the peptide β̣-endorphin in proopiomelanocortin (POMC) neurones in ovariectomised female guinea pigs (77,78) and increases the expression of this gene in the arcuate nucleus after chronic treatment (79). Because the Gq-mER is found in the anorectic POMC neurones, we have hypothesised that activation of this receptor would have a physiological effect on energy homeostasis. Indeed, the Gq-mER agonist STX attenuates the post-ovariectomy body weight gain in female guinea pigs. At doses similar to other SERMs, STX (2 or 6 mg/kg) lowers the body weight gain after ovariectomy similar to systemic treatment with oestradiol benzoate in a dose-dependent manner (37,79). Therefore, there are at least two receptors involved in the control of energy homeostasis, ERα and the Gq-mER (80), although we cannot rule out that some of the effects of the Gq-mER on energy homeostasis involve synergistic actions with ERα.
Oestradiol may also control energy homeostasis by regulating gene expression of neuropeptides, cation channels and/or channel modulators (signalling molecules) via both nuclear-initiated (ERα/β) and membrane-initiated signalling (Gq-mER). The potential cross-talk between rapid, membrane-initiated effects of E2 on signalling pathways (and channels) and oestrogenic gene regulation of neuropeptides, channels and signalling mechanisms ultimately determines neuronal function and activity. One probable mechanism for the control of gene expression by STX through the Gq-mER is the activation of CREB by PKA (Figure 1). Genes containing a CREB response element in their promoter regions are potential targets for STX-induced gene expression. However, recent data also suggests that cAMP can activate ERα and initiate ERE-mediated transcription via ERα associations with CREB and CREB binding protein (81,82). Other potential transcriptional mechanisms include MAPK activation via PKC, CaM-CaMKII activation via IP3-induced calcium release (Figure 1) and PI3K-Akt activation (83). To measure gene regulation via the Gq-mER using STX, we employed custom gene microarray analysis of arcuate tissue from STX-treated female guinea pigs coupled to quantitative real-time PCR. The analysis of gene expression with STX was used to evaluate the membrane-initiated versus nuclear-initiated signalling of E2. Because the Gq-mER activates signalling molecules that may impinge on channel function (PLC, PI3K, PKC and PKA) (35,83), this study was focused on the gene regulation of cation channels, signaling molecules and associated genes. As predicted, several relevant genes in the arcuate nucleus were regulated by long-term STX treatment.
Of particular interest in the context of energy homeostasis is the regulation of NPY from these orexigenic neurones. We have recently demonstrated that STX significantly reduces NPY mRNA expression in the arcuate nucleus (79) suggesting that STX exerts some of its anorectic effects by suppressing NPY. While the cellular mechanism is currently unknown, the anorectic effects of STX may be mediated, in part, by channel modulation in POMC neurones and control of gene expression in NPY neurones. However, in immortalised NPY neurones, E2 can suppress NPY gene expression via membrane-mediated PI3K-Akt signalling and via CREB-interactions with ERα at the CRE promoter half-site (84). STX is potentially activating this CREB-CRE mediated suppression of NPY gene expression through the activation of PKA. Another possible indirect effect on NPY activity and gene expression is the increase in GABAA receptor function, which is inhibitory. GABA neurotransmission relies on appropriate clustering of GABAA receptors to the post-synaptic neuronal membrane. In particular, GABAA receptor targeting to the membrane is facilitated by the GEC-1 protein, which connects the receptors to the cytoskeleton and facilitates translocation to the membrane (85). Indeed, long-term E2 and STX treatment both increased the expression of gec1, in the arcuate nucleus (79), confirming previous findings that E2 increases gec1 mRNA expression (86). An increase in gec1 gene expression could potentiate the GABAA-mediated activity in NPY neurones. The indirect modulation of GABA neurotransmission by E2 (and STX) may be an inhibitory oestrogenic mechanism in hypothalamic neurones.
The second indirect mechanism to control neuronal function is the E2-induced gene regulation of cation channels that control neuronal excitability and neurotransmitter release. We have previously shown that E2 induces gene expression of a Ca2+ channel (Cav3.1) subunit in the arcuate nucleus after 24 hr treatment (87) and after long-term E2 treatment (30 day) (79). The E2-induced increase in Cav3.1 subunit expression has previously been demonstrated to increase the peak T-type Ca2+ current by two-fold in arcuate neurones including POMC neurones (87) and increased the magnitude of voltage-dependent calcium currents in unidentified neurones in the ventromedial hypothalamus (88). Furthermore, the increase in Cav3.1 expression in the arcuate nucleus from E2 treatment does not occur in αERKO mice (89) indicating that the expression is under the control of the nuclear receptor. However, STX also significantly up-regulates the Cav3.1 subunit in the arcuate nucleus after long-term treatment (79) suggesting that these two receptor-mediated transcriptional effects converge at the Cav3.1 gene. Since the Gq-mER signalling pathway is found in POMC neurones, one can postulate that the up-regulation after STX-treatment is occurring in these neurones.
Furthermore, the increase in the T-type Ca2+ current facilitates the burst firing of neurones and causes an increase in neurotransmitter and secretory protein (α-melanocyte-stimulating hormone) release. (90). However, in order to deinactivate the T-type current, the neurones must be hyperpolarised below their resting membrane potential. An obvious mechanism to generate a hyperpolarised state is to up-regulate the expression of inhibitory ionotropic receptors and/or K+ channels. One such ionotropic receptor that STX up-regulates is the glycine receptor (91). STX increased the expression of the glycine receptor β subunit, which is the structural subunit for this ligand-gated Cl− channel. Glycine is a major inhibitory neurotransmitter and initiates a post-synaptic increase in chloride conductance through this receptor (92). K+ channels also function to hyperpolarise neurones. We have previously reported that E2 treatment will increase the gene expression of K+ channel subunits that control neuronal excitability in the guinea pig arcuate nucleus (91). E2 treatment increases the mRNA expression of the KCNQ5 channel subunit that underlies the M-current and the inwardly-rectifying K+ (Kir) channel Kir2.4 subunit. The M-current controls neuronal excitability by constitutively hyperpolarising the cell membrane (93) while the Kir2 family is the prime determinant of the resting membrane potential (94,95). Both of these currents are modulated by GPCR that activate the PIP2-PLC-PKC and PKA signalling pathways (93–98). The modulation of K+ channels by PKA is controlled by docking with the scaffold protein, A-kinase anchoring proteins (AKAP) (50). In the arcuate nucleus, E2 down-regulated AKAP11 after 24 hr and long-term treatment (79,91); however, long-term STX treatment increases the expression of the AKAP11 (79). The regulation of this gene by E2 or STX may affect PKA regulation of numerous K+ channels including those discussed in this review.
As stated, STX via the Gq-mER potentially activates a host of signalling pathways. Many of these signalling proteins, which are involved in the modulation of channel activity and gene expression, are also transcriptional targets for both the nuclear ER and Gq-mER receptors. For example, genes involved in calcium signalling pathways are regulated by E2 and STX treatment. Oestradiol treatment, both 24 hr and long-term, increases the expression of calmodulin-1 in the arcuate nucleus (79,91). STX does not regulate calmodulin but does regulate the calmodulin-dependent kinase, CaM kinase II. CaMK II is a modulator of ion channels (Ca2+, K+, Na+) (99) and is required for the Ca2+-sensitive production of long-term potentiation (LTP) in neurones from the hypothalamus (100). The regulation of these calcium signalling molecules may have multiple effects on gene expression, neuronal excitability and synaptic neurotransmitter release.
The PI3K pathway and its associated proteins are also a target for gene regulation by both E2 and STX. Peripheral signals such as leptin and insulin activate phoshatidylinositide 3-kinase (PI3K) via insulin receptor substrate in POMC neurones (101). Furthermore, the rapid attenuation of GIRK channel activity in POMC neurones by E2 involves the activation of PI3K (83). Oestradiol has recently been shown to up-regulate PI3K p85α in the dorsomedial portion of the ventromedial hypothalamic nucleus (adjacent to the arcuate nucleus) after 24 hr treatment but not in the arcuate nucleus. However, E2 up-regulated p55γ expression in the arcuate nucleus (83). Conversely, STX up-regulates the p85α subunit of PI3K and phosphatidylinositol transfer protein β (PITPβ) in the arcuate nucleus. PITPβ transports lipids (phosphatidylinositols) from their site of synthesis (endoplasmic reticulum) to the cellular membrane where they are the preferred substrates for the lipid kinases (PI3K, PI4K, etc.,) (102). Not only is PITPβ activity required for PI3K, this protein is also necessary for PLC-mediated signalling (103). Since PI3K- and PLC-mediated signalling are implicated in the membrane-mediated effects of E2 and other factors that control energy homeostasis in POMC neurones (leptin, insulin, etc.,), any changes in activity or expression of the transfer proteins may be another indirect mechanism for E2 to potentiate the effects of these peripheral signals on energy homeostasis.
Conclusions and Significance
The gonadal steroid E2 participates in numerous functions including reproduction, feeding, neuroprotection and cognition. The classical actions of E2 are to regulate gene transcription through binding to and activating nuclear receptors that stimulate or inhibit gene transcription at specific DNA binding sites. However, recently it has become clear that E2 can exert its action through multiple signalling mechanisms including membrane-initiated steroid signalling. In this review, we have summarised the evidence for E2 activating a novel hypothalamic signal transduction pathway for the control of neuronal excitability and gene transcription that is initiated by a Gq-mER. This novel pathway is in addition to the classical ERE-mediated and non-ERE-mediated transcriptional pathways that have been extensively investigated in central and peripheral tissues. The activation of the Gq-mER provides an additional mechanism for E2 to control gene expression not involving the classical ERs. Activations of the putative hypothalamic Gq-mER via STX is associated not only with new gene transcription but with attenuation in post-ovariectomy body weight gain in a dose-dependent manner. STX is currently being examined in other homeostatic functions and may be an excellent tool to delineate the effects of the Gq-mER on these hypothalamic functions. The Gq-mER clearly is a coupling mechanism between the control of gene transcription and rapid signalling events that together will ultimately determine the effects of E2 on hypothalamic functions. Also, we are beginning to understand the cross-talk between rapid signalling events and changes in gene expression. However, many challenges lie ahead to fully identify the nature of membrane steroid receptors, functional properties and integration with nuclear steroid receptors.
ACKNOWLEDGMENTS
The authors thank members of their laboratories who contributed to the work described herein, especially Drs. Chunguang Zhang and Anna Malyala. The work from the authors’ laboratories was supported by PHS grants NS 43330, NS 38809 and DK 68098.
REFERENCES
- 1.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 2.Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 3.Hammes SR, Levin ER. Extra-nuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 4.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 5.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JÅ. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 7.Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: Implications for female reproductive physiology. Horm Behav. 2001;40:169–177. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- 8.Stossi F, Likhite VS, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cylcin G2 promoter. J Biol Chem. 2006;281:16272–16278. doi: 10.1074/jbc.M513405200. [DOI] [PubMed] [Google Scholar]
- 9.Kininis M, Chen BS, Diehl AG, Issacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol. 2007;27:5090–5104. doi: 10.1128/MCB.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Malley BW, Tsai M-J. Molecular pathways of steroid receptor action. Biol Repro. 1992;46:163–167. doi: 10.1095/biolreprod46.2.163. [DOI] [PubMed] [Google Scholar]
- 11.Muramatsu M, Inoue S. Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem Biophys Res Commun. 2000;270:1–10. doi: 10.1006/bbrc.2000.2214. [DOI] [PubMed] [Google Scholar]
- 12.Gruber CJ, Gruber DM, Gruber IM, Wieser F, Huber JC. Anatomy of the estrogen response element. Trends Endocrinol Metab. 2004;15:73–78. doi: 10.1016/j.tem.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson JÅ, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ER and ER at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP. Determinants contributing to estrogen-regulated expression of SK3. Biochem Biophys Res Commun. 2003;303:660–668. doi: 10.1016/s0006-291x(03)00408-x. [DOI] [PubMed] [Google Scholar]
- 15.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 16.Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 17.Pietras RJ, Szego CM. Estrogen receptors in uterine plasma membrane. J Steroid Biochem. 1979;11:1471–1483. doi: 10.1016/0022-4731(79)90124-9. [DOI] [PubMed] [Google Scholar]
- 18.Towle AC, Sze PY. Steroid binding to synaptic plasma membrane: Differential binding of glucocorticoids and gonadal steroids. Journal of Steroid Biochemistry. 1983;18:135–143. doi: 10.1016/0022-4731(83)90079-1. [DOI] [PubMed] [Google Scholar]
- 19.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 20.Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29:199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- 21.Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: Effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 22.Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci U S A. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cato ACB, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Science's STKE. 2002;2002:re9–re21. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- 24.Yang S-H, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- 25.Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr Opin Neurobiol. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 26.Lagrange AH, Rønnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 28.Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watters JJ, Dorsa DM. Transcriptional effects of estrogen on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci. 1998;18:6672–6680. doi: 10.1523/JNEUROSCI.18-17-06672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 31.Kelly MJ, Rønnekleiv OK. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff DW, editor. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 361–380. [Google Scholar]
- 32.Rønnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Kelly MJ, Rønnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toran-Allerand CD. Estrogen and the brain: beyond ER-α, ER-β and 17β-estradiol. Ann NY Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- 35.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 37.Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy S, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 40.Weatherill PJ, Wilson APM, Nicholson RI, Davies P, Wakeling AE. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Steroid Biochem Mol Biol. 1988;30:263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- 41.Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)alpha and ERbeta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- 44.Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 47.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 48.Szegõ ÉM, Barabás K, Balog J, Szilágyi N, Korach KS, Juhász G, Abrahám IM. Estrogen induces estrogen receptor α-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons In Vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lagrange AH, Rønnekleiv OK, Kelly MJ. The potency of μ-opioid hyperpolarization of hypothalamic arcuate neurons is rapidly attenuated by 17β-estradiol. J Neurosci. 1994;14:6196–6204. doi: 10.1523/JNEUROSCI.14-10-06196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu Q, Moss RL. 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an Estrogen Membrane Receptor Coupled to a G-protein in Human Breast Cancer Cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 53.Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 54.Filardo EJ, Quinn JA, Bland KI, Frackelton ARJ. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 55.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-Mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinology. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 56.Owman C, Blay P, Nilsson C, Lolait SJ. Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt's lymphoma and widely distributed in brain and peripheral tissues. Biochem Biophys Res Commun. 1996;228:285–292. doi: 10.1006/bbrc.1996.1654. [DOI] [PubMed] [Google Scholar]
- 57.O'Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH, Kolakowski LFJ, George SR. Discovery of three novel G-protein-coupled receptor genes. Genomics. 1998;47:310–313. doi: 10.1006/geno.1998.5095. [DOI] [PubMed] [Google Scholar]
- 58.Spary EJ, Chapman SE, Maqbool A, Batten TFC. Novel oestrogen receptor GPR30: brain localisation and changes in expression during the rat oestrus cycle. Auton Neurosci. 2007;135:61–62. [Google Scholar]
- 59.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 60.Sakamoto H, Matsuda K-I, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M. Expression of GPR30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology. 2007;148:5842–5850. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- 61.Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milewicz A, Bidzinska B, Mikulski E, Demissie M, Tworowska U. Influence of obesity and menopausal status on serum leptin, cholecystokinin, galanin and neuropeptide Y levels. Gynecol Endocrinol. 2000;14:196–203. doi: 10.3109/09513590009167682. [DOI] [PubMed] [Google Scholar]
- 63.Geary N. Estradiol, CCK and satiation. Peptides. 2001;22:1251–1263. doi: 10.1016/s0196-9781(01)00449-1. [DOI] [PubMed] [Google Scholar]
- 64.Poehlman ET. Menopause, energy expenditure, and body composition. Acta Obstet Gynecol Scand. 2002;81:603–611. doi: 10.1034/j.1600-0412.2002.810705.x. [DOI] [PubMed] [Google Scholar]
- 65.Ahdieh HB, Wade GN. Effects of hysterectomy on sexual receptivity, food intake, running wheel activity, and hypothalamic estrogen and progestin receptors in rats. J Comp Physiol Psychol. 1982;96:886–892. [PubMed] [Google Scholar]
- 66.Colvin GB, Sawyer CH. Induction of running activity by intracerebral implants of estrogen in ovariectomized rats. Neuroendocrinology. 1969;4:309–320. doi: 10.1159/000121762. [DOI] [PubMed] [Google Scholar]
- 67.Shimomura Y, Shimizu H, Takahashi M, Sato N, Uehara Y, Fukatsu A, Negishi M, Kobayashi I, Kobayashi S. The significance of decreased ambulatory activity during the generation by long-term observation of obesity in ovariectomized rats. Physiol Behav. 1990;47:155–159. doi: 10.1016/0031-9384(90)90055-9. [DOI] [PubMed] [Google Scholar]
- 68.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 69.Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6:329–349. doi: 10.1016/0018-506x(75)90003-3. [DOI] [PubMed] [Google Scholar]
- 70.Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- 71.Czaja JA. Sex differences in the activational effects of gonadal hormones on food intake and body weight. Physiol Behav. 1984;33:553–558. doi: 10.1016/0031-9384(84)90370-6. [DOI] [PubMed] [Google Scholar]
- 72.McCaffrey TA, Czaja JA. Diverse effects of estradiol-17 beta: concurrent suppression of appetite, blood pressure and vascular reactivity in conscious, unrestrained animals. Physiol Behav. 1989;45:649–657. doi: 10.1016/0031-9384(89)90086-3. [DOI] [PubMed] [Google Scholar]
- 73.Jones MEE, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao SG, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 75.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 76.Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8:561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- 77.Thornton JE, Loose MD, Kelly MJ, Rønnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- 78.Bethea CL, Hess DL, Widmann AA, Henningfeld JM. Effects of progesterone on prolactin, hypothalamic beta-endorphin, hypothalamic substance P, and midbrain serotonin in guinea pigs. Neuroendocrinology. 1995;61:695–703. doi: 10.1159/000126897. [DOI] [PubMed] [Google Scholar]
- 79.Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 81.Lazennec G, Thomas JA, Katzenellenbogen BS. Involvement of cyclic AMP response element binding protein (CREB) and estrogen receptor phosphorylation in the synergistic activation of the estrogen receptor by estradiol and protein kinase activators. J Steroid Biochem Mol Biol. 2001;77:193–203. doi: 10.1016/s0960-0760(01)00060-7. [DOI] [PubMed] [Google Scholar]
- 82.Coleman KM, Dutertre M, El Gharbawy A, Rowan BG, Weigel NL, Smith CL. Mechanistic differences in the activation of estrogen receptor-alpha (ER alpha)- and ER beta-dependent gene expression by cAMP signaling pathway(s) J Biol Chem. 2003;278:12834–12845. doi: 10.1074/jbc.M212312200. [DOI] [PubMed] [Google Scholar]
- 83.Malyala A, Zhang C, Bryant D, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- 84.Titolo D, Mayer CM, Dhillon SS, Cai F, Belsham DD. Estrogen failitates both phosphatidylinositol 3-kinase/Akt and ERK1/2 mitogen-activated protein kinase membrane signaling required for long-term neuropeptide Y transcriptional regulation in clonal, immortalized neurons. J Neurosci. 2008;28:6473–6482. doi: 10.1523/JNEUROSCI.0514-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang H, Bedford FK, Brandon NJ, Moss SJ. Olsen RW. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- 86.Malyala A, Pattee P, Nagalla SR, Kelly MJ, Rønnekleiv OK. Suppression subtractive hybridization and microarray identification of estrogen regulated hypothalamic genes. Neurochem Res. 2004;29:1189–1200. doi: 10.1023/b:nere.0000023606.13670.1d. [DOI] [PubMed] [Google Scholar]
- 87.Qiu J, Bosch MA, Jamali K, Xue C, Kelly MJ, Rønnekleiv OK. Estrogen upregulates T-type calcium channels in the hypothalamus and pituitary. J Neurosci. 2006;26:11072–11082. doi: 10.1523/JNEUROSCI.3229-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee AW, Kyrozis A, Chevaleyre V, Kow L-M, Zhou J, Devidze N, Zhang Q, Etgen AM, Pfaff DW. Voltage-dependent calcium channels in ventromedial hypothalamic neurons of postnatal rats: modulation by oestradiol and phenylephrine. J Neuroendocrinol. 2008;20:188–198. doi: 10.1111/j.1365-2826.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- 89.Bosch MA, Hou J, Kelly MJ, Rønnekleiv OK. 17β-estradiol regulation of the mRNA expression of T-type calcium channel subunits: role of estrogen receptor α and estrogen receptor β. J Comp Neuro. 2009;512:347–358. doi: 10.1002/cne.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- 91.Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- 92.Kirsch J. Glycinergic transmission. Cell Tissue Res. 2006;326:535–540. doi: 10.1007/s00441-006-0261-x. [DOI] [PubMed] [Google Scholar]
- 93.Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001;90:1–19. doi: 10.1016/s0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- 94.Töpert C, Döring F, Wischmeyer E, Karschin C, Brockhaus J, Ballanyl K, Derst C, Karschin A. Kir2.4: a novel K+ inward rectifier channel associated with motorneurons of cranial nerve nuclei. J Neurosci. 1998;18:4096–4105. doi: 10.1523/JNEUROSCI.18-11-04096.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prüss H, Derst C, Lommel R, Veh RW. Differential distribution of individual subunits of strongly inwardly rectifying potassium channels (Kir2 family) in rat brain. Mol Brain Res. 2005;139:63–79. doi: 10.1016/j.molbrainres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- 97.Du X, Zhang H, Lopes C, Mirshashi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J Biol Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- 98.Perillan PR, Chen M, Potts EA, Simard JM. Transforming growth factor-beta 1 regulates Kir2.3 inward rectifier K+ channels via phospholipase C and protein kinase C-delta in reactive astrocytes from adult rat brain. J Biol Chem. 2002;277:1974–1980. doi: 10.1074/jbc.M107984200. [DOI] [PubMed] [Google Scholar]
- 99.Pitt GS. Calmodulin and CaMKII as molecular switches for cardiac ion channels. Cardiovasc Res. 2007;73:641–647. doi: 10.1016/j.cardiores.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 100.Fukunaga K, Horikawa K, Shibata S, Takeuchi Y, Miyamoto E. Ca2+/ calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J Neurosci Res. 2002;70:799–807. doi: 10.1002/jnr.10400. [DOI] [PubMed] [Google Scholar]
- 101.Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta. 2007;1771:677–691. doi: 10.1016/j.bbalip.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 103.Thomas GMH, Cunningham E, Fensome A, Ball A, Totty NF, Truong O, Hsuan JJ, Cockcroft S. An essential role for phosphatidylinositol transfer protein in phospholipase C-mediated inositol lipid signaling. Cell. 1993;74:919–928. doi: 10.1016/0092-8674(93)90471-2. [DOI] [PubMed] [Google Scholar]