Abstract
Recent theories have posited that the hippocampus and thalamus serve distinct, yet related, roles in episodic memory. Whereas the hippocampus has been implicated in long-term memory encoding and storage, the thalamus, as a whole, has been implicated in the selection of items for subsequent encoding and the use of retrieval strategies. However, dissociating the memory impairment that occurs following thalamic injury as distinguished from that following hippocampal injury has proven difficult. This study examined relationships between MRI volumetric measures of the hippocampus and thalamus and their contributions to prose and rote verbal memory functioning in 18 patients with intractable temporal lobe epilepsy (TLE). Results revealed that bilateral hippocampal and thalamic volume independently predicted delayed prose verbal memory functioning. However, bilateral hippocampal, but not thalamic, volume predicted delayed rote verbal memory functioning. Follow-up analyses indicated that bilateral thalamic volume independently predicted immediate prose, but not immediate rote, verbal recall, whereas bilateral hippocampal volume was not associated with any of these immediate memory measures. These findings underscore the cognitive significance of thalamic atrophy in chronic TLE, demonstrating that hippocampal and thalamic volume make quantitatively, and perhaps qualitatively, distinct contributions to episodic memory functioning in TLE patients. They are also consistent with theories proposing that the hippocampus supports long-term memory encoding and storage, whereas the thalamus is implicated in the executive aspects of episodic memory.
Keywords: Hippocampus, Thalamus, Verbal memory, MRI volumetrics, Temporal lobe epilepsy
Introduction
It is widely recognized that both the hippocampus and thalamus play a critical role in episodic memory functioning (Kopelman, 1995; Kopelman & Stanhope, 2002; Squire, 1982; Van der Werf, Witter, Uylings, & Jolles, 2000). However, the extent to which the hippocampus and thalamus make unique contributions to episodic memory functioning and the nature of their respective contributions remains somewhat unclear. Recent theories have proposed that the hippocampus and thalamus serve distinct, yet related roles, in episodic memory. Whereas the hippocampus supports long-term memory encoding and storage of the associations among the constituent elements of an experience (i.e., relational material) (Eichenbaum, 2004; Eichenbaum & Cohen, 2001; Morris et al., 2003; O’Reilly & Rudy, 2001; Rolls, 1990; Ryan & Cohen, 2003; Squire & Zola-Morgan, 1991), the thalamus has been implicated in the executive aspects of episodic memory. Specifically, it has been hypothesized that the anterior thalamic nuclei play a role in the selection of items for subsequent memory storage and are thus involved in encoding strategies (Aggleton & Brown, 1999; Van der Werf, Jolles, Witter, & Uylings, 2003), that the mediodorsal nucleus of the thalamus is involved in the coordination and selection of suitable, active retrieval strategies in conjunction with the prefrontal cortex (Van der Werf, Jolles, et al., 2003), and that the intralaminar and midline nuclei of the thalamus are implicated in the allocation of cortical activation necessary for the execution of encoding and retrieval processes (Van der Werf, Jolles, et al., 2003).
Although the hippocampus and thalamus are believed to support different episodic memory processes, dissociating the contributions of these brain structures to episodic memory has proven difficult. The hippocampus and thalamus share extensive reciprocal connections (Dolleman-van der Weel & Witter, 1996; Herkenham, 1978; Oikawa, Sasaki, Tamakawa, & Kamei, 2001; Su & Bentivoglio, 1990; van Groen & Wyss, 1990; Wouterlood, Saldana, & Witter, 1990), and injury to either structure may alter neuronal functioning in the other structure (Bertram & Zhang, 1999; Dolleman-van der Weel, Lopes da Silva, & Witter, 1997; Meyer, Obara, & Muramatsu, 1993). Moreover, injury to either the hippocampus or thalamus can result in comparably dense amnesic syndromes (Kopelman & Stanhope, 2002), a finding that led Aggleton and Brown (1999) to state that “the traditional distinction between temporal lobe and diencephalic amnesics is misleading; both groups have damage to the same functional system” (p. 426).
However, other research has illustrated subtle differences in the memory impairments exhibited by temporal lobe and diencephalic amnesics. It has been demonstrated that Korsakoff’s patients, who have sustained injury to the thalamus, exhibit heightened susceptibility to proactive interference, in addition to deficits in judging the temporal order of events, compared to temporal lobe amnesics (Squire, 1982). Additionally, lesions to the mediodorsal, midline nuclei, or intralaminar nuclei of the thalamus have been associated with executive dysfunction (e.g., impaired set-switching and inhibition), in addition to memory deficits (Van der Werf, Scheltens, et al., 2003), whereas hippocampal lesions have been shown to result in highly selective memory deficits (Eichenbaum & Cohen, 2001). These findings appear to suggest that thalamic injury results in deficits in the executive aspects of episodic memory (i.e., the selection of to-be-encoded information and retrieval strategies). However, it remains unclear whether the thalamus proper directly subserves some of these mnemonic processes or whether the observed deficits arise secondarily from a disconnection of the medial temporal lobe from the prefrontal cortex following diencephalic injury, a theory that was put forth by Warrington and Weiskrantz (1982).
The current study aims to quantify the relative contributions of magnetic resonance imaging (MRI) volumetric measures of the hippocampus and whole thalamus to episodic memory functioning in patients with temporal lobe epilepsy (TLE). Patients with TLE present a suitable population for such an investigation because episodic memory impairments are prevalent in these patients when compared to healthy controls (Leritz, Grande, Bauer, 2006) and when compared with patients with extra-temporal or generalized seizures (Bergin, Thompson, Baxendale, Fish, Shorvon, 2000). Moreover, both the hippocampus and thalamus are involved in the propagation of seizure activity and exhibit pathologic changes in TLE. Electrophysiological studies in animal models of TLE and in human clinical TLE patients have implicated the hippocampus and other temporal limbic structures in seizure initiation (Spencer & Spencer, 1996), whereas the thalamus is believed to play a role in seizure modulation (Bertram et al., 2001; Guye et al., 2006). MRI and positron emission tomography (PET) studies have also revealed structural and metabolic abnormalities of the temporal lobe (Hermann, Seidenberg, & Bell, 2002; Rubin et al., 1995), hippocampus (Henry et al., 1993; Seidenberg et al., 2005), and thalamus (Juhasz et al., 1999; Keller, Wilke, Wieshmann, Sluming, & Roberts, 2004) in TLE patients. Concerning the latter, a recent study concluded that thalamic atrophy in TLE patients was most prominent in those thalamic subnuclei having strong connections with the hippocampus, such as the anterior portion of the thalamus (Bonilha, Rorden, Castellano, Cendes, & Li, 2005).
Whereas several TLE studies have demonstrated relationships between structural volumetric measures of hippocampal integrity and performance on measures of episodic memory (Griffith et al., 2003), far fewer have examined atrophy of extra-hippocampal structures in relation to memory functioning in TLE patients (e.g., Bonilha et al., 2007; Martin et al., 1999).
Regarding thalamic-memory relationships, Seidenberg et al. (2008) reported that smaller whole thalamic volume was associated with poorer performance in episodic memory, as well as in several other cognitive domains (e.g., confrontation naming, IQ, executive functioning), in TLE patients. Additionally, Rausch, Henry, Ary, Engel, and Mazziotta (1994) reported that asymmetric resting PET glucose metabolism of the whole thalamus and lateral temporal lobe, but not mesial temporal lobe, independently predicted verbal memory functioning in TLE patients. Also of relevance, Kopelman et al. (2001) found significant correlations of hippocampal and thalamic volume with measures of recall and recognition in memory disordered patients with diencephalon, temporal lobe, or frontal lobe injury. Although noteworthy, these studies do not indicate whether measures of hippocampal and thalamic integrity make independent quantitative contributions to episodic memory functioning in TLE patients. They also provide limited information regarding the qualitative nature of the contributions of the hippocampus and thalamus to episodic memory.
Our study investigated these issues by directly examining the relative contributions of volumetric measures of the hippocampus and whole thalamus to verbal episodic memory in TLE patients. To this end, we employed statistical models in which hippocampal and whole thalamic MRI volume served as potential predictors of performance on one measure of delayed prose verbal memory and two measures of delayed rote verbal memory. Measures of delayed prose and rote verbal memory were specifically chosen because prose verbal memory ostensibly relies on encoding and storage processes of relational material in long-term episodic memory, in addition to the executive aspects of episodic memory (e.g., the organization of propositionally-or logically-related material at encoding and the retrieval of this material at recall), whereas rote verbal memory (e.g., paired associates) predominately relies on encoding and storage processes in long-term episodic memory (Lillywhite et al., 2007; Rausch et al., 1994; Saling et al., 1993). Based on contemporary neurobiological theories of episodic memory (Aggleton & Brown, 1999; Van der Werf, Jolles et al., 2003), we hypothesized that hippocampal and whole thalamic volume would independently predict delayed prose verbal memory functioning in the TLE sample, whereas hippocampal, but not thalamic, volume would predict delayed rote verbal memory functioning.
Method
Participants
Data for the current study were derived from eighteen participants with TLE who participated from July 1988 to November 1999 in a larger study of the neuro-developmental impact of epilepsy (Hermann et al., 2002; Hermann et al., 2003) conducted at the University of Wisconsin Hospital Department of Neurology (UW). Initial selection criteria included: (1) chronological age from 14 to 60 years, (2) complex partial seizures of definite or probable temporal lobe origin (see details below), (3) absence of MRI abnormalities other than atrophy on clinical reading, and (4) no other neurological disorder. A board-certified neurologist with special expertise in epileptology reviewed patients’ medical records. This review, blinded to all MRI volumetric and neuropsychological data, included seizure semiology, previous EEGs, clinical neuroimaging reports, and all available medical records. On the basis of this review, each patient was classified as having seizures of definite, probable, or possible temporal lobe origin. Definite temporal lobe epilepsy was defined by continuous video/EEG monitoring of spontaneous seizures demonstrating temporal lobe seizure onset; probable temporal lobe epilepsy was determined by review of clinical semiology with features reported to reliably identify complex partial seizures of temporal lobe origin versus onset in other regions (e.g., the frontal lobe) in conjunction with interictal EEGs, neuroimaging findings, and developmental and clinical history. Only patients meeting criteria for definite or probable temporal lobe epilepsy were eligible for study participation, while patients with possible temporal lobe epilepsy were excluded.
Demographic and seizure characteristics of the participants are listed in Table 1. The demographic characteristics of these participants closely resemble those of other participants in the overall study (Hermann et al., 2002; Hermann et al., 2003) and closely match those of other TLE patients who have been evaluated for epilepsy surgery at UW (Griffith et al., 2003; Griffith et al., 2004). Of note, for those patients in the current sample who underwent intensive video/EEG monitoring, seizure onset was localized to the left temporal lobe in five patients and to the right temporal lobe in nine patients. One patient had bilateral temporal lobe onset.
Table 1.
Demographics, seizure characteristics, and memory performance in the TLE sample
| Demographics | |
| Gender (female/male) | 13/5 |
| Handedness (left/right) | 3/15 |
| Age | 34.5 (13.2) [14–58]a |
| Years of education | 12.7 (2.3) [9–17] |
| WAIS-IIIb Full Scale IQ | 92.8 (13.6) [62–121] |
| Seizure characteristics | |
| Participants with video EEG monitoring (left/right/bilateral) | 5/9/1 |
| Participants without video EEG monitoring | 3 |
| Initial precipitating incident (yes/no)c | 11/5 |
| Anti-epileptic drug regimen (monotherapy/polytherapy)c | 5/12 |
| Age at onset (years) | 13.7 (8.1) [2–35] |
| Duration of epilepsy (years) | 20.9 (14.7) [1–55] |
| Seizure frequency—past year (weekly or greater/monthly) | 8/10 |
Mean (SD) [range].
Wechsler Adult Intelligence Scale—third edition.
Totals reflect missing data.
All participants gave informed consent for participation in these procedures as a part of this UW Institutional Review Board-approved protocol.
Magnetic resonance image acquisition and processing
MRIs were obtained on a 1.5-T GE Signa MR scanner. Sequences acquired for each participant included: (1) T1-weighted, three-dimensional SPGR acquired with the following parameters: TE = 5, TR = 24, flip angle = 40, NEX = 2, FOV = 26, slice thickness = 1.5mm, slice plane = coronal, matrix = 256 x 192; (2) Proton Density (PD), and (3) T2-weighted images acquired with the following parameters: TE = 36 (for PD) or 96 ms (for T2), TR = 3000 ms, NEX = 1, FOV = 26, slice thickness = 3.0 mm, slice plane = coronal, matrix = 256 x 192, and an echo train length = 8.
Following acquisition, MRIs were processed within Brain Research: Analysis of Images, Networks, and Systems (BRAINS 2), a semi-automated software package designed for analysis of structural and functional neuroimaging data (Magnotta et al., 2002). The T1 weighted images were spatially normalized so that the anterior-posterior axis of the brain was realigned parallel to the anterior commisure - posterior commisure line, and the inter-hemispheric fissure was aligned on the other two axes. A six-point linear transformation was used to warp the standard Talairach atlas space (Talairach & Tournoux, 1988) onto the resampled image. Images from the three pulse sequences were then co-registered using a local adaptation of automated image registration software (Woods, Cherry, & Mazziotta, 1992). Following alignment of the image sets, the PD and T2 images were re-sampled into 1mm cubic voxels (T1 scans were acquired with 1mm voxels). Next, an automated algorithm for each image modality classified each voxel into gray matter, white matter, CSF, blood, or “unclassified” (i.e., voxels that were not identified as grey matter, white matter, CSF, or blood) (Harris et al., 1999; Magnotta et al., 2002). This process yielded a segmented brain image for each participant, permitting continuous volumetric tissue measurements within the ROIs of interest.
Regions of interest and volume computation
Automated neural networks identifying the hippocampus, whole thalamus, and total intracranial volume were applied to segmented images using guidelines established and validated by the University of Iowa (Magnotta et al., 1999; Pantel et al., 2000; Spinks et al., 2002). Per established guidelines, the resulting regions of interest (ROIs) were then manually inspected and trimmed, when necessary, by a researcher (C.S.) in order to obtain the most anatomically valid volumetric measure of the hippocampus, whole thalamus, and total intracranial volume. Hippocampal ROIs included grey matter of the pes or head of the hippocampus, the body, and the tail (see Pantel et al. (2000) for description of hippocampal boundaries and method validation). Thalamic ROIs included grey matter of the whole thalamus (see Spinks et al. (2002) for description of thalamic boundaries and method validation). Total intracranial volume ROIs included three tissue classes (gray matter, white matter, CSF) within the border of the dura. Total intracranial volume ROIs excluded the dural venous sinuses, including the superior sagital, transverse, straight, and sigmoid sinuses, and blood vessels visible on sulci and fissures. Tracing of the brain stem was discontinued when the cerebral arteries first appeared posteriorly (Magnotta et al., 1999). Left, right, and combined bilateral hippocampal and thalamic volume, in addition to total intracranial volume, were then computed from the ROIs generated in BRAINS 2.
A fourth ROI measuring gross volume of bilateral occipital lobe grey matter was also obtained. To calculate this, the volume of grey matter within the occipital Talairach box of each participant was measured in BRAINS 2 (Magnotta et al., 2002). This served as a “control” volumetric measure that was not expected to correlate with any of the memory measures and thus would reinforce interpretations that hippocampal- or thalamic-memory relationships were indeed specific to the hippocampus or thalamus, rather than overall brain integrity.
Volumetric measures of each structure (i.e., left and right hippocampus and thalamus, and bilateral occipital grey matter) were divided by total intracranial volume, in order to adjust for differences in the size of ROIs due to body size and age-related brain atrophy. Means and standard deviations of raw (uncorrected) volumetric measures are listed in Table 2.
Table 2.
Means and standard deviations of raw (uncorrected) ROI volumetric measures
| Region of Interest | Left (cm3) | Right (cm3) | Bilateral (cm3) |
|---|---|---|---|
| Hippocampus | 1.61 (.49)a | 1.26 (.35) | 2.87 (.72) |
| Thalamus | 5.76 (.67) | 6.00 (.87) | 11.76 (1.51) |
| Occipital grey matterb | -- | -- | 61.52 (9.38) |
| Total Intracranial Vol. | -- | -- | 1,320.63 (185.02) |
Mean (SD).
Represents the volume of grey matter within the occipital Talairach box.
Memory testing
All participants completed Logical Memory (LM) I (immediate) and II (30-minute delay) and Verbal Paired Associates (VPA) I and II of the Wechsler Memory Scale—III (WMS-III; Wechsler, 1997), in addition to the six-trial Verbal Selective Reminding Task (VSRT) immediate and delayed recall (Buschke, 1973). All memory measures were administered in a standardized fashion in accordance with their test manuals (Buschke, 1973; Wechsler, 1997). On LM, participants verbally recalled two stories without cues, either immediately (LM I) or 30 minutes after having been read the story aloud (LM II). Raw LM scores reflected the total number of correct story details recalled by participants and served as indexes of immediate and delayed prose verbal memory functioning. On VPA, participants verbally recalled unrelated word-pairings upon cueing with the first word in the pairing, either immediately (LM I) or 30 minutes following a learning phase for the word-pairings (LM II). On VSRT, participants verbally recalled an unrelated list of words without cues, either immediately (VSRT immediate recall) or 30 minutes following a six-trial learning phase for the word list (VSRT delayed recall). Raw scores on VPA and VSRT reflected the total number of correct words recalled. These measures served as indexes of immediate and delayed memory for rote verbal information.
Statistical analyses
To examine associations between variables, Pearson correlations were calculated between adjusted left, right, and bilateral hippocampal and thalamic MRI volume and the three delayed memory measures. Subsequently, hierarchical multiple regressions were conducted to examine the unique contributions of bilateral hippocampal and thalamic volume to performance on each of the three delayed memory measures (i.e., LM II, VPA II, VSRT delayed recall). Bilateral, as opposed to separate left and right, volumetric measures were used, given that the TLE sample consisted of a mixed group of patients with left, right, and bilateral seizure onset. As such, the expected differences in correlations of left and right hippocampus and thalamus with memory are generally obfuscated. The proportion of unique variance in each of the three memory measures attributable to bilateral hippocampal volume was determined by first entering a block of predictors composed of bilateral thalamic volume and second by entering a block composed of bilateral hippocampal volume. Conversely, the proportion of unique variance in each of the three memory measures attributable to bilateral thalamic volume was determined by first entering a block of predictors composed of bilateral hippocampal volume and second by entering a block composed of bilateral thalamic volume. The change in the coefficient of determination ( R2) from the first block of predictors to the second block represented the percentage of unique variance in a given memory measure attributable to the volumetric measure of interest. A two-tailed α of 0.05 was adopted for all analyses.
Results
Correlations among volumetric measures and among memory measures
Table 3 lists Pearson correlations between volumetric measures of the left, right, and bilateral hippocampus and thalamus and performance on the delayed memory measures. Left, right, and bilateral hippocampal volume showed significant, positive correlations with each other (all p’s < .05), as did left, right, and bilateral thalamic volume (all p’s < .01). Hippocampal volumes were not significantly correlated with thalamic volumes (all p’s > .10).
Table 3.
Pearson correlations between MRI volumes and memory measures
| Left HC Vola | Right HC Vol | Bilateral HC Vol | Left TH Vol | Right TH Vol | Bilateral TH Vol | LM delay | VSRT delay | |
|---|---|---|---|---|---|---|---|---|
| Left HC Vol | — | |||||||
| Right HC Vol | .51** | — | ||||||
| Bilateral HC Vol | .93† | .80† | — | |||||
| Left TH Vol | .11 | .24 | .18 | — | ||||
| Right TH Vol | −.17 | .22 | −.02 | .93† | — | |||
| Bilateral TH Vol | −.05 | .24 | .07 | .98† | .99† | — | ||
| LM delay | .37 | .57** | .51** | .61† | .52** | .57** | — | |
| VSRT delay | .41* | .65† | .57** | .37 | .28 | .32 | .72† | — |
| VPA delay | .39 | .42* | .46* | .34 | .23 | .29 | .82† | .56** |
HC, hippocampus; LM, Logical Memory; TH, thalamus; Vol, MRI structural volume; VPA, verbal paired associates; VSRT, verbal selective reminding task
p < .10
p < .05
p < .01
All delayed memory measures were positively correlated with one another (all p’s < .05).
Correlations between volumetric and memory measures
Left, right, and bilateral volumetric measures of the hippocampus and thalamus positively correlated with performance on each of the three delayed memory measures; however, only some of these correlations reached statistical significance (see Table 3). LM II scores correlated with left (p = .007), right (p = .027), and bilateral (p = .014) thalamic volume, in addition to right (p = .013) and bilateral (p = .031) hippocampal volume. However, LM II scores were not significantly correlated with left hippocampal volume (p > .10). VSRT delayed recall scores correlated with right (p = .004) and bilateral (p = .014) hippocampal volume and showed a trend towards significance with left hippocampal volume (p = .093). However, correlations between VSRT delayed recall and left, right, and bilateral thalamic volume (all p’s > .10) were not significant. Last, correlations between VPA II scores with right (p = .083) and bilateral (p = .056) hippocampal volume showed trends toward statistical significance; however, correlations between VPA II scores with left hippocampal volume and left, right, and bilateral thalamic volume were not significant (all p’s > .10). The “control” volumetric measure of bilateral grey matter in the occipital Talairach box was not associated with performance on any of the delayed memory measures (all p’s > .20), as expected.
Variance in memory measures uniquely attributable to bilateral hippocampal or thalamic volume
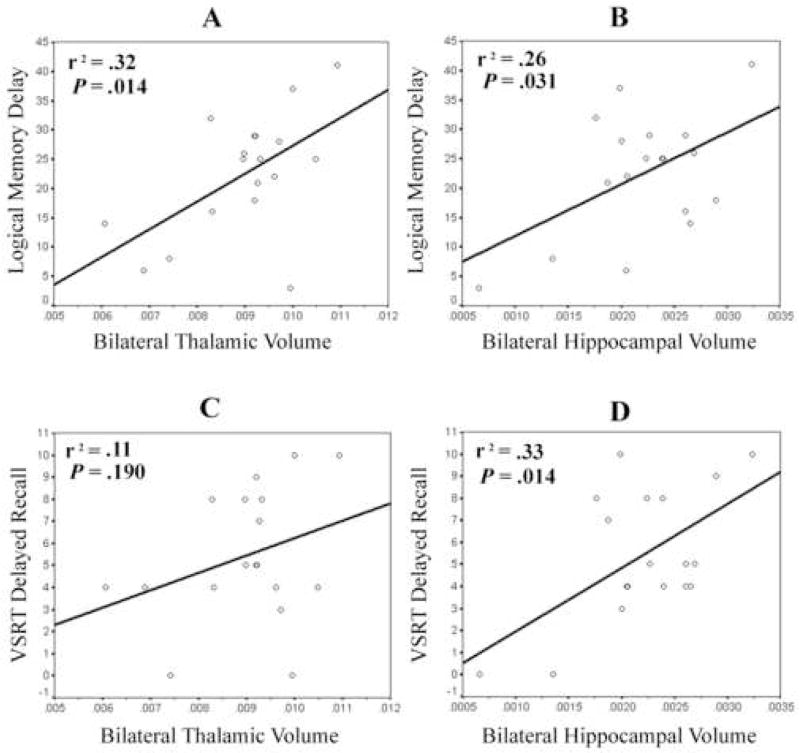
For LM II scores, bilateral hippocampal volume accounted for 26% of the variance before statistically controlling for bilateral thalamic volume (F(1,16) = 5.57, p = .031), and 22% of the variance after controlling for bilateral thalamic volume (F(1,15) = 7.23, p = .017). Similarly, bilateral thalamic volume accounted for 32% of the variance before controlling for bilateral hippocampal volume (F(1,16) = 7.65, p = .014), and 28% of the variance after controlling for bilateral hippocampal volume (F(1,15) = 9.37, p = .008). Entered together in one predictor block, bilateral hippocampal and thalamic volume explained 54% of variance in LM II scores (F(2,15) = 8.93, p = .003) (see Figure 3). Thus, it follows that there is only 4% shared variance in LM II scores between the hippocampus and thalamus (54% total variance minus 28% unique variance from thalamus and 22% unique variance from hippocampus equals 4% shared variance).
Figure 3.
Scatterplots and best-fit lines of simple regression for thalamic or hippocampal volume predicting performance on LM II and VSRT delayed recall scores. Note. r2 values marked * were statistically significant (p < .05).
A different pattern of predicted variance was observed for VSRT delayed recall scores, in that bilateral thalamic volume did not account for a significant percentage of the variance either before (11%) (F(1,16) = 1.87, p = .190) or after (8%) (F(1,15) = 2.03, p = .174) statistically controlling for bilateral hippocampal volume. In contrast, bilateral hippocampal volume accounted for 33% of the variance before controlling for bilateral thalamic volume (F(1,16) = 7.70, p = .014), and 30% of the variance after controlling for bilateral thalamic volume (F(1,15) = 7.59, p = .015). The full model with both predictors of bilateral hippocampal and thalamic volume explained 41% of variance in VSRT delayed recall scores (F(2,15) = 5.11, p = .020) (see Figure 3).
A similar pattern of predicted variance emerged for VPA II scores, although the observed effects only trended towards statistical significance. Bilateral hippocampal volume accounted for 21% of the variance in VPA II scores before controlling for bilateral thalamic volume (F(1,16) = 4.24, p = .056) and 19% of the variance after controlling for bilateral thalamic volume (F(1,15) = 3.97, p = .065), whereas bilateral thalamic volume only accounted for only 8% of the variance before controlling for bilateral hippocampal volume (F(1,16) = 1.43, p = .250) and 7% of the variance after controlling for bilateral hippocampal volume (F(1,15) = 1.34, p = .266). Together, bilateral hippocampal and thalamic volume accounted for a non-significant percentage (27%) of the variance in VPA II scores (F(2,15) = 2.83, p = .090).
Further analyses examined relationships of bilateral hippocampal and thalamic volume with performance on the three measures of immediate memory (LM I, VSRT immediate recall, and VPA I). Bilateral hippocampal volume was not correlated with any of these immediate memory measures (all p’s > .10). Similarly, bilateral thalamic volume was not correlated with VPA I or VSRT immediate recall (all p’s > .10). However, bilateral thalamic volume was associated with LM I (r = .72, p = .001). A follow-up multiple regression analysis demonstrated that bilateral thalamic volume accounted for a significant percentage of variance (49%) in LM I scores after controlling for bilateral hippocampal volume (F(1,15) = 19.39, p = .001).
Discussion
The main findings of this study are that hippocampal and thalamic volume were both independent predictors of LM II scores in TLE patients, even after controlling for the volume of the other structure. However, hippocampal volume, but not thalamic volume, was a predictor of performance on VSRT delayed recall, regardless of whether the volume of the other structure was controlled for. Similarly, hippocampal volume showed a trend towards significance as a predictor of variance in VPA II scores, whereas thalamic volume fell markedly short of accounting for a significant percentage of variance. Follow-up analyses further demonstrated that bilateral thalamic volume independently predicted LM I scores, but not VPA I or VSRT immediate recall scores, whereas bilateral hippocampal volume was not associated with any of these immediate memory measures. These findings have implications for understanding the distinct quantitative, and potentially distinct qualitative, roles of the hippocampus and thalamus to verbal memory functioning in patients with TLE.
The quantitative contributions of hippocampal and thalamic volume to verbal episodic memory functioning
Hippocampal and thalamic volume uniquely explained 22% and 28%, respectively, of the variance in LM II scores. To our knowledge, this study, along with Seidenberg et al. (2008) and Kopelman et al. (2001), are the only studies to report that thalamic volume was predictive of memory functioning. Additionally, our results are consistent with prior TLE studies reporting that hippocampal volume explained 12% (Griffith et al., 2003), 29% (Martin et al., 1999), 34% (Reminger et al., 2004), and 36% (Kalviainen et al., 1997) of the variance in LM II scores. Our analyses extend upon these findings by demonstrating that the percentage of total explained variance roughly doubled to 54% when LM II scores were predicted from both hippocampal and thalamic volume in the current TLE sample. This marked increase in explained variance suggests that hippocampal and thalamic volume are largely non-redundant in their quantitative contributions to prose verbal memory functioning (only 4% of the variance is shared). These findings underscore the importance of both hippocampal and thalamic integrity in memory disordered TLE patients.
Of interest, the pattern of explained variance on the two measures of delayed rote verbal memory, VSRT delayed recall and VPA II, differed from that observed for delayed prose verbal memory in the TLE sample. Hippocampal volume, but not thalamic volume, was a significant predictor of performance on VSRT delayed recall, and hippocampal volume showed a trend towards accounting for a significant percentage (21%) of variance in VPA II scores, more than doubling the percentage (8%) of variance attributed to thalamic volume. This pattern is consistent with past studies reporting that hippocampal volume predicted delayed rote verbal memory functioning in TLE patients (Griffith et al., 2003; Kilpatrick et al., 1997; Pegna et al., 2002) and suggest that hippocampal integrity is more crucial to delayed rote verbal memory than thalamic integrity. However, caution should be exercised in interpreting the magnitude of differences in unique and explained variance between the thalamus and the hippocampus given the small sample size of this study.
The qualitative contributions of hippocampal and thalamic volume to verbal episodic memory functioning
The pattern of explained variance observed across the three verbal memory measures suggests that the hippocampus and thalamus have qualitatively distinct roles in verbal episodic memory functioning in TLE patients. If volumetric measures of these structures provided highly similar qualitative information regarding episodic memory functioning, it would be expected that hippocampal and thalamic volume would be comparably predictive of different types of episodic memory measures, regardless of the cognitive demands of those tasks. Instead, the pattern of predicted variance in memory measures appears to be consistent with theories that implicate the hippocampus in encoding and storing the constituent elements of an experience into a long-term memory representation (i.e., relational material) (Eichenbaum, 2004; Eichenbaum & Cohen, 2001; Morris et al., 2003; O’Reilly & Rudy, 2001; Rolls, 1990; Ryan & Cohen, 2003; Squire & Zola-Morgan, 1991), whereas the thalamus, as a whole, participates in the selection of to-be-encoded stimuli and retrieval strategies (Aggleton & Brown, 1999; Van der Werf, Jolles, et al., 2003). This interpretation is supported because hippocampal and thalamic volume each uniquely predicted delayed verbal prose memory functioning, which ostensibly relies on the encoding and storage of information, in addition to the selection of information as relevant or irrelevant prior to encoding and the strategic retrieval of propositionally- or logically-related material during recall. However, hippocampal, but not thalamic, volume predicted delayed verbal rote memory functioning, which ostensibly relies on the encoding and storage of arbitrary relational information but deemphasizes the selection of relevant to-be-encoded stimuli or the use of retrieval strategies (Saling et al., 1993; Rausch et al., 1994).
The proposition that the hippocampus and thalamus make qualitatively distinct contributions to verbal memory is further supported by comparison of measures of immediate recall. Thalamic, but not hippocampal, volume predicted immediate verbal prose memory functioning, whereas neither thalamic nor hippocampal volume predicted immediate verbal rote memory functioning. Similarly to delayed prose memory, immediate prose memory also presumably involves the organization of propositionally- or logically-related material during stimulus presentation and thus may be expected to be related to thalamic volume. In contrast, hippocampal volume might not be expected to predict immediate prose or rote memory functioning because the hippocampus is implicated in the representation of information in long-term memory more so than immediate memory. These results represent one of the few data illustrating directly the differential contributions of the hippocampus and thalamus across different types of episodic memory recall tasks. It may also suggest that memory impairment in TLE involves deficits related to the encoding and storage of relational material, in addition to the executive aspects of episodic memory (i.e., the selection of to-be-encoded information and retrieval strategies).
Study limitations and future directions
The current study has several limitations. The small sample size and other confounds due to the small sample size should be considered, including heterogeneity in seizure medication usage/dose, heterogeneity in the side of seizure onset, in addition to a widespread range of ages in the sample. Another effect of the small sample size is that interpretation of the differences in sizes of correlations and relevant contributions to variance must be made with caution.
Additionally, the large number of correlations conducted among and between brain volumetric measures and memory measures increased the likelihood of Type I errors (false positives), although following up correlations with the multiple regressions reduces this likelihood. Results should thus be regarded as tentative, and replication of the current results in larger TLE samples, which will increase statistical power and permit an examination of the uncontrolled variable confounds, is warranted.
Also of note, our results do not necessarily indicate that the thalamus per se supports all of the executive aspects of episodic memory. An alternative explanation is that thalamic volume loss is associated with volume loss of the dorsolateral prefrontal cortex, and that the dysfunction of this second brain region is partly responsible for poorer delayed prose memory functioning in TLE patients. Follow-up studies using experimental designs may provide stronger evidence for independent hippocampal and thalamic contributions to verbal episodic memory, as would studies aiming to delineate the contributions specific thalamic subnuclei (e.g., the anterior nuclei, the mediodorsal nucleus, and the intralaminar and midline nuclei) to prose memory functioning. Recent evidence also suggests that thalamic subnuclei may be involved to different extents in the pathological processes affecting patients with TLE (Bonilha et al., 2005) and may influence different types of memory (e.g., familiarity memory) (Kishiyama et al., 2005). However, methods to reliably delineate MRI volumes of thalamic subnuclei remain to be developed.
Conclusions
Hierarchical multiple regression models demonstrated that MRI volume of the hippocampus and thalamus each provided unique predictive information regarding delayed prose verbal memory functioning. However, hippocampal, but not thalamic, volume predicted delayed rote verbal memory functioning. Further analyses showed that bilateral thalamic volume independently predicted immediate prose, but not immediate rote, verbal memory functioning, whereas bilateral hippocampal volume was not associated with any of these immediate memory measures. The observed pattern of explained variance across the immediate and delayed memory measures suggests that the hippocampus and thalamus make quantitatively, and perhaps qualitatively, distinct contributions to episodic memory functioning. Our results are consistent with contemporary models of episodic memory that implicate the hippocampus in long-term memory encoding and storage of relational material (Eichenbaum, 2004; Eichenbaum & Cohen, 2001) and the thalamus in the selection of items to-be-encoded in episodic memory and retrieval strategies (Aggleton & Brown, 1999; Van der Werf, Jolles, et al., 2003). Our results also underscore the importance of thalamic atrophy to the memory deficits observed in TLE patients. Further study of the qualitative contributions of the thalamus to episodic memory in larger samples of TLE patients appears warranted.
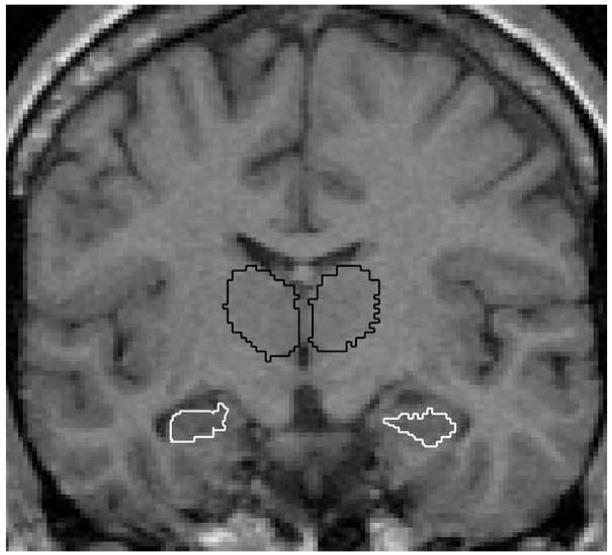
Figure 1.
Example of MRI scan depicting hippocampal (white) and thalamic (black) ROIs in the coronal view.
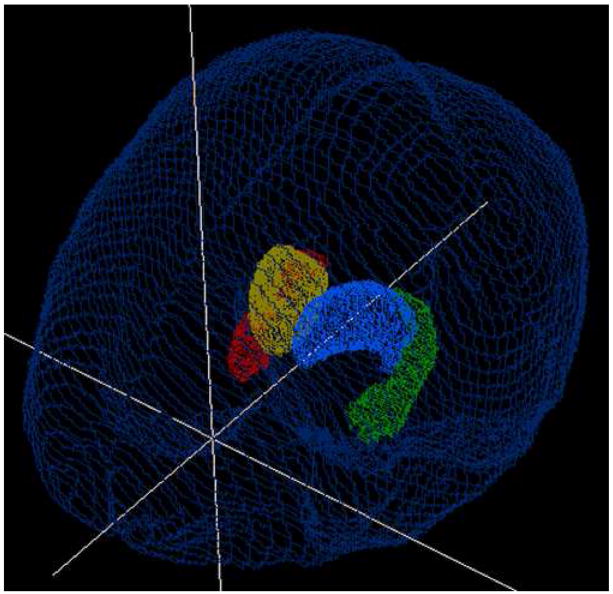
Figure 2.
(hardcopy version). Example of 3D hippocampal (white) and thalamic (grey) ROIs viewed from the right superior aspect.
Figure 2 (online version). Example of 3D hippocampal (green and red) and thalamic (blue and yellow) ROIs viewed from the right superior aspect.
Footnotes
This study was carried out at the Department of Neurology, University of Wisconsin and the Departments of Neurology and Psychology, University of Alabama-Birmingham.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal- anterior thalamic axis. Behavioral and Brain Sciences. 1999;22(3):425–444. [PubMed] [Google Scholar]
- Bergin PS, Thompson PJ, Baxendale SA, Fish DR, Shorvon SD. Remote memory in epilepsy. Epilepsia. 2000;41(2):231–239. doi: 10.1111/j.1528-1157.2000.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Mangan PS, Zhang D, Scott CA, Williamson JM. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42(8):967–978. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience. 1999;92(1):15–26. doi: 10.1016/s0306-4522(98)00712-x. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Alessio A, Rorden C, Baylis G, Damasceno BP, Min LL, Cendes F. Extrahippocampal gray matter atrophy and memory impairment in patients with medial temporal lobe epilepsy. Human Brain Mapping. 2007;28(12):1376–1390. doi: 10.1002/hbm.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Castellano G, Cendes F, Li LM. Voxel-based morphometry of the thalamus in patients with refractory medial temporal lobe epilepsy. Neuroimage. 2005;25(3):1016–21. doi: 10.1016/j.neuroimage.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behaviour. 1973;12:543. [Google Scholar]
- Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. Journal of Neuroscience. 1997;17(14):5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Witter MP. Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. The Journal of Comparative Neurology. 1996;364(4):637–650. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. New York: Oxford University Press; 2001. [Google Scholar]
- Griffith HR, Pyzalski RW, Seidenberg M, Hermann BP. Memory relationships between MRI volumes and resting PET metabolism of medial temporal lobe structures. Epilepsy & Behavior. 2004;5(5):669–676. doi: 10.1016/j.yebeh.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Pyzalski RW, O'Leary D, Magnotta V, Bell B, Dow C, et al. A Controlled Quantitative MRI Volumetric Investigation of Hippocampal Contributions to Immediate and Delayed Memory Performance. Journal of Clinical & Experimental Neuropsychology. 2003;25(8):1117–1127. doi: 10.1076/jcen.25.8.1117.16731. [DOI] [PubMed] [Google Scholar]
- Guye M, Régis J, Tamura M, Wendling F, McGonigal A, Chauvel P, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129(7):1917–1928. doi: 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, et al. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. Journal of Computer Assisted Tomography. 1999;23(1):144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Henry TR, Mazziotta JC, Engel J. Interictal metabolic anatomy of mesial temporal lobe epilepsy. Archives of Neurology. 1993;50(6):582–589. doi: 10.1001/archneur.1993.00540060022011. [DOI] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. The Journal of Comparative Neurology. 1978;177(4):589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth R, Ruggles K, et al. The Neurodevelopmental Impact of Childhood-onset Temporal Lobe Epilepsy on Brain Structure and Function. Epilepsia. 2002;43(9):1062–1071. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth RD, Wendt G, et al. Extratemporal quantitative MR volumetrics and neuropsychological status in temporal lobe epilepsy. Journal of the International Neuropsychological Society. 2003;9(3):353–362. doi: 10.1017/S1355617703930013. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Bell B. The neurodevelopmental impact of childhood onset temporal lobe epilepsy on brain structure and function and the risk of progressive cognitive effects. Progress in Brain Research. 2002;135:429–438. doi: 10.1016/S0079-6123(02)35040-4. [DOI] [PubMed] [Google Scholar]
- Juhasz C, Nagy F, Watson C, da Silva EA, Muzik O, Chugani DC, et al. Glucose and [11C]flumazenil positron emission tomography abnormalities of thalamic nuclei in temporal lobe epilepsy. Neurology. 1999;53(9):2037–2045. doi: 10.1212/wnl.53.9.2037. [DOI] [PubMed] [Google Scholar]
- Kälviäinen R, Partanen K, Aikiä M, Mervaala E, Vainio P, Riekkinen PJ, et al. MRI-based hippocampal volumetry and T2 relaxometry: correlation to verbal memory performance in newly diagnosed epilepsy patients with left-sided temporal lobe focus. Neurology. 1997;48(1):286–287. doi: 10.1212/wnl.48.1.286. [DOI] [PubMed] [Google Scholar]
- Keller SS, Wilke M, Wieshmann UC, Sluming VA, Roberts N. Comparison of standard and optimized voxel-based morphometry for analysis of brain changes associated with temporal lobe epilepsy. NeuroImage. 2004;23(3):860–868. doi: 10.1016/j.neuroimage.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Kilpatrick C, Murrie V, Cook M, Andrewes D, Desmond P, Hopper J. Degree of left hippocampal atrophy correlates with severity of neuropsychological deficits. Seizure. 1997;6:213–218. doi: 10.1016/s1059-1311(97)80008-8. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. The Korsakoff’s syndrome. British Journal of Psychiatry. 1995;166:154– 173. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Lasserson D, Kingsley D, Bello F, Rush C, Stanhope N, et al. Structural MRI volumetric analysis in patients with organic amnesia, 2: correlations with anterograde memory and executive tests in 40 patients. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71(1):23–8. doi: 10.1136/jnnp.71.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD, Stanhope N. Anterograde and retrograde amnesia following frontal lobe, temporal lobe, or diencephalic lesions. In: Squire LR, Schacter DL, editors. The Neuropsychology of Memory. 3. New York: The Guilford Press; 2002. pp. 47–60. [Google Scholar]
- Kishiyama MM, Yonelinas AP, Kroll NE, Lazzara MM, Nolan EC, Jones EG, Jagust WJ. Bilateral thalamic lesions affect recollection- and familiarity-based recognition memory judgments. Cortex. 2005;41(6):778–88. doi: 10.1016/s0010-9452(08)70296-x. [DOI] [PubMed] [Google Scholar]
- Leritz EC, Grande LJ, Bauer RM. Temporal lobe epilepsy as a model to understand human memory: the distinction between explicit and implicit memory. Epilepsy & Behavior. 2006;9(1):1–13. doi: 10.1016/j.yebeh.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Lillywhite LM, Saling MM, Briellmann RS, Weintrob DL, Pell GS, Jackson GD. Differential contributions of the hippocampus and rhinal cortices to verbal memory in epilepsy. Epilepsy & Behavior. 2007;10(4):553–9. doi: 10.1016/j.yebeh.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Andreasen NC, Schultz SK, Harris G, Cizadlo T, Heckel D, et al. Quantitative In Vivo Measurement of Gyrification in the Human Brain: Changes Associated with Aging. Cerebral Cortex. 1999;9(2):151–160. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Computer Medical Imaging and Graphics. 2002;26(4):251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Martin RC, Hugg JW, Roth DL, Bilir E, Gilliam FG, Faught E, et al. MRI extrahippocampal volumes and visual memory: correlations independent of MRI hippocampal volumes in temporal lobe epilepsy patients. Journal of the International Neuropsychological Society. 1999;5(6):540–548. doi: 10.1017/s1355617799566083. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Obara K, Muramatsu K. Diaschisis. Neurological Research. 1993;15(6):362–366. doi: 10.1080/01616412.1993.11740164. [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, et al. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2003;358(1432):773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Conjunctive Representations in Learning and Memory: Principles of Cortical and Hippocampal Function. Psychological Review. 2001;108(2):311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Oikawa H, Sasaki M, Tamakawa Y, Kamei A. The circuit of Papez in mesial temporal sclerosis: MRI. Neuroradiology. 2001;43(3):205–210. doi: 10.1007/s002340000463. [DOI] [PubMed] [Google Scholar]
- Pantel J, O'Leary DS, Cretsinger K, Bockholt HJ, Keefe H, Magnotta VA, et al. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus. 2000;10(6):752–758. doi: 10.1002/1098-1063(2000)10:6<752::AID-HIPO1012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Pegna AJ, Caldara-Schnetzer AS, Perrig SH, Lazeyras F, Khateb A, Mayer E, et al. Is the right amygdala involved in visuospatial memory? Evidence from MRI volumetric measures. European Neurology. 2002;47(3):148–155. doi: 10.1159/000047973. [DOI] [PubMed] [Google Scholar]
- Rausch R, Henry TR, Ary CM, Engel J, Mazziotta J. Asymmetric interictal glucose hypometabolism and cognitive performance in epileptic patients. Archives of Neurology. 1994;51(2):139–144. doi: 10.1001/archneur.1994.00540140045013. [DOI] [PubMed] [Google Scholar]
- Reminger SL, Kaszniak AW, Labiner DM, Littrell LD, David BT, Ryan L, et al. Bilateral hippocampal volume predicts verbal memory function in temporal lobe epilepsy. Epilepsy & Behavior. 2004;5(5):687–695. doi: 10.1016/j.yebeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1990;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rubin E, Dhawan V, Moeller JR, Takikawa S, Labar DR, Schaul N, et al. Cerebral metabolic topography in unilateral temporal lobe epilepsy. Neurology. 1995;45(12):2212–2223. doi: 10.1212/wnl.45.12.2212. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. Evaluating the neuropsychological dissociation evidence for multiple memory systems. Cognitive, Affective & Behavioral Neuroscience. 2003;3(3):168–185. doi: 10.3758/cabn.3.3.168. [DOI] [PubMed] [Google Scholar]
- Saling MM, Berkovic SF, O'Shea MF, Kalnins RM, Darby DG, Bladin PF. Lateralization of verbal memory and unilateral hippocampal sclerosis: evidence of task-specific effects. Journal of Clinical and Experimental Neuropsychology. 1993;15(4):608–618. doi: 10.1080/01688639308402582. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Kelly KG, Parrish J, Geary E, Dow C, Rutecki P, et al. Ipsilateral and Contralateral MRI Volumetric Abnormalities in Chronic Unilateral Temporal Lobe Epilepsy and their Clinical Correlates. Epilepsia. 2005;46(3):420–430. doi: 10.1111/j.0013-9580.2005.27004.x. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Hermann B, Pulsipher D, Morton J, Parrish J, Geary E, Guidotti L. Thalamic Atrophy and Cognition in Unilateral Temporal Lobe Epilepsy. Journal of the International Neuropsychological Society. 2008;14(3):384–393. doi: 10.1017/S1355617708080399. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Implication of seizure termination location in temporal lobe epilepsy. Epilepsia. 1996;37(5):455–458. doi: 10.1111/j.1528-1157.1996.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Spinks R, Magnotta VA, Andreasen NC, Albright KC, Ziebell S, Nopoulos P, et al. Manual and Automated Measurement of the Whole Thalamus and Mediodorsal Nucleus Using Magnetic Resonance Imaging. NeuroImage. 2002;17(2):631–642. [PubMed] [Google Scholar]
- Squire LR. Comparisons between forms of amnesia: some deficits are unique to Korsakoff’s Syndrome. Journal of Experimental Psychology. 1982;8(6):560–571. doi: 10.1037//0278-7393.8.6.560. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Su HS, Bentivoglio M. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. The Journal of Comparative Neurology. 1990;297(4):582–593. doi: 10.1002/cne.902970410. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme, Publishing Group; 1988. [Google Scholar]
- Van der Werf YD, Jolles J, Witter MP, Uylings HB. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003;39(4–5):1047–1062. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HBM, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41(10):1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Uylings HBM, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38(5):613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. The Journal of Comparative Neurology. 1990;302(3):515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. Amnesia: a disconnection syndrome? Neuropsychologia. 1982;20(3):233–48. doi: 10.1016/0028-3932(82)90099-9. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. New York: Psychological Corperation; 1997. [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. The Journal of Comparative Neurology. 1990;296(2):179–203. doi: 10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]