Abstract
The California Hass avocado (Persea Americana) is an example of a domesticated berry fruit that matures on the tree during its growing season but ripens only after being harvested. Avocados are typically harvested multiple times during the growing season in California. Previous research has demonstrated potential health benefits of avocados and extracts of avocado against inflammation and cancer cell growth, but seasonal variations in the phytochemical profile of the fruits being studied may affect the results obtained in future research. Therefore in the present study, avocados were harvested in January, April, July and September 2008 from four different growing locations in California (San Luis Obispo, Ventura, Riverside and San Diego), and analyzed fortotal fat content, fatty acid profile, carotenoids and vitamin E. A significant increase in total carotenoid and fat content of avocados from all regions was noted as the season progressed from January to September. Four carotenoids not previously described in the avocado were quantified. The total content of carotenoids was highly correlated with total fat content (r=0.99, p<0.001) demonstrating a remarkable degree of constancy of carotenoid intake per gram of fat content in the California Hass avocado.. Future clinical research on the health benefits of the avocado should specify the time of harvest, degree of ripening, growing area and the total phytochemical profile of the fruit or extract being studied. These steps will enable researchers to account for potential nutrient-nutrient interactions that might affect the research outcomes.
Keywords: avocado, fruits, carotenoids, vitamin E
INTRODUCTION
The avocado (Persea americana) is a berry fruit with a dark green leathery skin and a very large seed. Ninety-five percent of American avocado production is located in Southern California. The California Hass avocado originated from a single mother tree in La Habra, California in 1935, is the most common avocado cultivar (1). Avocados are picked when they are mature but unripe, and so have the advantage of being left on the tree during the season and harvested repeatedly. Therefore, while they are eaten in the ripe state, they can have different phytochemical composition when harvested at different times during the year.
Increasing evidence of health benefits of the avocado is both driving increased consumption and stimulating research on potential health benefits. We previously reported that California Hassavocados have the highest content of lutein among commonly eaten fruits as well as several related carotenoids and tocopherol. Furthermore, we demonstrated that a lipophilic extract of avocado containing these carotenoids and tocopherols inhibited the growth of both androgen-dependent (LNCaP) and androgen-independent (PC-3) human prostate cancer cell lines in vitro. Incubation of PC-3 prostate cancer cells with the avocado extract led to G2/M cell cycle arrest accompanied by an increase in p27 protein expression (2). In the course of our studies, we also noted variations in the content of carotenoids and fat depending on time of harvest of avocados. Ding et al. (3) demonstrated that avocado extract selectively induced apoptosis in human oral cancer cell lines by modulation of reactive oxygen species. Castillo-Juarez et al. (4) demonstrated antibacterial activity of a methanolic avocado extract against Helicobacter pylori, a cause of gastritis implicated in the etiology of gastric cancer. Avocado/soybean unsaponifiables (ASU) is an extract prepared from avocado and soybean oil. The product has been approved as a prescription drug in France for several years and has now been introduced in Denmark as a food supplement. ASU has been examined in vitro and in animal studies that have shown an anti-inflammatory effect and a stimulatory effect on proteoglycan synthesis in chondrocytes. Four randomized, double-blind, placebo-controlled clinical trials have been published. These studies indicate that ASU has an effect on the symptoms of knee and hip osteoarthritis but not on the structural changes caused by osteoarthritis (5). These studies suggest that combinations of phytochemicals from the avocado fruit may offer potential health benefits in both inflammatory diseases and cancer prevention (6), but much more research is needed. In order to conduct clinical studies on the health benefits of avocado consumption, any variation in the composition of the avocado must be adequately considered. Therefore, in the present study, California Hass avocados harvested in January, April, July, and September 2008 from four different locations (San Luis Obispo, Ventura, Riverside and San Diego) were studied to characterize their contents of carotenoids, fatty acids, and tocopherol using HPLC, gas chromatography, and mass spectrometry. In addition, four carotenoids not previously documented in the Hass avocado were characterized and quantified.
MATERIALS AND METHODS
Reagents and Standards
All solvents used were HPLC grade (Fisher Scientific, Fairlawn, NJ). Other reagents were either HPLC (ammonium acetate, triethylamine), or GC (butylated hydroxytoluene), or reagent (potassium hydroxide, sodium carbonate, sodium sulfate, acetyl chloride and sodium chloride) grade. All-trans-antheraxanthin, all-trans-violaxanthin, 9′-cis-neoxanthin, all-trans-α-carotene (6′R) and echinenone were purchased from CaroteNature (Lupsingen, Switzerland). All-trans-zeaxanthin, all-trans-lutein and β-cryptoxanthin were purchased from Indofine (Hillsborough, NJ); all-trans-β-carotene, α-tocopherol, γ-tocopherol and fatty acid standards from Sigma (St. Louis, MO).
Sampling
Hass avocado samples were provided by the California Avocado Commission (CAC, Irvine, CA) and were shipped from San Diego, Riverside, Ventura and San Luis Obispo Counties in California to UCLA Center for Human Nutrition in January, April, July and September 2008. Avocados arrived in cartons containing 60 fruits of similar size. The samples were immediately sealed hermetically and stored at room temperature upon arrival to promote ripening. The ripeness of the fruit was characterized as previous described (2). Chemical extractions were performed immediately after fruit ripening. They were analyzed within 4–8 days after arrival once ripened. For each extraction and HPLC analysis, twelve similarly ripened avocados were randomly selected and triplicate analyses were performed for each sample. Among the thirty-six fruits from each carton five of them were weighed. Avocados were peeled, and a sampling syringe was used to collect a sample from near the middle of the flesh. Pieces of equal weight (about 0.16 g each) were combined, homogenized, and extracted immediately.
Fat Content Measurement
The method for the fat content measurement was modified from AOAC methods (7, 8). Briefly, an avocado sample was separated into peel, flesh and seed. The flesh was homogenized, and 2 g of homogenized avocado flesh was weighed and extracted with 50 ml of 95% ethanol/hexane (1:1). The mixture was shaken vigorously and stirred for 45 min then filtered. The residue of avocado flesh was rinsed three times with 20 ml of 95% ethanol/hexanes (1:1), filtered and the filtrates combined. The hexane fraction of the filtrate was separated from 95% of ethanol in a separation funnel, then the 95% ethanol fraction was extracted twice with 25 ml hexane each time. All hexane fractions were combined and treated with 3 g of anhydrous sodium sulfate for 10 min. The hexane fraction was filtered, and rinsed with 10 ml of fresh hexane. The filtrate was evaporated under reduced pressure at 60 °C and the residue was dried at 95–100°C for 30 min, cooled and dried in a vacuum desiccator until a constant weight was obtained, and recorded as the fat content of the sample.
Derivatization Of Fatty Acids And Gas Chromatography
The derivatives of fatty acid methyl esters were prepared using a methylation solution of methanol and benzene at a ratio of 1:4 (v/v). Homogenized avocado flesh sample (100 mg) was mixed with 5 mL of the methylation mixture and 100 μL of a 100 mg/mL heptadecanoic acid (C17:0) internal standard. The methylation reaction was started by adding 200 μL of acetyl chloride and heated at 100 °C for one hour. The reaction vials were shaken vigorously after 3 min of heating to mix all the components into a single phase. After heating, the vials were cooled to room temperature, neutralized with 4 mL of 6% sodium carbonate, and then shaken again. Upon centrifuge separation, the upper layer was removed and subjected to GC analysis. The analysis was performed on a GC equipped with a 25 m × 0.25 mm ID fused silica capillary column. The FID and injector port were maintained at 260 °C, with column heating starting from 150 °C and increasing to 200 °C at 5 °C per minute. The flow rates of the carrier gases were 1 mL/min helium, 30 mL/min hydrogen, and 300 mL/min air. The free fatty acids were identified by comparison of the peak retention times with those of commercial free fatty acid methyl ester standards: palmitic acid, palmitoleic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, and arachidic acid. Fatty acid composition was determined by the ratio of an individual fatty acid peak area to the total area of all fatty acid peaks. Duplicate injections were made per sample.
HPLC analysis of carotenoids and tocopherol
Extractions of avocado flesh were performed using previously described methods (9) with minor modifications. All extractions, saponifications and analyses were carried out at room temperature under subdued light. Briefly, approximately 2 g of flesh from 12 avocado fruits were mixed with 4 mL acetone containing 0.05% (w/v) 2, 6-Di-tert-butyl-4-methylphenol (BHT), 0.2 g sodium carbonate and 2 g sodium sulfate, and homogenized at 4°C for 1.5 min at medium speed. The organic phase was then separated and the extraction of the residue was repeated until the extract was colorless. The extracts were combined and concentrated under nitrogen to a volume of 10 ml, which was then saponified for 2 h under nitrogen after adding 5 ml of methanolic potassium hydroxide (15% w/v). An aliquot of the mixture (3 mL) was then diluted with 1 ml of sodium chloride solution (10%) and partitioned into methylene chloride (2 mL) and water. The organic phase was washed three times with water, evaporated to dryness under nitrogen and reconstituted in methanol/Methyl tert-butyl ether (MTBE) (85:15) for analysis by HPLC.
A Hewlett Packard 1050 HPLC instrument (Hewlett-Packard Company, Wilmington, DE) with an autosampler, a C30 carotenoids column (250 × 4.6mm, 5 μm, YMC, Waters) and a multiple wavelength detector was used to analyze samples at 445 nm. The HPLC mobile phase consisted of methanol/water (96:4, v/v) containing 0.05 M ammonium acetate and a volume fraction of 0.05% triethylamine (solvent A), and methyl tert-butyl ether (solvent B). The gradient was as follows: 1) 10 minute gradient of solvent A from 100% to 90%; 2) 9 minute gradient to 69% solvent A and 31% solvent B; 3) 16 minute gradient to 35% solvent A and 65% solvent B; and 4) a 0.1 minute hold at 65% solvent A and 35% solvent B. For tocopherol analysis, a Bakerbond narrow-pore C18 column (250 × 4.6 mm, 5 μm, J.T. Baker) was used as previously described (2) with modifications of solvents and gradient. Briefly, solvent A consisted of methanol/water (96:4, v/v) containing 0.05 M ammonium acetate. Solvent B was acetonitrile and solvent C was ethyl acetate. Each of the three solvents contained a volume fraction of 0.05% triethylamine. The gradient was as follows: 1) 3 minute hold at 100% solvent A; 2) 42 minute gradient to 30% solvent A, 60% solvent B, and 10% solvent C; 3) 1 minute hold; and 4) 8 minute gradient back to the initial composition. A multiple wavelength detector was used to analyze tocopherol at 292 nm. For both carotenoids and α-tocopherol analyses, the flow rate was held at 1.0 ml/min and temperature of the column at 30°C. An aliquot of 50 μl of sample was injected. Data were analyzed with the Hewlett Packard Chemstation® software.
Concentrations of violaxanthin, 9′-cis-neoxanthin, lutein, zeaxanthin, β-cryptoxanthin, α- and β-carotene, α-tocopherol were determined by HPLC using external calibration. Concentrations of neoxanthin, neochrome, lutein-5, 6-epoxide and chrysanthemaxanthin/were determined using the curves of violaxanthin, 9′-cis-neoxanthin, lutein, and lutein, respectively. Concentrations of the stock solutions were determined spectrophotometrically using Beer’s Law. Absorption coefficients and wavelengths were provided by the manufacturer or obtained from published values (10). Calibration standards were prepared from the stock solutions by dilution. To determine recovery of the extraction, saponification, and chromatography procedures, a known concentration of echinonone in ethanol solution was added to the fruit sample before homogenization. The final concentration of echinonone was obtained from its calibration curve and the percentage recovery is calculated.
LCMS identification of un-characterized carotenoids peaks
LCMS analysis was carried out to confirm the molecular weights and identities of the carotenoids by a Surveyor HPLC system equipped with a diode array absorbance detector (Thermo Finnigan, San Jose, USA). Solvent, gradient and column were the same as that of in HPLC analysis except the column was maintained at 25 °C. After passing through the flow cell of the DAD, the column elute was split and 0.2 ml/min was directed to a LCQ Advantage ion trap mass spectrometer fitted with an electrospray (ESI) interface. Analyses utilized the positive ion mode (m/z M + H+) for detection of carotenoids. Preliminary analyses were carried out using full scan, data dependent MS/MS scanning from m/z 250–1000. The capillary temperature was 275 °C, sheath gas and auxiliary gas were 45 and 0 units/min respectively, and source voltage was 4 kV. Identities of the compounds were obtained by matching their molecular ions (m/z) obtained by LC-ESI-MS with literature data.
RESULTS AND DISCUSSION
The average weights of the fruits from Ventura, San Luis Obispo, San Diego and Riverside for each of the 4 seasonal crops were not significantly different (164±11.7, 170 ±9.9, 171 ±8.2, and 166±9.3 g, respectively).
Carotenoids Identification
The identification of carotenoids for which authentic standards were not available was carried out by HPLC through the combined use of chromatographic retention time, and visible absorption spectrum obtained with an HPLC using an on-line photodiode array detector. A numerical notation %III/II, which describes the ratio of the peak height of band III and band II as a percent, and the intensities of a cis-isomer peak, determined as the ratio of its absorbance to that of the second absorption band, known as Q ratio or DB/DII were used for identification of individual carotenoids and cis-isomers, respectively. An epoxide test, or the acid- catalyzed epoxide-furanoid oxide rearrangement was also performed to support the identification. Table 1 shows the spectroscopic characteristics of carotenoids. In addition, LC/MS data were also used to confirm the mass distribution that aided in identification.
Table 1.
Spectroscopic Characteristics of Carotenoids in Avocado Flesh
| Untreated | Treated with Acid | ||||
|---|---|---|---|---|---|
| Peak no. | Carotenoids | λmax (nm) | III/II% | λmax (nm) | III/II% |
| 1 | all-trans-neoxanthin | 415, 440, 470 | 93.0 | 398, 422, 450 | |
| 2 | all-trans-violaxanthin | 417, 440, 470 | 96.2 | ||
| 3 | all-trans-neochrome | 399, 422, 448 | 90.3 | ||
| 4 | 9′-cis-neoxanthin | 410, 436, 467 | 92.5 | 398, 422, 450 | |
| 5 | all-trans-lutein-5,6-epoxide | 416, 440, 468 | 96.0 | 398, 424, 450 | |
| 6 | chrysanthemaxanthin | 398, 424, 450 | 95 | ||
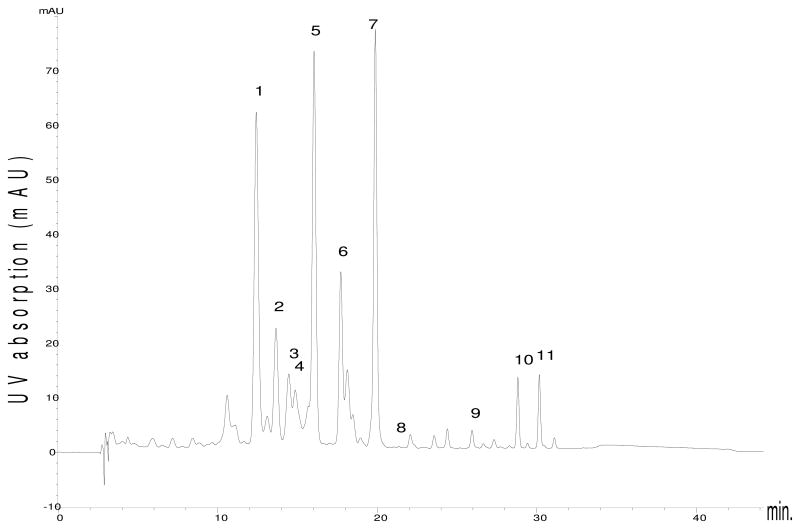
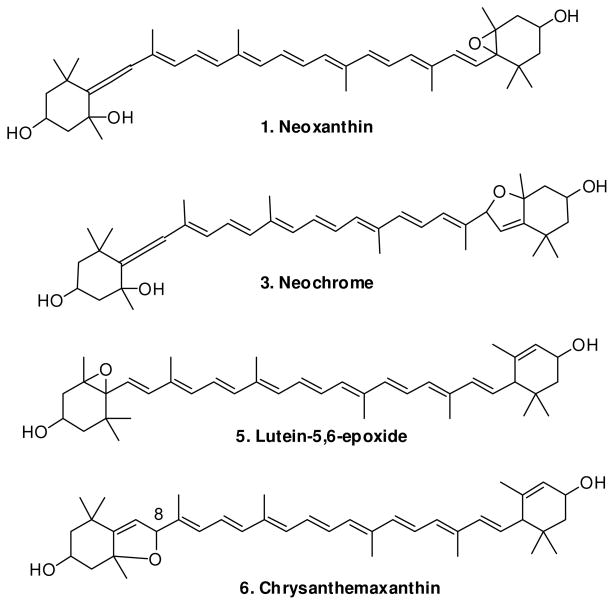
A typical chromatogram of the carotenoids present in avocado flesh is shown in Figure 1. We have previously described (2) and in the present study confirmed the presence of lutein (7), zeaxanthin (8), β-cryptoxanthin (9), α-carotene (10), and β-carotene (11) in the California Hass avocado. In the present study, we identified the following carotenoids (see Table 1): all-trans neoxanthin (1), all-trans violaxanthin (2), all-trans neochrome (3), 9′-cis-neoxanthin (4), lutein-5, 6 epoxide (5), and chrysanthemaxanthin (6).
Figure 1.
HPLC Chromatograms of carotenoids in flesh of Hass avocado, monitored at 445 nm. Peaks numbered according to the elution sequence: 1: All trans-neoxanthin, 2: All trans-violaxanthin, 3: all trans-neochrome, 4: 9′-cis-neoxanthin, 5: all trans-lutein-5, 6-epoxide, 6: chrysanthemaxanthin, 7: lutein, 8: zeaxanthin, 9: β-cryptoxanthin, 10: α-carotene, and 11: β-carotene.
1 had an m/z of 601 consistent with all-trans neoxanthin. Its on-line ultraviolet-visible (UV-Vis) absorption spectrum showed well-defined bands with their maxima located at 415, 440 and 470 nm. The absorption spectrum of 2 and its retention time corresponded to the all-trans-violaxanthin reference standard. 3 was identified as all-trans neochrome with its UV-Vis maxima at 399, 422 and 448 nm and a 90.3. % of the peak height of band III to band II. 4 was identified as 9′ cis-neoxanthin based on absorption maxima at 410, 436 and 467 nm compared with 9′ cis-neoxanthin reference standard measured at 412, 436 and 465 nm and coelution with the standard. Treatment of neoxanthin with trace amounts of alcoholic hydrochloric acid (HCl) was reported to yield a mixture of four carotenoids (11). The major product was neochrome whose visible absorption maxima were shifted by ca. 15 nm to shorter wavelengths compared to neoxanthin which is typical for a rearrangement of (5, 6-) epoxide to the corresponding (5, 8-) furanoid oxide. We treated 9′-cis-neoxanthin with trace amount of methanolic HCl and observed two new peaks at lower retention times compared to their 5′,6′-epoxide. These two 5′,8′-furanoids had identical UV/Vis absorption maxima at 399, 422 and 450 nm. In addition, one of the two peaks had the same retention time as that of 3. It is known that C 30 columns are capable of separating stereoisomers (12), and that acid catalyzes carotenoid cis/trans isomerization. Therefore, the two peaks likely represent stereoisomers of all-trans-neochrome (8′R, 8′S). The absorption spectrum of 5 consisted of three well-defined bands at 416, 440, 468 nm which were consistent with the literature and a %III/II measured as 96.0. The m/z of 5 was 584. Treatment of the flesh extract with trace amount of acid resulted in the disappearance of 5 and an appearance of two peaks located at higher retention times, superimposed with untreated 6 and the peak next to it, which by UV spectra analysis indicated a coelution of three carotenoids. UV/vis absorption maxima of the two peaks were identical (398, 420, 448 nm) and exhibited a hypsochromic shift of 18 nm by which we confirmed the presence of one 5, 6-epoxidegroup, or lutein-5, 6-epoxide in the molecule. The 5,8-epoxyderivatives, the stereoisomers chrysanthemaxanthin (8S) or flavoxanthin (8R) were the products. Based on the published elution order in a similar C30 column, the first eluted peak was identified as chrysanthemaxanthin (13). The peak has the same retention time and the UV absorption maxima as that of 6, whose m/z+ is 584.
We also treated all-trans-violaxanthin with acid and observed six very low intensity HPLC peaks with retention times ranging from 12 to 24 min. (data not shown). However, none of the compounds was predominant. Acid-catalyzed epoxide-furanoid rearrangement of violaxanthin generates multiple products including stereoisomers of luteoxanthin (8′R, 8′S), auroxanthin (8R, 8′R; 8R, 8′S; 8S, 8′S) (12). Our acid treated avocado flesh extract exhibited four distinct peaks from carotenoids 1–6: two of them were the same peaks from acid-catalyzed neoxanthin and the other two represent chrysanthemaxanthin and flavoxanthin. It is interesting to note that avocado skin extract, which does not contain neochrome and chrysanthemaxanthin, exhibited the same four peaks after the same acid treatment. Thus, we have uniquely characterized trans-neoxanthin (1), neochrome (3), lutein-5, 6-epoxide (5) and chrysanthemaxanthin (6) in the present study.
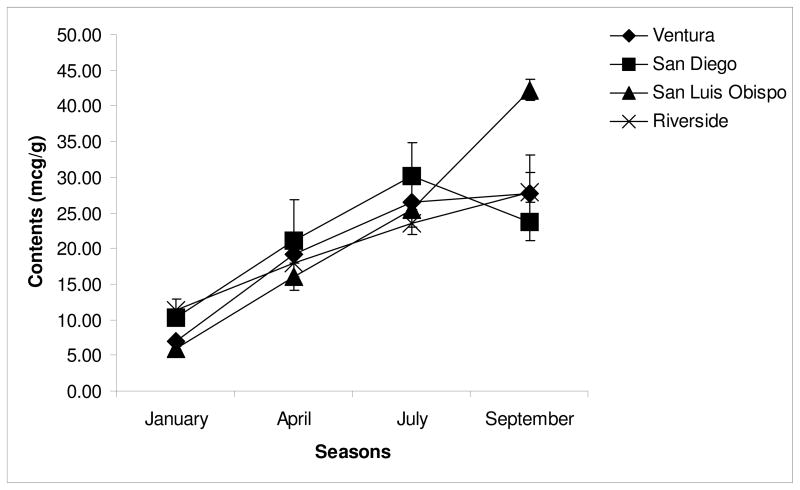
The levels of the eleven carotenoids in the flesh of the avocado samples from four growing locations across four harvesting times (January, April, July and September, 2008) are shown in Table 2. It was apparent that xanthophylls predominated over carotenes. In most samples, lutein was present in the highest concentration, followed by neoxanthin and then lutein-5, 6-epoxide in the avocado flesh. Variations in carotenoid contents from season to season and from samples within the same harvest were observed. San Luis Obispo samples had the largest variations in total carotenoids content from beginning to end of the season (5.9 vs. 42.2 μg/g, Figure 3). In the San Luis Obispo samples, among all the carotenoids quantified, levels of both neoxanthin and α-carotene increased by 26-fold, while levels of violaxanthin, 9′-cis-neoxanthin, lutein-5, 6-epoxide and β-carotene by more than 10-fold from January to September. In this region, the level of neoxanthin in September reached the highest level as observed at 12.0 μg/g, while the contents of lutein and lutein-5, 6-epoxide were also the highest (8.4 and 9.0 μg/g, respectively). The actual level of carotenoids in avocado might be a little bit higher than the reported data because an average recovery from extraction, saponification and HPLC was 77%.
Table 2.
Levels of Carotenoids in Avocado Flesh (μg/g)
| January | April | July | September | |
|---|---|---|---|---|
| Ventura | ||||
| neoxanthin | 0.46±0.02 | 5.32±0.32 | 6.16±0.94 | 5.28±1.10 |
| violaxanthin | 0.53±0.06 | 2.37±0.36 | 2.43±0.34 | 2.11±0.51 |
| neochrome | 0.37±0.00 | 0.66±0.05 | 1.20±0.16 | 1.45±0.04 |
| 9″-cis-neoxanthin | 0.23±0.01 | 0.94±0.09 | 1.25±0.06 | 1.51±0.07 |
| lutein-5,6-epoxide | 0.75±0.02 | 3.50±0.21 | 5.78±0.77 | 5.12±0.57 |
| chrysanthemaxanthin | 0.31±0.13 | 0.78±0.05 | 2.00±0.24 | 2.71±0.26 |
| lutein | 3.72±0.24 | 4.56±0.19 | 6.30±0.70 | 8.03±0.15 |
| zeaxanthin | 0.04±0.02 | 0.05±0.00 | 0.09±0.01 | 0.15±0.08 |
| β-cryptoxanthin | 0.30±0.05 | 0.18±0.03 | 0.21±0.05 | 0.21±0.02 |
| α-carotene | 0.03±0.02 | 0.32±0.02 | 0.45±0.07 | 0.54±0.07 |
| β-carotene | 0.21±0.03 | 0.56±0.04 | 0.58±0.11 | 0.62±0.05 |
| San Luis Obispo | ||||
| neoxanthin | 0.46±0.04 | 3.45±0.59 | 6.27±0.08 | 11.97±0.29 |
| violaxanthin | 0.44±0.03 | 1.77±0.12 | 2.72±0.07 | 4.75±0.10 |
| neochrome | 0.40±0.07 | 0.79±0.07 | 0.93±0.04 | 1.52±0.03 |
| 0.19±0.05 | 0.75±0.04 | 1.44±0.07 | 2.16± 0.01 | |
| lutein-5,6-epoxide | 0.78±0.15 | 2.51±0.23 | 5.22±0.25 | 8.99±0.59 |
| chrysanthemaxanthin | 0.42±0.17 | 1.21±0.49 | 1.29±0.15 | 2.52±0.05 |
| lutein | 2.83±0.27 | 4.33±0.24 | 5.92±0.25 | 8.42±0.23 |
| zeaxanthin | 0.06±0.02 | 0.05±0.00 | 0.04 0.01 | 0.06±0.01 |
| β-cryptoxanthin | 0.20±0.03 | 0.20±0.04 | 0.25±0.01 | 0.18±0.02 |
| α-carotene | 0.04±0.04 | 0.28±0.01 | 0.52± 0.04 | 0.65±0.04 |
| β-carotene | 0.10±0.04 | 0.69±0.07 | 0.88±0.02 | 1.01±0.07 |
| San Diego | ||||
| neoxanthin | 1.26±0.13 | 3.15±1.53 | 6.34±1.01 | 5.18±0.67 |
| violaxanthin | 1.11±0.13 | 1.87±0.56 | 2.59±0.50 | 1.89±0.23 |
| neochrome | 0.55±0.04 | 0.90±0.19 | 1.47±0.23 | 1.13±0.12 |
| 0.44±0.04 | 0.95±0.37 | 1.56±0.20 | 1.19±0.16 | |
| lutein-5,6-epoxide | 1.69±0.09 | 3.42±1.25 | 6.73±1.17 | 4.87±0.46 |
| chrysanthemaxanthin | 0.92±0.14 | 1.77±0.34 | 2.45±0.42 | 1.96±0.37 |
| lutein | 3.63±0.04 | 7.26±1.04 | 6.92±0.96 | 6.29±0.49 |
| zeaxanthin | 0.01±0.00 | 0.17±0.07 | 0.06±0.02 | 0.07±0.00 |
| β-cryptoxanthin | 0.23±0.04 | 0.39±0.10 | 0.21± 0.03 | 0.18±0.03 |
| α-carotene | 0.15± 0.03 | 0.46±0.11 | 0.89±0.09 | 0.48±0.10 |
| β-carotene | 0.33± 0.06 | 0.78±0.15 | 0.88±0.03 | 0.52±0.11 |
| Riverside | ||||
| neoxanthin | 1.27±0.24 | 4.12±0.68 | 4.82±0.56 | 6.29±1.49 |
| violaxanthin | 1.270.23 | 1.89±0.26 | 2.32±0.21 | 2.23±0.52 |
| neochrome | 0.55±0.08 | 0.79±0.08 | 1.10±0.05 | 1.61±0.25 |
| 0.48±0.02 | 0.86±0.14 | 1.21±0.11 | 1.46±0.24 | |
| lutein-5,6-epoxide | 1.74±0.19 | 3.37±0.39 | 4.94±0.29 | 6.19±1.35 |
| chrysanthemaxanthin | 0.87±0.30 | 1.35±0.05 | 2.08±0.08 | 2.72±0.38 |
| lutein | 4.31±0.29 | 4.39±0.18 | 5.42±0.11 | 6.02±0.82 |
| zeaxanthin | 0.01±0.02 | 0.05±0.01 | 0.05±0.01 | 0.04±0.01 |
| β-cryptoxanthin | 0.27±0.03 | 0.23±0.01 | 0.17±0.01 | 0.18±0.02 |
| α-carotene | 0.22±0.02 | 0.34±0.04 | 0.59±0.07 | 0.50±0.08 |
| β-carotene | 0.41±0.07 | 0.57±0.05 | 0.82±0.06 | 0.59±0.09 |
Figure 3.
Variation of total carotenoids in the flesh of avocados from different locations and seasons.
The color of avocado flesh varies from dark green just under the skin to pale green in the middle section of the flesh to yellow near the seed. Ashton et al (14) reported that in the three equally divided flesh sections, the total carotenoid concentrations were greatest in the dark green flesh after harvest and during ripening over ten days, and we confirmed these observation in our study. Since the content of carotenoids increases as one gets close to the peel, consumers should be advised to nick and peel the skin from the avocado prior to slicing the avocado, or to cut the fruit in half, remove the seed, peel after nicking the skin and then slice the fruit to get the nutrient-rich outer section of the avocado.
In addition to carotenoids, levels of α-tocopherol were also measured in flesh (Table 3). Variations in tocopherol content from different locations in the same season are much less compared to the season-to-season variations. It is worthy of note that highest level was observed in the same season as that of total carotenoids from July through September.
Table 3.
Levels of α-Tocopherol in Avocado Flesh (μg/g)
| Ventura | San Luis Obispo | San Diego | Riverside | |
|---|---|---|---|---|
| January, 2008 | 20.13 ± 0.29 | 17.85 ± 0.50 | 20.92 ± 2.42 | 16.27 ± 2.68 |
| April, 2008 | 19.05 ± 0.41 | 23.12 ± 4.84 | 21.73 ± 2.57 | 17.73 ± 1.53 |
| July, 2008 | 25.67 ± 4.06 | 23.31 ± 3.03 | 27.57 ± 2.27 | 23.96 ± 2.55 |
| September, 2008 | 27.05 ± 3.74 | 26.21 ± 0.96 | 20.87 ± 1.83 | 25.74 ± 1.82 |
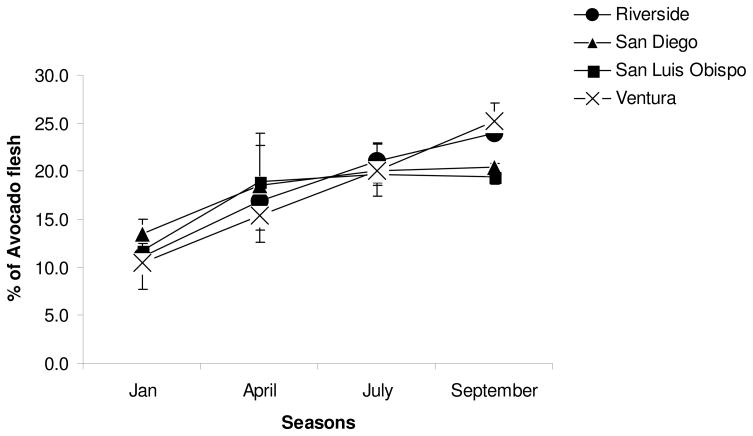
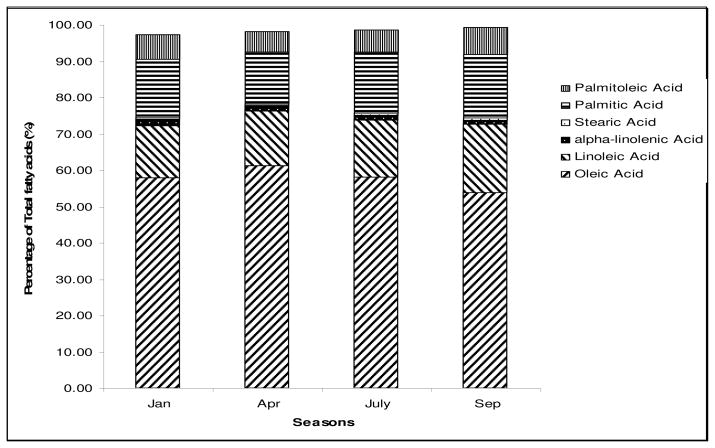
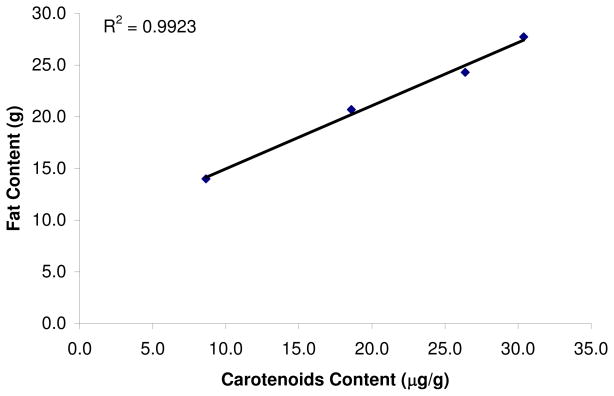
The fat content of the fruit increased over the growing season with maturation (see Figure 4). The fatty acid composition did not change so that the total amount of monounsaturated fat delivered per avocado increased over the growing season (see Figure 5). The carotenoids content is well correlated with the fat content (Figure 6).
Figure 4.
Variation of fat content in the flesh of avocados from different locations and seasons.
Figure 5.
Variation of the average fatty acids content in the flesh of avocados from different seasons
Figure 6.
Correlation of the average total carotenoids content vs. total fat content of the avocado flesh
In summary, California Hass avocados grown in different regions of Southern California have a similar phytochemical profile. However, there are significant and coincident increases in both total fat and carotenoids in fruit harvested later in the season. This seasonal maturation on the tree is characteristic of several common fruits among them the avocado and the banana. Research on the potential health benefits of the avocado should consider the time of harvest, degree of ripening, growing area of the particular fruits being studied, and the total phytochemical profile. These steps will enable researchers to account for potential nutrient-nutrient interactions that might affect their research outcomes.
Figure 2.
New identified carotenoids structures in avocado flesh
Acknowledgments
This research was supported by California Avocado Commission through an unrestricted educational grant and a NIH grant no. AT003960. The study was conducted and analyzed independently at the UCLA Center for Human Nutrition.
LITERATURE CITED
- 1.Whiley AW, Schaffer B. The Avocado: botany, production, and uses. CABI Publishing; New York: 2002. Chapter 1: History, Distribution and Uses; p. 1. [Google Scholar]
- 2.Lu QY, Arteaga JR, Zhang Q, Huerta S, Go VL, Heber D. Inhibition of prostate cancer cell growth by an avocado extract: role of lipid-soluble bioactive substances. J Nutr Biochem. 2005;16:23–30. doi: 10.1016/j.jnutbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Ding H, Han C, Guo D, Chin YW, Ding Y, Kinghorn AD, D’Ambrosio SM. Selective induction of apoptosis of human oral cancer cell lines by avocado extracts via a ROS-mediated mechanism. Nutr Cancer. 2009;61:348–356. doi: 10.1080/01635580802567158. [DOI] [PubMed] [Google Scholar]
- 4.Castillo-Juarez I, Gonzalez V, Jaime-Aguilar H, Martinez G, Linares E, Bye R, Romero I. Anti-Helicobacter pylori activity of plants used in Mexican traditional medicine for gastrointestinal disorders. J Ethnopharmacol. 2009;122:402–405. doi: 10.1016/j.jep.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Ernst E. Avocado-soybean unsaponifiables (ASU) for osteoarthritis - a systematic review. Clin Rheumatol. 2003;22:285–288. doi: 10.1007/s10067-003-0731-4. [DOI] [PubMed] [Google Scholar]
- 6.Ding H, Chin YW, Kinghorn AD, D’Ambrosioa SM. Chemopreventive characteristics of avocado fruit. Seminars in Cancer Biology. 2007;17:386–394. doi: 10.1016/j.semcancer.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz W. Official methods of Analytical of AOAC International. 17. AOAC international; Gaithersburg, MD, USA: 2000. AOAC Official Method 989.05 Fat in milk; p. 33.2.26. [Google Scholar]
- 8.Horwitz W. Official methods of Analytical of AOAC International. 17. AOAC international; Gaithersburg, MD, USA: 2000. AOAC Official Method 996.06 Fat (Total, saturated, unsaturated) in food; p. 41.1.28A. [Google Scholar]
- 9.Khachik F, Beecher GR, Goli MB, Lusby WR. Separation and Quantitation of Carotenoids in Foods. Methods in Enzymology. 1992;213:347–359. doi: 10.1016/0076-6879(92)13136-l. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JB, Kline MC, Gill LM, Yen JH, Duewer DL, Sniegoski LT, Sharpless KE. Preparation and value assignment of standard reference material 968c fat-soluble vitamins, carotenoids, and cholesterol in human serum. Clinica Chimica Acta. 2001;305:141 – 155. doi: 10.1016/s0009-8981(00)00429-0. [DOI] [PubMed] [Google Scholar]
- 11.Cholnoky L, Gyorgyfy K, Ronai A, Szabolcs J, Toth G, Galasko G, Mallams AK, Waight ES, Weedon BCL. Carotenoids and Related Compounds .21. Structure of Neoxanthin (Foliaxanthin) Journal of the Chemical Society C-Organic. 1969:1256. [Google Scholar]
- 12.Asai A, Terasaki M, Nagao A. An epoxide-furanoid rearrangement of spinach neoxanthin occurs in the gastrointestinal tract of mice and in vitro: Formation and cytostatic activity of neochrome stereoisomers. Journal of Nutrition. 2004;134:2237–2243. doi: 10.1093/jn/134.9.2237. [DOI] [PubMed] [Google Scholar]
- 13.Melendez-Martinez AJ, Britton G, Vicario IM, Heredia FJ. HPLC analysis of geometrical isomers of lutein epoxide isolated from dandelion (Taraxacum officinale F. Weber ex Wiggers) Phytochemistry. 2006;67:771–777. doi: 10.1016/j.phytochem.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Ashton OB, Wong M, McGhie TK, Vather R, Wang Y, Requejo-Jackman C, Ramankutty P, Woolf AB. Pigments in avocado tissue and oil. J Agric Food Chem. 2006;54:10151–10158. doi: 10.1021/jf061809j. [DOI] [PubMed] [Google Scholar]