Abstract
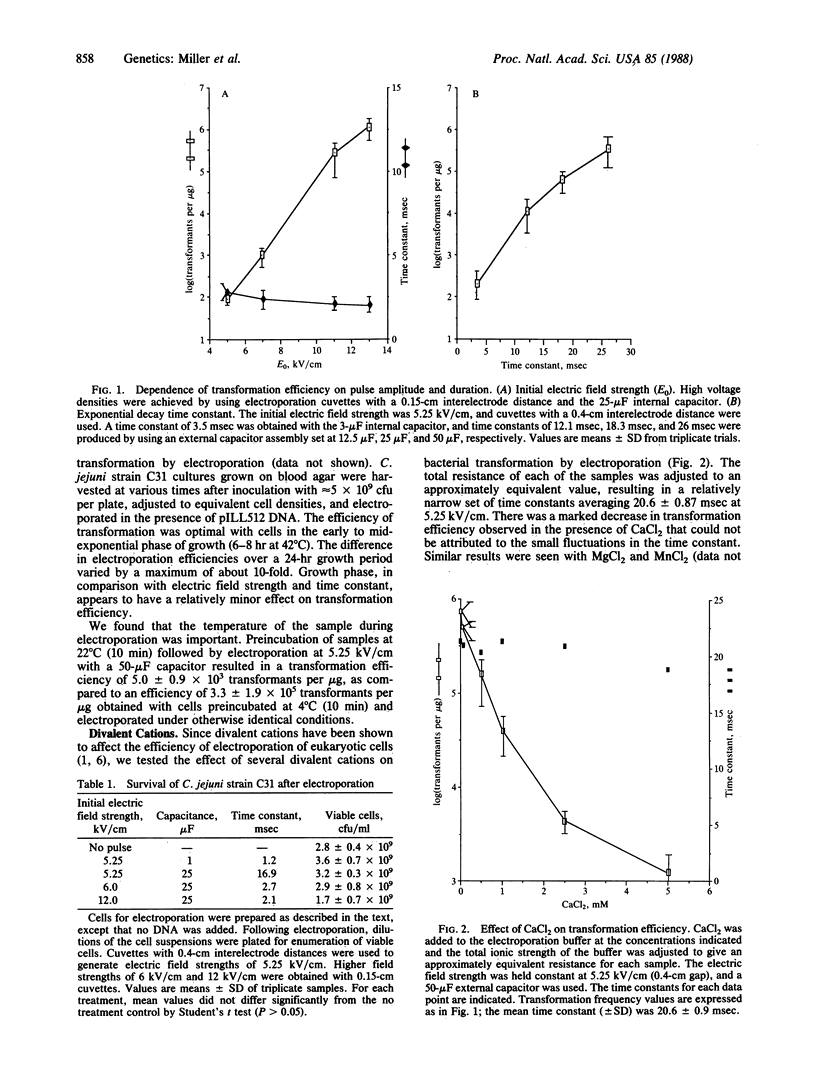
Electroporation permits the uptake of DNA by mammalian cells and plant protoplasts because it induces transient permeability of the cell membrane. We investigated the utility of high-voltage electroporation as a method for genetic transformation of intact bacterial cells by using the enteric pathogen Campylobacter jejuni as a model system. This report demonstrates that the application of high-voltage discharges to bacterial cells permits genetic transformation. Our method involves exposure of a Campylobacter cell suspension to a high-voltage exponential decay discharge (5-13 kV/cm) for a brief period of time (resistance-capacitance time constant = 2.4-26 msec) in the presence of plasmid DNA. Electrical transformation of C. jejuni results in frequencies as high as 1.2 x 10(6) transformants per microgram of DNA. We have investigated the effects of pulse amplitude and duration, cell growth conditions, divalent cations, and DNA concentration on the efficiency of transformation. Transformants of C. jejuni obtained by electroporation contained structurally intact plasmid molecules. In addition, evidence is presented that indicates that C. jejuni possesses DNA restriction and modification systems. The use of electroporation as a method for transforming other bacterial species and guidelines for its implementation are also discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Taylor D. N., Feldman R. A. Epidemiology of Campylobacter jejuni infections. Epidemiol Rev. 1983;5:157–176. doi: 10.1093/oxfordjournals.epirev.a036256. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Yakobson E. Location and nucleotide sequence of the transfer origin of the broad host range plasmid RK2. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3595–3598. doi: 10.1073/pnas.80.12.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Voltage-induced pore formation and hemolysis of human erythrocytes. Biochim Biophys Acta. 1977 Dec 1;471(2):227–242. doi: 10.1016/0005-2736(77)90252-8. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Harel J., Tompkins L. Gene transfer from Escherichia coli to Campylobacter species: development of shuttle vectors for genetic analysis of Campylobacter jejuni. J Bacteriol. 1987 Nov;169(11):5320–5323. doi: 10.1128/jb.169.11.5320-5323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Trieu-Cuot P., Courvalin P. Structural relationship between the genes encoding 3'-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):135–150. doi: 10.1016/s0769-2609(85)80040-5. [DOI] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs C. D., Bates G. W. Stable transformation of tobacco by electroporation: evidence for plasmid concatenation. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5602–5606. doi: 10.1073/pnas.83.15.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Gerbaud G., Lambert T., Courvalin P. In vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 1985 Dec 16;4(13A):3583–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tur-Kaspa R., Teicher L., Levine B. J., Skoultchi A. I., Shafritz D. A. Use of electroporation to introduce biologically active foreign genes into primary rat hepatocytes. Mol Cell Biol. 1986 Feb;6(2):716–718. doi: 10.1128/mcb.6.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. I., Caldwell M. B., Lee E. C., Guerry P., Trust T. J., Ruiz-Palacios G. M. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. K., Neumann E. Electric field mediated gene transfer. Biochem Biophys Res Commun. 1982 Jul 30;107(2):584–587. doi: 10.1016/0006-291x(82)91531-5. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Pilwat G., Riemann F. Dielectric breakdown of cell membranes. Biophys J. 1974 Nov;14(11):881–899. doi: 10.1016/S0006-3495(74)85956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U., Vienken J. Electric field-induced cell-to-cell fusion. J Membr Biol. 1982;67(3):165–182. doi: 10.1007/BF01868659. [DOI] [PubMed] [Google Scholar]



